Abstract
Oxidative stress, a redox imbalance between the endogenous reactive species and antioxidant systems, is common to numerous pathological conditions such as cancer, central nervous system injuries, radiation injury, diabetes etc. Therefore, compounds able to reduce oxidative stress have been actively sought for over 3 decades. Superoxide is the major species involved in oxidative stress either in its own right or through its progeny, such as ONOO−, H2O2, ·OH, CO3·−, and ·NO2. Therefore, the very first compounds developed in the late 1970-ies were the superoxide dismutase (SOD) mimics. Thus far the most potent mimics have been the cationic meso Mn(III) N-substituted pyridylporphyrins and N,N′-disubstituted imidazolylporphyrins (MnPs), some of them with kcat(O2·−) similar to the kcat of SOD enzymes. Most frequently studied are ortho isomers MnTE-2-PyP5+, MnTnHex-2-PyP5+, and MnTDE-2-ImP5+. The ability to disproportionate O2·− parallels their ability to remove the other major oxidizing species, peroxynitrite, ONOO−. The same structural feature that gives rise to the high kcat (O2·−) and kred (ONOO−), allows MnPs to strongly impact the activation of the redox-sensitive transcription factors, HIF-1α, NF-κB, AP-1, and SP-1, and therefore modify the excessive inflammatory and immune responses. Coupling with cellular reductants and other redox-active endogenous proteins seems to be involved in the actions of Mn porphyrins.
While hydrophilic analogues, such as MnTE-2-PyP5+ and MnTDE-2-ImP5+ are potent in numerous animal models of diseases, the lipophilic analogues were developed to cross blood brain barrier and target central nervous system and critical cellular compartment, mitochondria. The modification of its structure, aimed to preserve the SOD-like potency and lipophilicity, and diminish the toxicity, has presently been pursued. The pulmonary radioprotection by MnTnHex-2-PyP5+ was the first efficacy study performed successfully with non-human primates. The Phase I toxicity clinical trials were done on amyotrophic lateral sclerosis patients with N,N′-diethylimidazolium analogue, MnTDE-2-ImP5+ (AEOL10150). Its aggressive development as a wide spectrum radioprotector by Aeolus Pharmaceuticals has been supported by USA Federal government. The latest generation of compounds, bearing oxygens in pyridyl substituents is presently under aggressive development for cancer and CNS injuries at Duke University and is supported by Duke Translational Research Institute, The Wallace H. Coulter Translational Partners Grant Program, Preston Robert Tisch Brain Tumor Center at Duke, and National Institute of Allergy and Infectious Diseases.
Metal center of cationic manganese porphyrins easily accepts and donates electrons as exemplified in the catalysis of O2·− dismutation. Thus such compounds may be equally good anti- and pro-oxidants; in either case the beneficial therapeutic effects may be observed. Moreover, while the in vivo effects may appear antioxidative, the mechanism of action of MnPs that produced such effects may be pro-oxidative; the most obvious example being the inhibition of NF-κB. The experimental data therefore teach us that we need to distinguish between the mechanism/s of action/s of MnPs and the effects we observe.
A number of factors impact the type of action of MnPs leading to favorable therapeutic effects: levels of reactive species and oxygen, levels of endogenous antioxidants (enzymes and low-molecular compounds), levels of MnPs, their site of accumulation, and the mutual encounters of all of those species. The complexity of in vivo redox systems and the complex redox chemistry of MnPs challenge and motivate us to further our understanding of the physiology of the normal and diseased cell with ultimate goal to successfully treat human diseases.
Keywords: Mn porphyrins, SOD mimics, peroxynitrite scavengers, pro-oxidants, antioxidants, oxidative stress, reactive species, MnTE-2-PyP5+, MnTnHex-2-PyP5+, MnTDE-2-ImP5+, AEOL10113, AEOL10150
Introduction
A seminal paper of Gershman and Gilbert in 1954 drew the parallel between the effects of oxygen and those of ionizing radiation, and proposed that the damaging effects of oxygen are primarily due to oxygen radicals [1]. Another seminal paper of McCord and Fridovich in 1969 assigned the action of copper proteins, haemocuprein/hepatocuprein/cerebrocuprein to superoxide dismuting ability; superoxide dismutases were born [2]. Babior and coworkers early reports in 1970-ies on NADPH oxidase showed that reactive species, O2·− and H2O2, constitute inflammatory response to injury or toxin exposure [3–5]. NADPH oxidases [6–9] are now known to be widely distributed in many types of cells and cellular compartments under physiological conditions. Superoxide and peroxide are considered important players in cell signaling, proliferation, survival and death [10,11]. Species that arise from O2·− and H2O2, as well as other species such as nitrated lipids [10,11], S-nitrosoproteins [12], lipid radicals, cysteine radical and others, have been implicated in redox-based signaling pathways too [10,11,13–17]. The balance between the reactive species and the endogenous antioxidants allows cell to function normally. If the excess of reactive species and/or loss of endogenous antioxidant defenses occurs, and the subsequent oxidative stress is not managed properly, cells undergo apoptosis or necrosis. Oxidative stress has been implicated in the numerous pathological disorders, such as radiation injury, cancer, diabetes, injuries of central nervous system, etc. Therefore the synthetic and natural antioxidants have been aggressively sought. In particular, drugs have been developed to target mitochondria, subcellular compartments responsible for healthy cell functioning and for its metabolic failure [18].
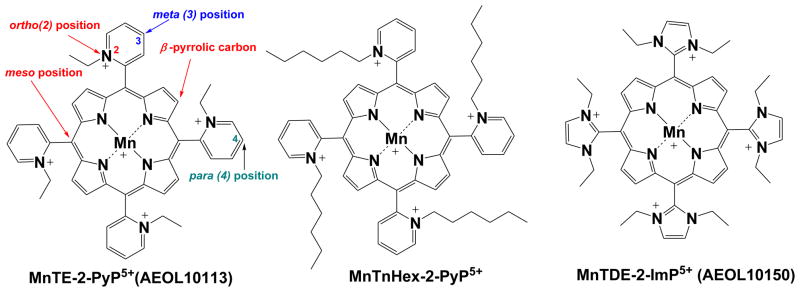
Metalloporphyrins are superior compounds as oxidative stress modulators for the same reasons nature is using them as the key active sites in numerous enzymes and proteins such as hemoglobin, myoglobin, cyt P450, oxygenases, oxidases, nitric oxide synthases, guanylyl cyclase etc: (1) macrocyclic porphyrin ligand provides complexes of extreme stabilities assuring the integrity of the metal site which in turn allows metal (primarily Mn, or Fe) to accomplish their redox-based actions; their stability is so high that its determination is precluded; (2) possibilities are endless for structural modifications aimed at fine tuning of their redox-based properties, bioavailability and toxicity. The first studies of the ability of metalloporphyrins to interact with small reactive molecules were reported by Pasternack, Halliwell, Faraggi, Weinraub and others in the late 1970s and early 1980s [19–27]. In the early1990-ies, Irwin Fridovich started exploring Mn porphyrins as potential SOD mimics [28]. The two decade-long exploration has resulted in the design of appropriate ligands around the metal site which (1) provide equal kinetic and thermodynamic facilitation for the O2·− dismutation as seen with superoxide dismutases; (2) are widely bioavailable; and (3) are fairly non-toxic. Those cationic Mn porphyrins that are potent SOD mimics have electron-deficient metal site which is in need of electron-donating ligands. They thus favor approach and/or binding of the electron rich anionic reactive species, such as O2·−, ONOO−, ONOOCO2−, CO3·−, ClO−. Further, this very cationic nature allows them to accumulate in critical cellular compartments, mitochondria, driven there by mitochondrial membrane negative potential. The negatively charged phospholipids may be at least in part the driving force for MnP accumulation in the central nervous system. We have detailed the design of such compounds based on the structure-activity relationships as well as their biology and pharmacology in our recent reviews [18,29–31]. The most in vivo studied Mn porphyrins are listed in Figure 1.
