Abstract
The eukaryotic translation initiation factor 5A (eIF5A) is the only cellular protein that contains an unusual amino acid, hypusine [Nε-(4-amino-2-hydroxybutyl)-lysine]. eIF5A and its hypusine/deoxyhypusine modification are vital for eukaryotic cell proliferation. Hypusine is formed posttranslationally by two enzymatic steps catalyzed by deoxyhypusine synthase and deoxyhypusine hydroxylase. Deoxyhypusine hydroxylase catalyzes a stereo-specific hydroxylation of the deoxyhypusine residue in the eIF5A intermediate protein, eIF5A(Dhp). The enzyme is totally specific for this protein and does not act on short peptides (<50 amino acids). The assay measures the conversion of the radiolabeled deoxyhypusine residue to a hypusine residue in eIF5A. Optimum conditions for the reaction and two detection methods for the product, hypusine-containing eIF5A, are described in this chapter. The first, and most reliable, method is the measurement of the amount of [3H]hypusine in the protein hydrolysate after its separation from [3H]deoxyhypusine, by ion exchange chromatography. This method does require specialized equipment. The second method is based on counting the total TCA soluble radioactivity after sodium periodate oxidation of the reaction mixture, since the radiolabeled 4-amino-2-hydroxy butyl moiety of the hypusine residue is cleaved and is released from protein as radiolabeled β-propionaldehyde and formaldehyde by periodate oxidation.
Keywords: Polyamine, Spermidine, Deoxyhypusine hydroxylase, Deoxyhypusine, Hypusine, Monooxygenase, eIF5A, Posttranslational modification, Protein hydroxylation
1. Introduction
Deoxyhypusine hydroxylase (deoxyhypusine monooxygenase, DOHH) (EC. 1.14.99.29) catalyzes the final step in the posttranslational synthesis of an unusual amino acid, hypusine [Nε-(4-amino-2-hydroxybutyl)-lysine] (see a recent review (1)). This enzyme converts the deoxyhypusine-containing eIF5A intermediate, eIF5A(Dhp), to the mature hypusine-containing form, eIF5A(Hpu), by stereo-specific hydroxylation at the C2 of the aminobutyl side chain of the deoxyhypusine residue ((2, 3), Scheme 1). DOHH exhibits a strict substrate specificity toward its protein substrate (4, 5).
DOHH is a non-heme diiron enzyme that is inhibited by iron chelators (6). It is a HEAT-repeat protein consisting of eight tandem α-helical hairpin structures (3). It has a structure and mechanism distinct from those of other 2-oxoacid and iron-dependent dioxygenases, such as prolyl or lysyl hydroxylases. Rather, DOHH resembles the monooxygenases with a diiron active center, such as methane monooxygenase (7). Cultured cells or tissues normally contain DOHH enzyme, but little or no substrate protein, deoxyhypusine-containing eIF5A intermediate, eIF5A(Dhp). Thus, radiolabeled eIF5A(Dhp) must be prepared and added as a substrate to measure the enzyme activity. The cDNAs for eIF5A (8), DHS (9), and DOHH (3) have been cloned, and recombinant proteins can be purified by simple methods (3, 9–11). Radiolabeled eIF5A(Dhp) can be prepared by an in vitro DHS reaction using purified recombinant eIF5A(Lys) and DHS (12).