Figure 1.
The most frequently studied redox-active Mn porphyrins for treating oxidative stress disorders, MnTE-2-PyP5+, MnTnHex-2-PyP5+ and MnTDE-2-ImP5+.
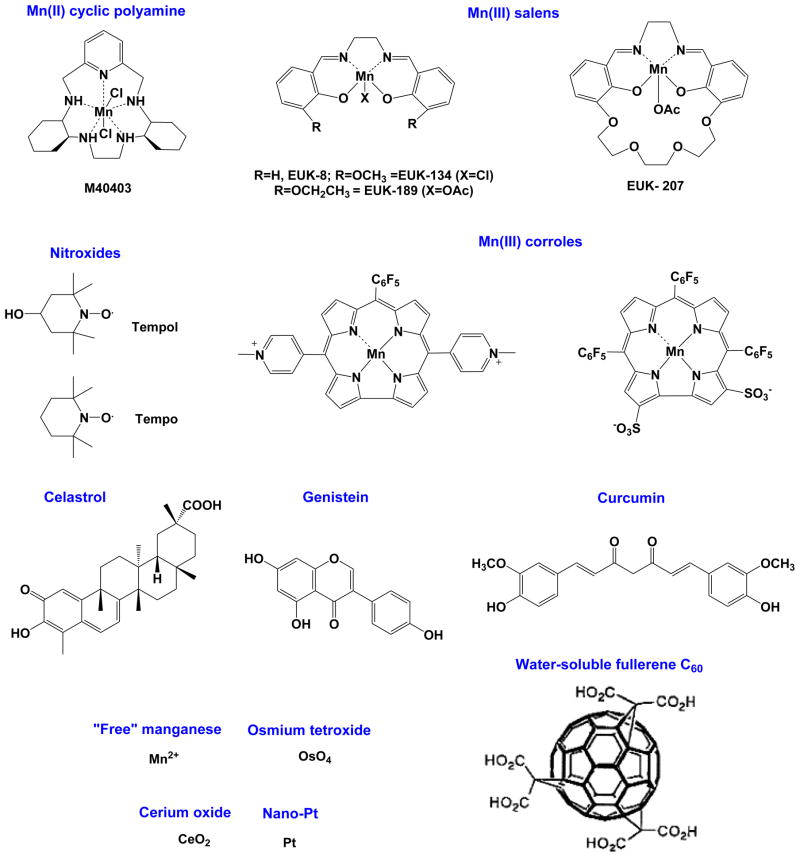
Figure 2 shows other redox-active compounds that are able to suppress oxidative stress. The core-modified porphyrins, corroles are also listed. Corroles have one less meso position when compared to porphyrins, and therefore different redox properties and bioavailability [18,29–31]. Primarily for the reasons cited above, and because of the remarkable efficacy of metalloporphyrins, the researchers that have explored Mn salen derivatives and Mn cyclic polyamines are exploring now the potential of porphyrin-based therapeutics also [18].
Figure 2.
Redox-active compounds which are able to diminish oxidative stress injuries. Shown are modified porphyrins, corroles with one less meso position; the methyl analogue of MnTE-2-PyP5+ (Figure 1) [18], and the anionic corrole with sulfonato groups on pyrrolic positions whose Ga complex is explored as anticancer drugs and Mn and Fe complexes for treating ROS-related injuries [18]. The non-porphyrin-based SOD mimics [18,29–31], metal oxides and metal nanoparticles are presented too. Mn2+ in its own right, when complexed in vivo to low-molecular weight ligands such as lactate or oxo/hydroxo/acetate ligands, exerts high SOD-like activity [18]. Cerium oxide nanoparticles [32,33], osmium tetroxide, OsO4 [34] and platinum nanoparticles [35] have shown SOD-like efficacy in vivo. Natural compounds, primary those bearing phenol functionalities reportedly bear potential to reduce oxidative stress also, as exemplified here with genistein [36], curcumin [37] and celastrol [38]. Among them curcumin has a marginal SOD-like activity [18].
The efficacy of Mn porphyrins-based SOD mimics is related to their ability to easily accept and donate electrons. During O2·− dismutation, the most potent catalysts are able to oxidize and reduce O2·− with similar-to-identical rate constants, k1 and k2 (eqs [1] and [2]), and thus carry potential to be equally efficacious pro- and antioxidants. It is therefore no wonder that more data are emerging indicating the pro-oxidative action of MnPs as therapeutically beneficial under certain, not yet fully understood conditions (see further below). The same is valid for various redox-active compounds. For details, the reader is directed to the reviews [18,29–31], and to the contributions in a Special Issue of Anticancer Agents in Medicinal Chemistry, titled “SOD enzymes and their mimics in cancer: pro- vs antioxidative mode of action” [39–44].
Design of Mn porphyrins-based efficacious and bioavailable SOD mimics
Maximizing thermodynamic and kinetic properties of MnPs
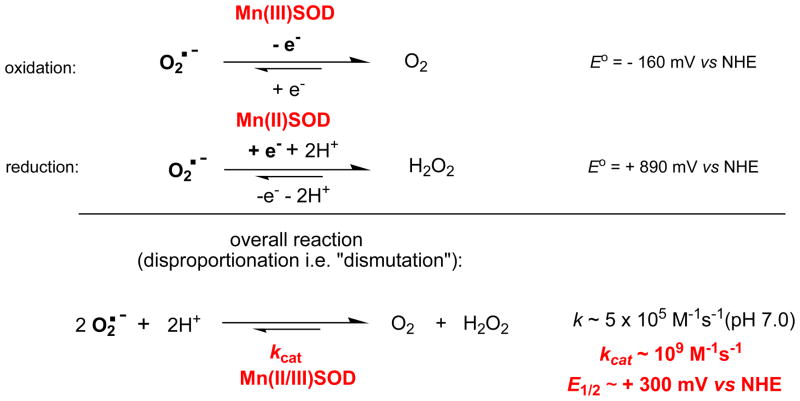
The natural strategy in developing potent SOD mimics has been to mimic the thermodynamics and kinetics of the O2·− dismutation catalyzed by SOD enzymes. The enzymes (regardless of the type of the metal site) dismute O2·− with log kcat = 8.84 − 9.30, and at metal-centered reduction potential, E1/2 ~ +300 mV vs NHE. This is a potential around the midway between the potential for the O2·− reduction (+890 mV vs NHE) and oxidation (−160 mV vs NHE). Thus, the equal thermodynamic facilitation is afforded for each step of the dismutation process (equations 1 and 2). Consequently both rate constants are identical (Figure 3) [45–47].
Figure 3.
The dismutation of O2·− catalyzed by superoxide dismutases. The enzyme-catalyzed dismutation is ~3 oders of magnitude faster than O2·− self-dismutation, and occurs at potential that is midway between the potential for O2·− oxidation to oxygen, and its proton-dependent reduction to H2O2. Under physiological conditions peroxide is removed in a subsequent step by peroxide-removing enzymes such as glutathione peroxidases and catalases.
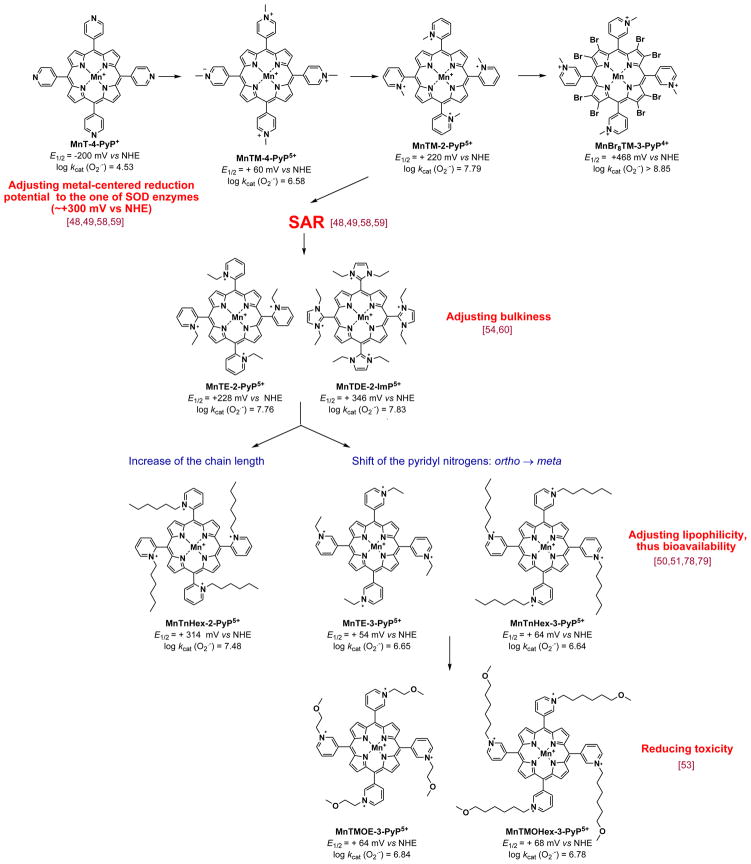
Using this approach we explored which modifications on unsubstituted Mn porphyrins, MnT-2-PyP+, MnT-4-PyP+ and MnTPP+, are needed to shift the potential for ~ 0.5 to 0.7 V from ~ −250 mV to ~ +300 mV vs NHE (Figures 3 and 4). The MnPs with very negative E1/2 ≤ −160 mV vs NHE are electron-rich (such as MnTBAP3− with E1/2 = −194 mV vs NHE). Thus they can not easily accept electrons in a first, rate-limiting step (eq 1) of the dismutation process and consequently can not catalyze O2·− dismutation.
Figure 4.
Historical overview of the optimization of the MnP structure for suppressing oxidative stress. Among structures shown, the most potent SOD mimic is the meta isomer of the Mn(II) β-octabrominated meso-tetrakis(N-methylpyridyl porphyrin, MnBr8TM-3-PyP4+; its log kcat ≥ 8.85 is nearly identical to the one of SOD enzymes. The kcat of SODs obtained by different researchers ranged from 8.84 to 9.30 (Table 1). Yet, with highly positive E1/2 of +468 mV vs NHE, Mn in MnBr8TM-3-PyP4+ is in +2 oxidation state. The metal complex is thus insufficiently stable and readily falls apart. While not of practical importance as a therapeutic, its developmenmt justifies the use of porphyrin as an excellent ligand for optimizing SOD mimics.
In order to make MnPs prone to accept electrons, metal center must be made electron-deficient. Such modification was achieved via the attachment of electron-withdrawing, quaternized cationic pyridyls to porphyrin meso positions. Consequently, MnTM-4-PyP5+ (Figure 4) was synthesized with positive charges in para positions with respect to meso carbons. E1/2 was shifted for 260 mV more positive relative to nonquatermized, MnT-4-PyP+ (E1/2 = + 60 mV vs NHE). Such E1/2 indicates that MnTM-4-PyP5+ is fairly prone to accept electrons in the first step of O2·− dismutation, and is thus a modestly strong SOD mimic (Table 1, Figure 4). The positive charges guide the anionic superoxide to the porphyrin ring, enhancing its SOD potency (Table 1).
Table 1.
Metal-centered reduction potential for the MnIII/MnII redox couple, E1/2 in mV vs NHE, rate constant for O2·− dismutation, kcat (O2·−), and rate constant for peroxynitrite reduction, kred(ONOO−) by MnP. Also given is the lipophilicity of MnPs expressed in terms of thin-layer chromatographic retention factor, Rf, and partition between n-octanol and water, log POW. For comparison, the kcat and E1/2 for Cu,ZnSOD are listed also.
| Compound | E1/2/mV vs NHEa | log kcat(O2•− )b | log kred(ONOO−)c | Rfd | log Powe | References |
|---|---|---|---|---|---|---|
| MnTM-2-PyP5+ | +220 | 7.79 | 7.28 | 0.030 | −7.86f | 48 |
| MnTE-2-PyP5+ | +228 | 7.76 | 7.53 | 0.060 | −6.89e | 49,50,56 |
| MnTnHex-2-PyP5+ | +314 | 7.48 | 7.11 | 0.380 | −2.76f | 50 |
| MnTnHep-2-PyP5+ | +342 | 7.65 | 0.460 | −2.10f | 18,29,51 | |
| MnTnOct-2-PyP5+ | +367 | 7.71 | 7.15 | 0.490 | −1.24f | 50 |
| MnTE-3-PyP5+ | +54 | 6.65 | 0.100 | −5.98e | 52 | |
| MnTnHex-3-PyP5+ | +66 | 6.64 | 0.550 | −2.06f | 51 | |
| MnTM-4-PyP5+ | +60 | 6.58 | 6.63 | 0.015 | 48 | |
| MnTMOE-3-PyP5+ | +64 | 6.84 | 0.120 | 53 | ||
| MnTMOHex-3-PyP5+ | +68 | 6.78 | 0.195 | 53 | ||
| MnTDE-2-ImP5+ | +346 | 7.83e | 7.43 | 0.138 | 54 | |
| MnBr8TM-3-PyP4+ | +468 | ≥8.85 | 18 | |||
| MnTBAP3− | −194 | 3.16 | 5.02 | 18,55 | ||
| Cu,ZnSOD | ~ +300 | 8.84–9.30 | 45–47 |
E1/2 is measured in 0.05 M phosphate buffer, pH 7.8, 0.1 M NaCl.