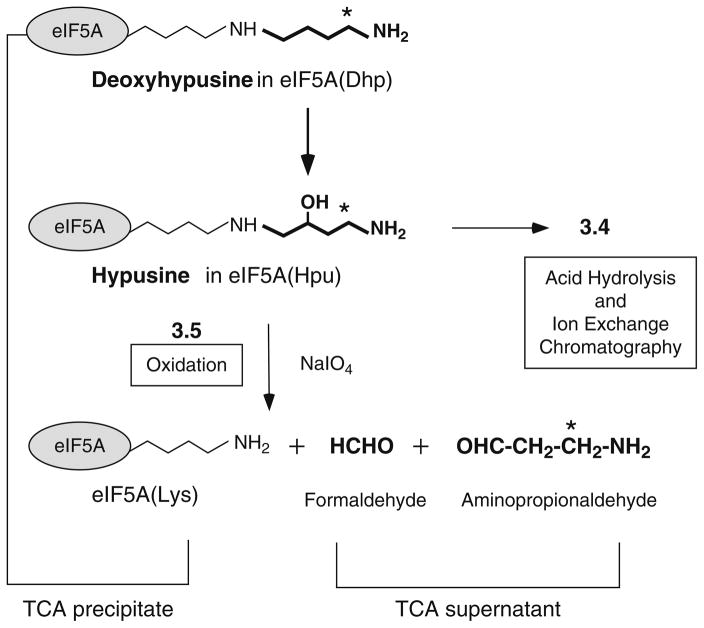
Typical reaction conditions for deoxyhypusine hydroxylase are described, and two methods are presented for measurement of the hypusine formed (Fig. 1). The [3H]hypusine formed in eIF5A can be measured after acid hydrolysis of the proteins and its separation from [3H]deoxyhypusine by ion exchange chromatographic separation (Fig. 2) (6, 13). This first method gives the most accurate estimate of the amount of [3H]hypusine formed. However, it requires an amino acid analyzer (or HPLC system) with a high resolution ion exchange column (14) to separate two similar amino acids, hypusine and deoxyhypusine. For those who do not have access to such a separation system, an oxidation method can be employed for an estimation of DOHH activity. Unlike deoxyhypusine, hypusine contains a vicinal amino (secondary) and hydroxyl group, which can be cleaved by periodate oxidation (15–17). Thus, the radiolabeled 4-amino-2-hydroxybutyl side chain of the hypusine residue of the reaction product, eIF5A(Hpu), is cleaved to radiolabeled β-propionaldehyde and formaldehyde by periodate oxidation (Fig. 1), whereas the deoxyhypusine-containing substrate protein is not cleaved. The radiolabeled β-propionaldehyde released from the hydroxylated protein, eIF5A(Hpu), is soluble in TCA and can be measured after its separation from proteins by TCA precipitation.
Fig. 1.
Biosynthesis of hypusine by two enzymatic steps and degradation of hypusine residue by periodate oxidation. Deoxyhypusine hydroxylase catalyzes hydroxylation of the deoxyhypusine residue at C2 of its aminobutyl side chain. Sodium (meta) periodate causes oxidative cleavages (due to vicinal amino hydroxyl groups) of the hypusine side chain to release β-propionaldehyde, which is measured as the radioactivity in the TCA supernatant, whereas the unreacted substrate protein eIF5A([3H]Dhp) is precipitated with TCA. The asterisk indicates the position of radioactivity derived from [1,8-3H]spermidine.
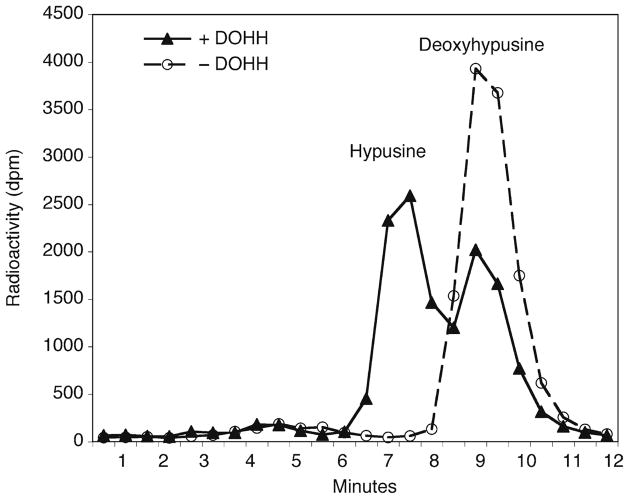
Fig. 2.
Ion exchange chromatographic separation of deoxyhypusine and hypusine. The proteins in the DOHH reaction mixture were precipitated with TCA and hydrolyzed in 6 N HCl. The amino acids in the hydrolyzed proteins were separated by ion exchange chromatography on DC6A column using PA Buffer B. Hypusine is eluted from the column 2 min earlier than deoxyhypusine.
2. Materials
2.1. Equipment
Plastic Eppendorf tubes (1.5 ml size) with screw caps, resistant to heating at 110°C.
Dry heating block (108°C) for acid hydrolysis.
SpeedVac concentrator (e.g., Savant) with a NaOH trap.
An amino acid analyzer with an ion exchange column (e.g., 0.4 × 7.5 cm, DC-6A cation exchange resin) (see Note 1).
Liquid scintillation fluid that tolerates high salt buffers (Optiphase High Safe 3 from PerkinElmer works well).
2.2. Reagent Stock Solutions
Use deionized or ultrapure water for preparation of all solutions.
Buffer A (50 mM Tris–HCl buffer pH 7.5, 1 mM DTT).
Mammalian protease inhibitor cocktail not containing EDTA (Sigma or Roche).
M Tris–HCl buffer (pH 7.5).
PBS (phosphate buffered saline).
Spermidine (100 mM), store frozen at −20°C in aliquots.
eIF5A(Dhp) substrate (2 μM, 40,000 dpm/μl, if the specific activity of [1,8-3H]spermidine used is 20 Ci/mmol). Store aliquots of solutions in 50 mM Tris–HCl pH 7.5, 1 mM DTT, 1 mg/ml of BSA at −20°C.
DTT (100 mM), the solution of 100 mM DTT (15.4 mg/ml in water) is stored frozen at −20°C in aliquots.
BSA (50 mg/ml in water), store frozen at −20°C in aliquots.
TCA solution (10% trichloroacetic acid). Store at 4°C.
6 N HCl solution made by 1:1 dilution of concentrated HCl (Subheading 3.3, step 1).
Buffer B (13.2 mM sodium citrate buffer, 1.5 N NaCl, pH 5.55, 0.1% Phenol) and PA buffer C (53 mM sodium citrate buffer, 3.0 N NaCl, pH 5.55, 0.1% Phenol). Buffer is stored at room temperature and filtered, and Brij (0.1%) is added before use.
0.2 M Na2HPO4, 45 mM Citric acid buffer pH 6.4.
0.3 M Sodium (meta)periodate, NaIO4. Make fresh solution just before use.
3. Methods
3.1. Preparation of eIF5A([3H] Dhp) Substrate Protein (High Specific Activity) by In Vitro DHS Reaction (see Note 2)
Set up a large scale DHS reactions in a mixture (2 ml) containing 0.2 M Glycine NaOH pH 9.2 buffer, 1 mM DTT, 1 mM NAD, 35 μM eIF5A(Lys) (1.2 mg, 70 nmol), 37.5 μM [1,8-3H]spermidine (75 nmol, 1.5 mCi, if the specific activity is 20 Ci/mmol), and 1 × 105 units of DHS (~0.1 mg of freshly prepared human recombinant enzyme) and incubate at 37°C for 3 h.
Take 20 μl of reaction mixture to estimate the amount of radiolabeled eIF5A in the TCA precipitate after thorough washing in TCA containing 1 mM polyamines, to calculate the total yield from the large scale reaction (see Note 3).
The unreacted [1,8-3H]spermidine is removed from the labeled protein substrate by repeated ammonium sulfate precipitation as follows. To the reaction mixture (2 ml), add 0.55 g (45% saturation) of finely ground ammonium sulfate, mix well until all the ammonium sulfate dissolves, and keep on ice for 20 min. Remove the precipitated proteins by centrifugation in a microfuge at 15,000 × g, at 4°C for 20 min. Add ammonium sulfate to the supernatant to 80% saturation to precipitate eIF5A. After centrifugation, remove the supernatant completely and dissolve the precipitate in 2 ml of Buffer A containing 1 mM unlabeled spermidine. This sample is precipitated again with ammonium sulfate (80% saturation), and this step is repeated three more times to remove all the unreacted [1,8-3H]spermidine.