SOD activity is measured by the cyt c assay in 0.05 M phosphate buffer, pH 7.8, 25 ± 1 °C.
Measurements in 0.05 M phosphate buffer, pH 7.4, 37 + 0.1 °C.
Rf is obtained on plastic-backed silica-gel thin-layer chromatography plates eluted with solvent system 1:1:8 = KNO3(sat): H2O:MeCN.
The log POW values are calculated using the equation log POW = 0.96 × nC − 8.82, where nC is a number of carbon atoms in the alkyl chain [51].
The log POW values experimentally determined [51].
MnTM-4-PyP5+ is a planar molecule and associates to a large extent with nucleic acids. If the association with nucleic acids is extensive, the approach of O2·− to MnP is precluded and its SOD-like activity fully suppressed. Also interaction with nucleic acids in its own right imposes toxicity [48]. The next step in development of SOD mimics was to move nitrogens from the para onto ortho positions, closer to the Mn center [48] (Figure 4). This modification resulted in enhanced electron-withdrawing effects. It also gained a bulkier molecule, MnTM-2-PyP5+, where the methyl groups are now stuck in vertical positions with respect to the porphyrin plane, because their rotation is greatly limited due to the steric hindrance imposed by β-pyrrolic carbons (Figure 1). As a consequence, its association with nucleic acids is diminished. Cationic charges placed closer to the metal site, in ortho positions, are more efficient in guiding anionic O2·− towards metal site (Figure 4) [57]. Such molecule has E1/2 = +220 mV vs NHE, which is another 160 mV more positive relative to E1/2 of MnTM-4-PyP5+ [48]. Combined, favorable thermodynamics and enhanced electrostatic guidance of O2·− towards metal site, resulted in high kcat (Table 1). The critical impact of electrostatics on O2·− dismutation catalyzed by MnPs, which accounts for the enhancement of kcat for 2 orders of magnitude, and is the reminiscent of the electrostatic effects observed with SOD enzymes, has been detailed elsewhere [18,29,58]. Also the impact of electronic effects on kcat has been reported [59].
Electron-withdrawing effects can also be achieved by introducing the chlorines and bromines on β-pyrrolic positions. Fully β-brominated or β-chlorinated MnPs with substituted pyridyls in meso positions are not stable complexes, and thus have been explored only for mechanistic purposes [18,29–31]. Partially halogenated MnPs require extensive chromatography to be isolated in a pure state; such purification is precluded for drug scaling up.
Optimizing bulkiness of the molecule to diminish its unfavorable interactions with nucleic acids
In a subsequent step of the drug development, the ethyl analogue, MnTE-2-PyP5+ was synthesized. It has enhanced bulkiness relative to MnTM-2-PyP5+ (Figure 4), which diminishes further its interactions with nucleic acids, and thus toxicity [48,49]. MnTE-2-PyP5+ (AEOL10113) has been the most frequently explored MnP in different models of oxidative stress [18,29,30]. Based on the same principles, an even bulkier MnP was synthesized with similarly high kcat (O2·−). It is Mn(III) meso-tetrakis(N,N′-diethylimidazolium-2-yl)porphyrin, MnTDE-2-ImP5+ (AEOL110150) (Figures 1 and 4, Table 1) [54,60]. Instead of 4 pyridyl rings, this compound bears 4 imidazolyl rings with two nitrogens in ortho positions that are substituted with ethyl groups placed above and below the porphyrin plane. Thus, the compound has no positional isomers, unlike all ortho N-substituted pyridylporphyrins which have 4 positional isomers (atropoisomers) [56]. The bulkiness of MnTDE-2-ImP5+ decreases its toxicity due to its weaker interactions with negatively charged biomolecules (such as nucleic acids), but it also diminishes its ability to cross the membranes [61]. The placement of ethyl groups above and below the porphyrin plane hinders charges, which are delocalized over the imidazolyl rings. Along with bulkiness this may reduce its transport into mitochondria. MnTDE-2-ImP5+ bears the same number of positive charges in ortho positions, i.e. in a close proximity to the Mn site and therefore possesses the same redox abilities in removing O2·− and ONOO− as the other two MnPs (Table 1). With better resolved patenting and licensing rights, Federal Government and are aggressively developing MnTDE-2-ImP5+ as a wide spectrum radioprotector (see below).
In cells, MnIIIP5+ would undergo facile reduction to MnIIP4+ in the first step of dismutation process with ascorbate, glutathione, tetrahydrobiopterin or flavoproteins due to their abundancy. In a subsequent step MnIIP4+ would reduce O2·− to H2O2 [18,29,30,62,63] (see below under the reactivity of MnPs). Thus in vivo, MnPs may mimic superoxide reductases (such as rubredoxin oxidoreductase) rather than SOD enzymes [18].
Developing simple SOD-deficient E. coli model to assess in vivo efficacy of those MnPs that have high kcat
Once the compounds with high log kcat of ≥ 6 are synthesized, their efficacy is tested in a simple prokaryotic organism Escherichia coli, before they are forwarded to animal studies. The SOD-deficient E. coli lacks cytosolic SOD enzymes and can grow aerobically well only when the medium is substituted with mimics of those enzymes [18,29,30,61,64–66]. Thus, it is a superoxide-specific model, and an excellent tool to predict with high likelihood the efficacy of the compounds in mammalian models of oxidative stress injuries.
Developing SOD-deficient yeast (Saccharomyces cerevisiae) model as an additional tool to evaluate the therapeutic potential of SOD mimics
Our present studies indicate that prokaryotic organism E. coli is by far the most sensitive organism to MnP toxicity. While 5 μM MnTnHex-2-PyP5+ is already toxic to E. coli [52,53,61] (Figure 7), at 30 μM it is neither toxic to eukaryotic S. cerevisiae, nor to eukaryotic mammalian and rodent cancer cell lines [30,67–70, Aird et al, unpublished, Fels et al unpublished]. Our present understanding is that each compound which is a powerful SOD mimic and nontoxic to E. coli (within reasonable concentration range) is undoubtedly an excellent candidate for clinical development. However, if the compound exerts toxicity to SOD-deficient E. coli, but protects SOD-deficient S. cerevisiae when it grows aerobically, it may still be a powerful therapeutic for mammals. The differences in the structure of prokaryotic cell wall and eukaryotic cell membrane may affect differentially their sensitivity towards the toxicity of lipophilic MnPs. A comparative evaluation of antioxidant potency, bioavailability and toxicity of SOD mimics in both of those systems may provide a more conclusive insight into the potential of an SOD mimic for clinical development.