Dissolve the proteins from the fourth ammonium sulfate precipitation in 2 ml of Buffer A and dialyze against ice-cold Buffer A for 3 h to remove the salts and unreacted radioactive spermidine.
To 5 μl of the dialyzed sample and add 250 μg of carrier BSA and 0.1 ml of 10% TCA. Keep on ice for 10 min and centrifuge the precipitate in microfuge (15,000 × g at 4°C, 10 min).
Separate the supernatant and dissolve the pellet in 0.1 ml of 0.1 N NaOH solution.
Count radioactivity in the supernatant and in the pellet to determine the radiopurity of the substrate protein (see Note 4).
If the substrate protein is sufficiently pure (>98% TCA precipitable), dilute the substrate to 2 μM and add carrier BSA to 1 mg/ml and store in aliquots at −20°C.
3.2. Preparation of the Enzyme Extract from Cultured Cells or Tissues
For preparation of a crude enzyme extract, sonicate freshly harvested cell pellets in Buffer A (50 mM Tris–HCl buffer (pH 7.5), 1 mM DTT) containing protease inhibitor cocktail not containing EDTA, (0.2 ml buffer for 107 cells), or homogenize tissues in the same buffer (1 ml per 1 g wet tissue).
Centrifuge the lysates in a refrigerated microfuge (at 15000 × g) for 20 min and take supernatants for protein determination and enzyme assays. Use 100–500 μg of total protein per assay as an enzyme source (see Note 5).
3.3. Deoxyhypusine Hydroxylase Assay
-
Just before the assay, make a master mixture by multiplying each volume by the sample number (n) and keep on ice in an Eppendorf tube.
Stock solution volume per assay final concentration1 M Tris–HCl pH 7.5 0.8 μl 20 mM 100 mM DTT 2.4 μl 6 mM 50 mg/ml BSA 0.8 μl 1 mg/ml eIF5A([3H]-Dhp) (2 μM) 2 μl 0.1 μM H2O 14 μl Volume of master mix per tube 20 μl Enzyme solution/lysate (see Note 6) 20 μl Total volume 40 μl Set up screw cap plastic Eppendorf tubes and number them. Add an enzyme sample in 20 μl to each tube on ice. Use 20 μl of Buffer A as a negative control and 2–10 units of purified DOHH (if available) as a positive control (see Note 7).
Start the reaction by adding 20 μl of the master mix to each tube. Mix gently and cap each tube.
Incubate the reaction mixtures in a 37°C water bath for 2 h.
3.4. Determination of Hypusine Formed after Ion Exchange Chromatographic Separation
Stop the reaction by adding 5 μl (250 μg) of carrier BSA and 0.2 ml of ice-cold 10% TCA solution. Gently mix and keep on ice for 20 min.
Centrifuge the TCA precipitated samples in a refrigerated microfuge (4°C) at 15,000 × g for 5 min and remove the supernatant and count radioactivity in the supernatant (see Note 8).
After removing all the TCA supernatant, add 0.4 ml of 6 N HCl to the pellet, tighten the cap and place in a heating block at 108°C overnight.
Dry the hydrolyzed samples using a SpeedVac concentrator set up with a NaOH trap.
Add 0.1 ml of water to each tube to dissolve dried residues. Count a 5 μl aliquot and apply an aliquot containing up to 50,000 dpm to the amino acid analyzer (see Note 9).
Separate hypusine from deoxyhypusine by running 13 min of PA Buffer B at the speed of 0.75 ml per min. Collect 26 tubes of 0.5 min fractions and count the total radioactivity in a liquid scintillation counter. In our system, the elution peak time for hypusine is 7.25 min and deoxyhypusine is approximately 9.25 min (Fig. 2) (see Note 10).
Sum the radioactivity of the hypusine peak (usually in 4–5 fractions) after subtraction of background radioactivity in the negative control, and multiply the number by a factor (100/volume injected) to estimate the total amount of hypusine formed in each reaction (see Note 11). Sum the radioactivity in both hypusine and deoxyhypusine peaks to estimate % conversion.
3.5. Determination of Protein-Bound Hypusine by Periodate Oxidation
If an amino acid analyzer or HPLC system is not available and steps 3 and 4 cannot be used, one can estimate hypusine formed by periodate oxidation of the reaction mixture (see Note 12).
To half of the reaction mixture (use 20 μl), add 5 μl (250 μg) of carrier BSA and 100 μl of 10% TCA. Keep on ice for 20 min, spin and measure the radioactivity in the TCA supernatant (see Note 8).
To the other half of the reaction mixture (20 μl), add 40 μl of Sodium phosphate/citrate buffer.
Add 15 μl of sodium (meta)periodate (0.3 M) solution and mix well and keep at RT for 1 h.
Add 5 μl (250 μg) of carrier BSA and add 0.2 ml of 10% TCA and keep on ice for 20 min.
Centrifuge the precipitates in the microfuge at 4°C at 15,000 × g for 5 min.
Take all the supernatant and count the radioactivity in the supernatant. Subtract the radioactivity in the TCA supernatant of unoxidized sample from that of the oxidized sample to calculate the hypusine formed (see Note 13).
Acknowledgments
This research was supported by the Intramural Research Program of the NIDCR, National Institutes of Health.
Footnotes
We use a Dionex D-400 analyzer with a column (0.4 × 7.5 cm) of cation exchange resin, DC-6A (13, 14). The resin has hydrophobic as well as ionic interactions with amino acids and polyamines. Since hypusine is less hydrophobic than deoxyhypusine due to a hydroxyl group addition, it is eluted 2 min earlier than deoxyhypusine under our separation conditions. Elution conditions on our analyzer are temperature 66°C, flow rate 0.75 ml/min, and we collect 0.5 min fractions directly into 7 ml glass scintillation vials.
Alternatively, the deoxyhypusine containing eIF5A(Dhp) can be prepared from mammalian cells cultured in the presence of (1,8-3H)spermidine and metal chelating inhibitors of DOHH (2, 16). However, the yield of radiolabeled eIF5A((3H)Dhp) is very low, compared to that from the in vitro DHS reaction. Endogenous spermidine in cells (~10 nmol per mg protein) dilutes the specific activity of (1,8-3H)spermidine added to the medium. This dilution has to be taken into consideration in estimation of specific activity of radiolabeled substrate protein prepared from cells.
Under this reaction condition, approximately 15% of eIF5A(Lys) is converted to the deoxyhypusine protein and a total of 10 nmol (2 × 108 dpm, if the specific activity of (1,8-3H)spermidine used is 20 Ci/mmol) is obtained. Since unreacted eIF5A(Lys) does not interfere with the DOHH reaction (4), it is not necessary to separate the labeled eIF5A(Dhp) from the eIF5A(Lys) protein.
It is critical to get rid of all unreacted (1,8-3H)spermidine (to a level not more than 1–2% of total radioactivity in the substrate preparation) by repeated ammonium sulfate precipitation and dialysis. It is important to keep the background radioactivity in the TCA supernatant low especially when the oxidation method is used for estimation of hypusine.
Fresh cultured cells or tissues contain approximately 1–10 units of DOHH activity per mg protein (2). 1 unit of DOHH activity is defined as that converting 1 pmol of eIF5A((3H)Dhp) to eIF5A((3H)Hpu) in 2 h at 37°C.
The protein concentration of cell or tissue crude extracts should be high (10–25 mg/ml) to test activity of lysates containing up to 200–500 μg protein in 20 μl. To test the activity of less than 20 μl of extract, dilute it with buffer A to 20 μl.