Figure 7.
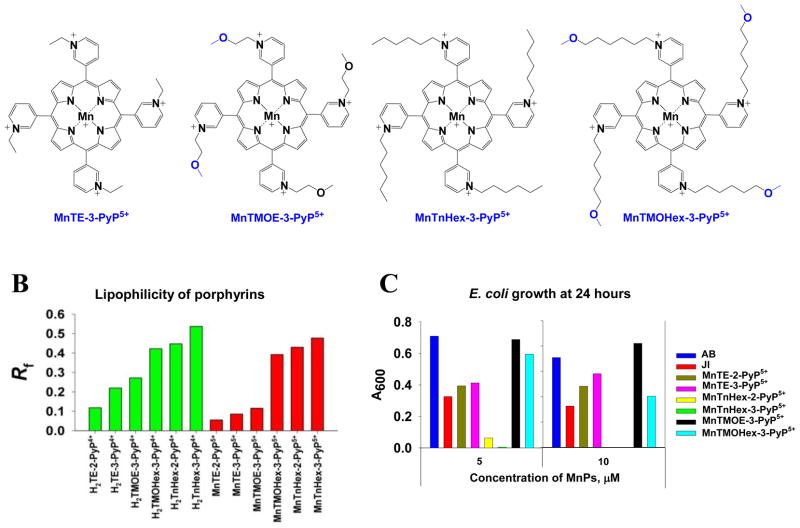
(A) The structures of meta isomers, where hexyl and ethyl compounds are derivatized with methoxy groups: MnTMOHex-3-PyP5+ and MnTMOE-3-PyP5+. The modification was meant to decrease the toxicity of MnTnHex-3-PyP5+. Ethyl analogue was originally intended for comparison, but appeared of unexpectedly high efficacy in vivo. This is explained as a result of favorably balanced several structural features; (B) The lipophilicities of metal-free porphyrins and their ligands expressed in terms of chromatographic retention factor, Rf. Lipophilicity of Mn complexes is lower than of their metal-free ligands due to the higher solvation of the metal site. The effect is more drastic with porphyrins bearing shorter substituents. Longer-chained analogues, alkyl and methoxyalkyl are more lipophilic than their shorter-chained analogues. Introduction of methoxy group reduces lipophilicity of longer alkyl analogue, H2TnHex-3-PyP4+ and its Mn complex; (C) The effect of the MnPs on the growth of SOD-deficient E. coli in minimal five amino acids-medium. The growth was measured as absorbance at 600 nm. The data are the average of 5 different studies where each compound was tested in triplicates. The data at 5 and 10 μM MnPs were provided; for the rest see ref 53. The growth of JI132 and AB1152 alone is shown also. At 5 μM MnTMOHex-3-PyP5+ is similarly potent as MnTMOE-3-PyP5+, and more efficacious than MnTE-2-PyP5+. The alkyl analogue, MnTnHex-3-PyP5+ is already very toxic at 5 μM. At 10 μM MnTMOHex-3-PyP5+ becomes toxic. MnTMOE-3-PyP5+ is the most efficacious of all compounds tested. Adapted from [53].
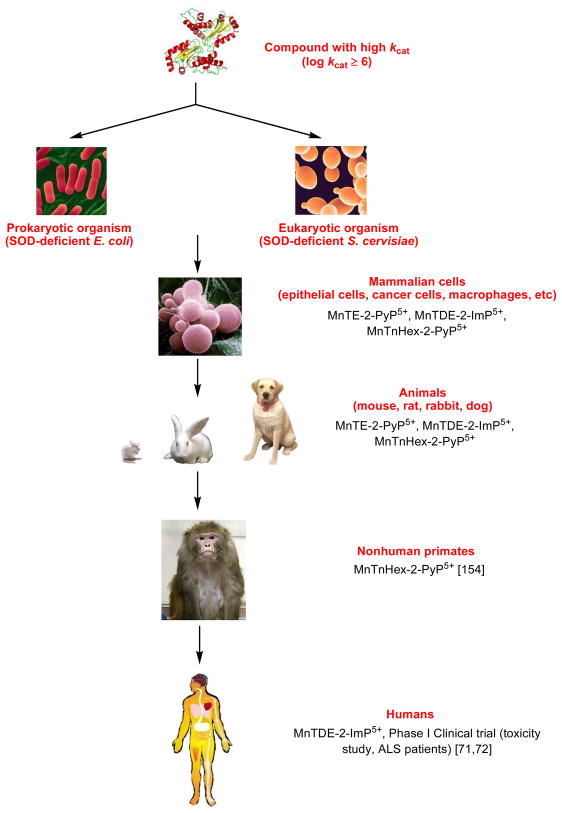
Figure 5 shows the stepwise efforts in optimizing and evaluating the MnP structure for clinical use starting from its design, and thorough studies of its aqueous chemistry (synthesis, purification and characterization), over the simple in vivo models - prokaryotic E. coli and eukaryotic S. cerevisiae- all the way up to nonhuman primates and eventually humans. The Phase I toxicity clinical trial was successfully conducted on amyotrophic lateral sclerosis patients with MnTDE-2-ImP5+. No toxicity was observed with doses well above the therapeutic dose [71,72]. The scaling up of MnTE-2-PyP5+ was completed, drug master filed (DMF) and toxicity studies are in progress (see under Therapeutic effects and clinical development of MnPs). The studies are also underway with oxygen-bearing derivatives whose favorable redox properties and bioavailibility are maintained, but toxicity diminished (Figure 4) [53,73].
Figure 5.
Historical overview of the development of MnPs from the conception, synthesis, testing in prokaryotic E. coli, eukaryotic S. cerevisiae and whole organisms (rats, mice and rabbits), over non human primates to humans. Few in vivo studies are referred here only. Other studies are listed in Table 2. For the details on dosing and outcome of the studies please see refs 18,29–31.
Optimizing properties that may improve MnPs bioavailability - lipophilicity
Ortho isomers of Mn(III) N-alkylpyridylporphyrins
From the time the very first efforts were made to synthesize the efficacious water-soluble SOD mimics [18,29,30,74], the questions arose whether such excessively charged and hydrophilic compounds would (1) cross the blood brain barrier; (2) cross the plasma membrane; and (3) enter critical cellular compartments, mitochondria, whose oxidative damage has the vast impact on the cell function. As the key role of mitochondria in diseases became obvious, a number of groups developed strategies to target mitochondria. It has been clearly shown [75–77] that the compound must have cationic charge and appropriate lipophilicity to accumulate in mitochondria, driven there by the mitochondrial membrane negative potential [75]. In order to judge if our compounds reach subcellular compartments, the analytical tools for their analyses in tissues and cellular compartments were developed. The very first method established was HPLC/fluorescence method where MnIIIP5+ was reduced with ascorbate to MnIIP4+. The exchange of Mn with Zn·followed and the fluorescence of ZnP was measured [78]. The method was very sensitive, and allowed the detection of as low as nM levels of MnTE-2-PyP5+ in plasma and various organs, as well as in cytosol, nucleus and mitochondria [78,79].
Enhancing lipophilicity by lengthening the alkyl chains
Longer members of the N-alkylpyridylporphyrin series, bearing alkyl chains with up to 8 carbon atoms were synthesized [50]. Their lipophilicity was measured either in terms of thin-layer chromatographic retention factor, Rf (ratio of MnP and solvent path on silica plates [51]), or partition between n-octanol and water, log POW. Both values are linearly related [51]. We frequently utilize Rf as it is easier to assess than log POW [51]. The lipophilicity increases 10-fold for each carbon atom added. Thus MnTE-2-PyP5+ is 13,500-fold less lipophilic than MnTnHex-2-PyP5+ and 450,000-fold less lipophilic than MnTnOct-2-PyP5+. The lipophilic analogues are more efficacious in vivo: MnTE-2-PyP5+ is ~120- and 1,000-fold less potent than are MnTnHex-2-PyP5+ and MnTnOct-2-PyP5+ [18,29,30,80]. E. coli study clearly showed that such improved efficacy arose at least in part from the increased accumulation of more lipophilic MnPs within a cell [52,61]. The preliminary ip pharmacokinetics shows higher accumulation of MnTnHex-2-PyP5+ in a mouse liver relative to MnTE-2-PyP5+ [Spasojevic et al, unpublished].
Enhancing lipophilicity by moving the pyridyl substitutents from ortho to meta positions relative to porphyrin meso carbon
A first hint that meta isomer MnTM-3-PyP5+ (Figure 4) offers similar protection to SOD-deficient E. coli as ortho analogue was reported in 1998 [48]. Efforts were originally directed towards designing SOD mimics of highest antioxidant capacity; therefore the meta isomers have been overlooked for a while [48,49]. Recently we quantified the lipophilicity of the series of MnPs and learned that the meta N-alkylpyridylporphyrins are ~10-fold more lipophilic than their ortho analogues (Table 1) [51]. The ~10-fold increased lipophilicity of MnTE-3-PyP5+ relative to MnTE-2-PyP5+, and therefore ~ 10-fold increased accumulation within E. coli compensated for its inferior SOD-like activity. Consequently both compounds are equally able to protect SOD-deficient E. coli when growing aerobically [52].
Enhancing lipophilicity via reduction of MnIIIP5+ to MnIIP4+ as a consequence of the loss of a single charge from the metal site
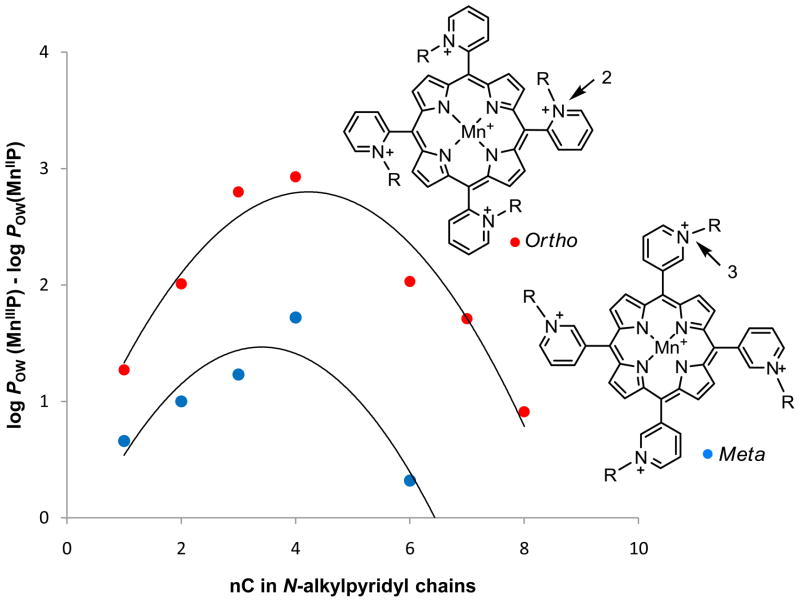
As already noted and due to their electron-deficiency, Mn(III) N-alkylpyridylporphyrins get readily reduced in vivo with cellular reductants and other redox active proteins. Upon reduction, single charge gets lost from the Mn site, which enhances MnP lipophilicity by as much as 3 orders of magnitude, depending upon the type of the isomer and the alkyl chain length [81]. The lipophilicity is enhanced more with ortho than with meta isomers, and the effect is peaking with MnTnBu-2(or 3)-PyP5+ as shown in Figure 6 [81]. Such gain in lipophilicity upon MnP reduction may in part explain their preferred mitochondrial accumulation and transport across the blood brain barrier.
Figure 6.
The lipophilicity gain upon reduction of ortho and meta isomers of Mn(III) N-alkylpyridylporphyrins as a function of a number of carbon atoms, nC. The gain is larger with ortho than with meta isomers, and is peaking with butyl compounds [81].
Enhancing mitochondrial accumulation
The HPLC/fluorescence method, originally developed for pharmacokinetic studies of MnTE-2-PyP5+ [78,79], appeared inferior to measure the levels of lipophilic compound, MnTnHex-2-PyP5+ in vivo. Due to the steric hindrance of the long alkyl chains for the approach of Zn to the porphyrin pyrrolic nitrogens, under experimentally achievable conditions the replacement of Mn did not progress to completion. Thus another, more sensitive and less elaborative LCMS/MS method was developed. After sample preparation, MnP was detected directly via mass spectroscopy [29,81]. The method was already used for the analyses of different MnPs in different tissues and cellular compartments [29,81]. In the first mouse heart mitochondrial study, MnTE-2-PyP5+ was found in mitochondria at > 5 μM. The study was preceded by Ferrer-Sueta et al work [63], where submitochondrial particles were protected by ≥ 4 μM MnTE-2-PyP5+ when exposed to the flux of ONOO−. Ongoing studies on the hydrophilic and lipophilic analogues are in progress to determine their mitochondrial vs cytosolic accumulation in yeast S. cerevisiae [70], and in mouse heart mitochondria. The increase in lipophilicity from methyl to hexyl analogue (Table 1) parallels the increase in their mitochondrial accumulation. The most hydrophilic member of the series, MnTM-2-PyP5+ distributes similarly between cytosol and mitochondria, while MnTnHex-2-PyP5+ distributes at > 90% in mitochondria relative to cytosol [70]. The MnTnHex-2-PyP5+ was further found to accumulate predominantly in mouse heart mitochondria at > 80% relative to cytosol [Spasojevic et al unpublished]. Without bearing mitochondrial-targeting sequence, a lipophilic cationic MnP, in its own right, distributes almost entirely into mitochondria (relative to cytosol), where it can suppress the oxidative stress [70,82,83, Batinic-Haberle et al, unpublished].