The recombinant human DOHH or yeast DOHH can be purified from BL21(DE3) cells transformed with pGEX-4T-3/hDOHH or pGEX-4T-3/yDOHH plasmids (3).
The radioactivity in the TCA supernatant would be either due to (1,8-3H)spermidine contaminant in the substrate preparation or due to proteolytic degradation of eIF5A((3H) Dhp). If the cell or tissue extract contains the protease inhibitor cocktail, the level of proteolytic degradation is usually quite low. If the extract is prepared from tissues or cells highly overexpressing DHS, one may observe the release of radioactivity due to reversal of deoxyhypusine synthesis (12). However, with normal cells or tissues, the reversal is negligible. One may include one control with a DOHH inhibitor (0.1 mM ciclopirox olamine) to obtain a value for DOHH-mediated release of radioactivity.
By estimating the total radioactivity in 100 μl of a hydrolyzed sample, one can calculate the volume of sample to apply to the column. It is recommended not to overload the column with too high radioactivity (>50,000 dpm) to avoid machine contamination from sample overloading and carry-over to the next run.
The radioactive impurity in the substrate preparation (mainly [1,8-3H]spermidine, should be <2% of total radioactivity of the substrate) can be removed by TCA precipitation after DOHH reaction. Thus, as many as 20 samples can be analyzed in sequence in PA Buffer B without much interference due to spermidine contaminant. However, if the background level of radioactivity in collected fractions increases, a 30 min wash with PA Buffer C and reequilibration (20 min) with PA Buffer B is recommended.
The radioactivity in the hypusine fraction in dpm can be converted to pmol as follows. For a batch of [1,8-3H] spermidine with 20 Ci/mmol specific activity, 1 pmol of spermidine is 4 × 104 dpm. Since only the aminobutyl side of [1,8-3H] spermidine is incorporated into deoxyhypusine, the specific activity of [3H]deoxyhypusine is 2 × 104 dpm per pmol.
In Subheading 3.3, step 2, one only need to count TCA soluble radioactivity released by periodate oxidation. This works when the radiolabeled substrate protein is highly pure and contain a minimum level (<2% of total radioactivity in the substrate preparation) of [1,8-3H] spermidine. If the substrate protein is contaminated with a significant amount of [1,8-3H] spermidine, this will cause a high background radioactivity in the TCA supernatant. In this case, it is necessary to distinguish between the radioactivity in the β-propionaldehyde from that of [1,8-3H] spermidine. A method employing reaction of aldehydes with dimedone (5,5-dimethyl-1,3-cyclo-hexanedione) and extraction of the aldehyde/dimedone adducts with toluene was reported (17). However, the method in Subheading 3.3, step 2 is simple and more accurate than the dimedone method, if the radiolabeled protein substrate is highly pure.
Under this condition, periodate oxidation completely cleaves the hypusine residue without causing any degradation of the deoxyhypusine residue or the eIF5A protein. The difference between the radioactivity in the TCA supernatant of the oxidized reaction mixture (step 6) and that of the oxidized sample in step 10 is the radioactivity of hypusine formed.