Enhancing nuclear accumulation
MnTE-2-PyP5+ was found to accumulate 3-fold more in nucleus relative to cytosol in a study where macrophages and LPS-stimulated macrophages were exposed for 1.25 hour to 34 μM MnP [30]. At least in part, the compound is driven into nucleus by negatively charged phosphates of nucleic acids.
Pharmacokinetic (PK) studies
The detailed PK study of MnTE-2-PyP5+ was performed with single 10 mg/kg ip injection. The plasma half-life is ~1 hour and the organ half-life between 60 and 135 hours [79]. MnTE-2-PyP5+ accumulates in all organs, most so in liver, kidneys and spleen. After initial built up, the levels in all organs drop continuously, but increase in brain beyond day 7.
Oral bioavailability
The PK via ip route was compared to PK via oral gavage; the same tmax was found [84]. Based on cmax, the oral availability of MnTE-2-PyP5+ is ~25% of its ip bioavailability. The PK studies are in progress on MnTnHex-2-PyP5+. The initial study indicates higher oral availability of MnTnHex-2-PyP5+ when compared to MnTE-2-PyP5+ [Spasojevic et al unpublished].
Transport of MnP across the blood brain barrier (BBB)
Under the same conditions, the lipophilic MnTnHex-2-PyP5+ crosses BBB 8- to 12-fold more than hydrophilic MnTE-2-PyP5+ [69, 82]. At least in part the porphyrin accumulation in brain is driven by negatively charged phospholipids [69, 82]. Brain accumulation explains its remarkable efficacy in a middle cerebral artery occlusion (stroke) and subarachnoid hemorrhage rodent models [69,85]. MnTnHex-2-PyP5+ was also protective in a rabbit cerebral palsy uterus ischemia model at only 0.12 mg/kg given iv twice, 30 minutes before and after ischemia. The hydrophilic MnTE-2-PyP5+, injected twice at 6 mg/kg, produced no effect [86]. For a drug to be able to rescue puppies, it must have the ability to cross several barriers: uterus, placenta, fetus and fetal BBB.
Optimizing properties that may diminish toxicity of MnPs
The ultimate goal of our studies is to have efficacious, bioavailable and nontoxic drug. Mn(III) meso tetrakis N-alkylpyridylporphyrins with longer alkyl chains are cationic and lipophilic which facilitates their mitocondrial accumulation and transport across the blood brain barrier. However, they bear cationic “heads” (cationic nitrogens) and hydrophobic alkyl chains and thus exert surfactancy (their aqueous solutions foam upon shaking) and detergent-like properties. Therefore, at higher concentrations they can damage membranes, whereby compromising the cell integrity. To disrupt the surfactancy of longer alkyl-chain analogues, oxygen atoms are placed within the alkyl chains. The strategy is analogous to replacing the sodium dodecyl sulfate (lauryl sulfate) in soaps and shampoos with ether-modified, less skin-irritating analogue, sodium laureth sulfate [87,88]. A first hint that oxygen insertion greatly diminishes the toxicity was reported in 2004 [54]. The porphyrins of similar antioxidant potency and with chains of equal length (equal number of atoms), MnTnBu-2-PyP5+ and MnTMOE-2-PyP5+, were compared. The methoxyethyl analogue was significantly less toxic to E. coli than MnTnBu-2-PyP5+ [54].
The first lipophilic oxygen derivatives, synthesized recently, were meta analogues (Figure 7A) [53]. Meta isomers were chosen for two reasons: (1) the synthesis is more facile, and (2) the meta isomers are ~ 10-fold more lipophilic than ortho analogues. For the latter reason the meta isomers appear to bear similar therapeutic potential as ortho compounds [52]. The Mn(III) meso-tetrakis(N-6′-methoxyhexylpyridinium-3-yl)porphyrin, MnTMOHex-3-PyP5+ was compared to Mn(III) meso-tetrakis(N-2′-methoxyethylpyridinium-3-yl)porphyrin, MnTMOE-3-PyP5+ (Figure 7A) [53]. The charges in meta isomer are still close to the Mn site assuring favorable thermodynamics and electrostatics for O2·− dismutation [52] (Table 1). The insertion of oxygens, however, decreased the lipophilicity of the methoxyhexyl compound, MnTMOHex-3-PyP5+ relative to hexyl analogue (Figure 7B, Table 1). Importantly, presence of one oxygen in alkyl chains also disrupts the surfactant-like property and thus diminishes the toxicity. 1 μM MnTnHex-3-PyP5+ exerts toxicity to SOD-deficient E. coli, while it was still not fully efficacious at 0.5 μM. The efficacy of methoxyhexyl analogue peaked at 5 μM (Figure 7C), and significant toxicity was observed at 10 μM. The methoxyethyl, MnTMOE-3-PyP5+ is significantly more efficacious than either its ethyl analogue, MnTE-3-PyP5+ (Figure 7C) or butyl porphyrin, MnTnBu-3-PyP5+ which bears the same number of atoms in pyridyl chains. Finally, MnTMOE-3-PyP5+ is more efficacious than our mostly in vivo studied compound, MnTE-2-PyP5+ (Figure 7C). We can assume at this point that such profound beneficial effect of MnTMOE-3-PyP5+ originates from a favorable interplay of the size of the molecule, length of pyridyl substituents, rotational flexibility (ortho isomer is more rigid, while meta is less so), bulkiness and presence of oxygens.
Research in progress shows that positioning of the oxygen at the end of the long flexible chains in MnTMOHex-3-PyP5+ allows the oxygen electron pairs to approach cationic Mn, which weakens the oxygen-to-alkyl chain bond, and thus facilitates hydrolysis of the methoxy group. Porphyrin species with 3 methoxyhexyl and one hydroxyhexyl groups were seen in mass spectrum. Also the position of oxygens at the end of long chains allows the access of the solvent, resulting in a more solvated, less lipophilic molecule when compared to either hexyl or heptyl (which has same number of atoms in chains) analogues (Figure 7B).
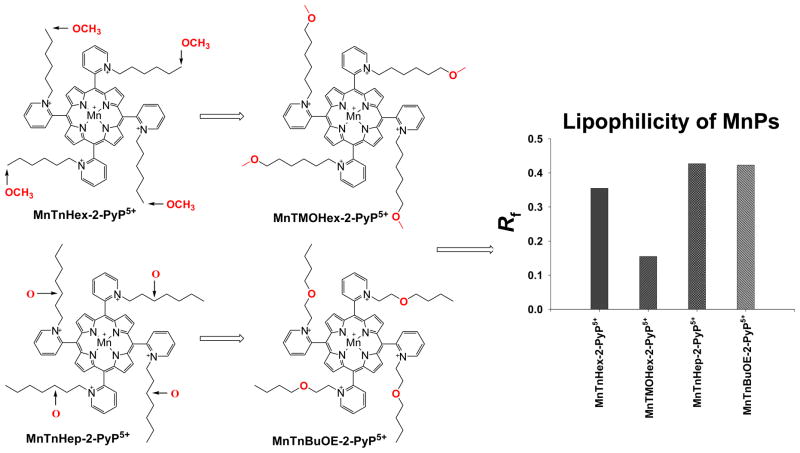
Much to our surprise the positioning of the oxygen closer to the porphyrin core hinders it from solvent molecules; the MnP is as lipophilic as oxygen non-bearing compound of identical length of pyridyl substituents, MnTnHep-2-PyP5+. Further, chains in ortho positions are less flexible. The oxygen is buried within a chain and its approach to a Mn site, which could have led to the hydrolysis of butyl group, is sterically precluded (Figure 8). Such synthetic strategy led to the compounds of remarkable properties, and in particular of diminished toxicity [Rajic et al, in preparation]. Needless to say that all those modified MnPs still preserve cationic charges in ortho positions which assures their redox-based actions and thus potency (Table 1).