References
- 1.Park MH. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A) J Biochem. 2006;139:161–169. doi: 10.1093/jb/mvj034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbruzzese A, Park MH, Folk JE. Deoxyhypusine hydroxylase from rat testis. Partial purification and characterization. J Biol Chem. 1986;261:3085–3089. [PubMed] [Google Scholar]
- 3.Park JH, Aravind L, Wolff EC, Kaevel J, Kim YS, Park MH. Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: a HEAT-repeat-containing metalloenzyme. Proc Natl Acad Sci USA. 2006;103:51–56. doi: 10.1073/pnas.0509348102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang KR, Kim YS, Wolff EC, Park MH. Specificity of the deoxyhypusine hydroxylase-eukaryotic translation initiation factor (eIF5A) interaction: identification of amino acid residues of the enzyme required for binding of its substrate, deoxyhypusine-containing eIF5A. J Biol Chem. 2007;282:8300–8308. doi: 10.1074/jbc.M607495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakus J, Wolff EC, Park MH, Folk JE. Features of the spermidine-binding site of deoxyhypusine synthase as derived from inhibition studies. Effective inhibition by bis- and mono-guanylated diamines and polyamines. J Biol Chem. 1993;268:13151–13159. [PubMed] [Google Scholar]
- 6.Park MH, Cooper HL, Folk JE. The biosynthesis of protein-bound hypusine (Nε-(4-amino-2-hydroxybutyl)lysine). Lysine as the amino acid precursor and the intermediate role of deoxyhypusine (Nε-(4-aminobutyl) lysine) J Biol Chem. 1982;257:7217–7222. [PubMed] [Google Scholar]
- 7.Vu VV, Emerson JP, Martinho M, Kim YS, Munck E, Park MH, Que L., Jr Human deoxyhypusine hydroxylase, an enzyme involved in regulating cell growth, activates O2 with a nonheme diiron center. Proc Natl Acad Sci USA. 2009;106:14814–14819. doi: 10.1073/pnas.0904553106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smit-McBride Z, Dever TE, Hershey JW, Merrick WC. Sequence determination and cDNA cloning of eukaryotic initiation factor 4D, the hypusine-containing protein. J Biol Chem. 1989;264:1578–1583. [PubMed] [Google Scholar]
- 9.Joe YA, Wolff EC, Park MH. Cloning and expression of human deoxyhypusine synthase cDNA. Struct function studies recombinant enzyme mutant proteins. J Biol Chem. 1995;270:22386–22392. doi: 10.1074/jbc.270.38.22386. [DOI] [PubMed] [Google Scholar]
- 10.Smit-McBride Z, Schnier J, Kaufman RJ, Hershey JW. Protein synthesis initiation factor eIF-4D. Funct comparison native unhypusinated forms protein. J Biol Chem. 1989;264:18527–18530. [PubMed] [Google Scholar]
- 11.Joe YA, Park MH. Structural features of the eIF-5A precursor required for post-translational synthesis of deoxyhypusine. J Biol Chem. 1994;269:25916–25921. [PubMed] [Google Scholar]
- 12.Park JH, Wolff EC, Folk JE, Park MH. Reversal of the deoxyhypusine synthesis reaction. Generation spermidine or homospermidine deoxyhypusine by deoxyhypusine synthase. J Biol Chem. 2003;278:32683–32691. doi: 10.1074/jbc.M304247200. [DOI] [PubMed] [Google Scholar]
- 13.Park MH, Cooper HL, Folk JE. Chromatographic identification of hypusine (Nε-(4-amino-2-hydroxyl)lysine) and deoxyhypusine (Nε-(4-aminobutyl)lysine) Meth Enzymol. 1983;94:458–462. [Google Scholar]
- 14.Folk JE, Park MH, Chung SI, Schrode J, Lester EP, Cooper HL. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980;255:3695–3700. [PubMed] [Google Scholar]
- 15.Park MH, Cooper HL, Folk JE. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci USA. 1981;78:2869–2873. doi: 10.1073/pnas.78.5.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbruzzese A, Park MH, Folk JE. Indirect assays for deoxyhypusine hydroxylase using dual-label ratio changes and oxidative release of radioactivity. Anal Biochem. 1986;154:664–670. doi: 10.1016/0003-2697(86)90044-8. [DOI] [PubMed] [Google Scholar]
- 17.Csonga R, Ettmayer P, Auer M, Eckerskorn C, Eder J, Klier H. Evaluation of the metal ion requirement of the human deoxyhypusine hydroxylase from HeLa cells using a novel enzyme assay. FEBS Lett. 1996;380:209–214. doi: 10.1016/0014-5793(96)00020-8. [DOI] [PubMed] [Google Scholar]