Figure 8.
Introduction of oxygens at different positions within alkyl chains dramatically affects the lipophilicity of MnPs, while maintaining the same redox-based abilities as exemplified with high kcat. Shown are the data related to the compounds that bear oxygens at the very end of the chains, MnTMOHex-2-PyP5+, and relatively close to the porphyrin core, MnTnBuOE-2-PyP5+, as well as their alkyl analogues of comparable chain length, MnTnHex-2-PyP5+ and MnTnHep-2-PyP5+ [18].
Mn porphyrins and reactive oxygen and nitrogen species
The exceptional ability of cationic Mn(III) N-alkylpyridylporphyrins to eliminate O2·− is primarily based on the electron-deficiency of the Mn site and the preferred reactivity of cationic porphyrins towards electron rich anionic species. Based on the same thermodynamics and kinetics that is operable for the O2·− dismutation, MnPs also effectively scavenge ONOO−, ClO−, and CO3·− (Figure 19) [18,29–31,89, Ferrer-Sueta et al unpublished]. This is exemplified by the linear relationship between the log kcat (O2·−) and log kred (ONOO−) for the series of ortho Mn(III) N-alkylpyridylporphyrins [90]. The relationship tells us that a compound which is a good SOD mimic [18,29–31] is also a potent peroxynitrite scavenger [90]. The electron density of the metal site may be best described in terms of metal-centered reduction potential, E1/2 for MnIIIP/MnIIP redox couple, which parallels the proton dissociation constants, pKa of the axially bound waters. The detailed spectral and electrochemical studies of ortho and meta MnTE-2(or 3)-PyP5+ with Mn in +2, +3, and +4 oxidation states, which are relevant for the in vivo redox reactions of MnPs, have recently been reported [91,92].
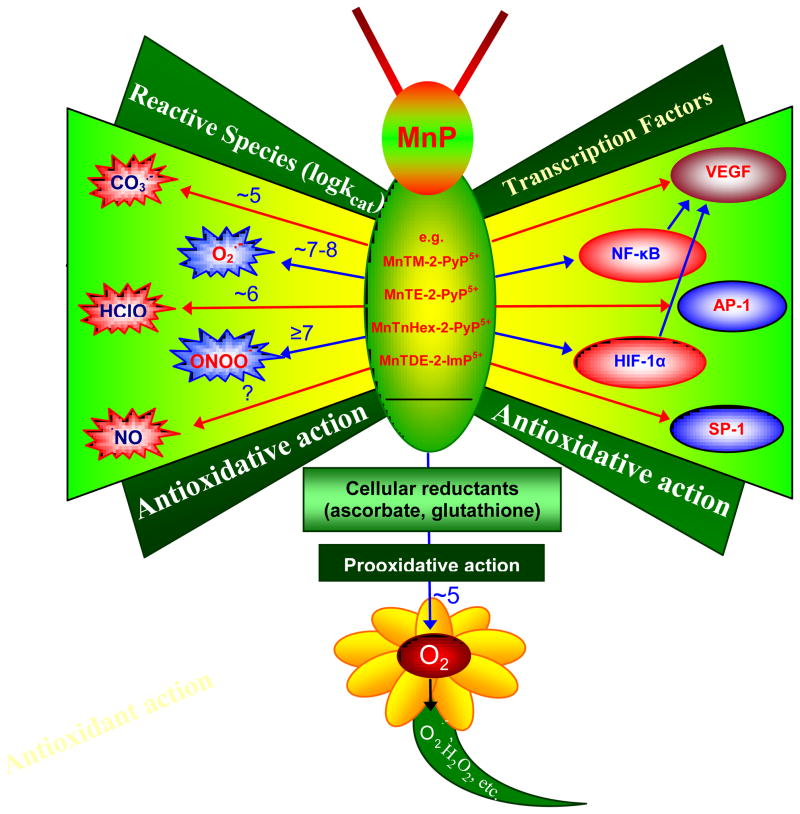
By reducing the levels of reactive species, MnPs reduce the oxidative damage to biological molecules as a consequence of primary oxidative insult [18]. Reactive species are required for upregulation of cellular transcriptional activity [18]. Thus, by removing those species MnPs can modulate transcriptional activity also, and in turn suppress excessive inflammation, i.e. continuous oxidative stress, and secondary oxidative damage of biological targets. Such action is antioxidative in nature. However, the pro-oxidative action of MnPs, which leads to the inhibition of transcription factors activation, has also been suggested based on their aqueous chemistry and cellular studies, and is detailed further below [30, 93–95].
Due to the thermodynamic and kinetic reasons stated above, the cationic compounds with E1/2 ≥ +50 mV vs NHE are able to efficiently remove anionic reactive species such as O2·− and ONOO−. Substantial experimental evidence was provided that anionic porphyrins, whose E1/2 is more negative than is the potential for oxidation of superoxide at −160 mV vs NHE, cannot be SOD mimics [18,29–31,58,96]. Such is MnTBAP3− (also known as MnTCPP3−) with E1/2 = −194 mV vs NHE. With such E1/2 both MnP and superoxide prefer oxidation in a first step of dismutation. Regardless, the manuscripts still continue to incorrectly assign SOD-like activity to this porphyrin. Most recently, Esposito and Cuzzocrea exemplify Mn porphyrin-based SOD mimics with the MnTBAP3− structure, misleading the readers [97]. . We showed that MnTBAP3− has the ability to reduce ONOO−, which action is still ~2 orders of magnitude lower than that of ortho cationic N-substituted pyridylporphyrins [55]. Peroxynitrite is a strong oxidant and can oxidize even those metalloporphyrins whose metal center is not very electron-deficient and thus not eager to bind electron-donating ligands such as ONOO−. The role of this and other anionic and neutral porphyrins, as well as modified porphyrins (corroles and texaphyrins [see review in ref 18,98,99]) with respect to peroxynitrite scavenging, may require further thermodynamic and kinetic considerations. Aside from ONOO− - related reactivity, their differential accumulation in tissues and subcellular compartments is critical to their actions and must be accounted for.
Mn porphyrins and transcription factors
Antioxidative action
The same property that allows MnPs to operate as SOD mimics allows them to modulate cellular transcriptional activity. Cationic, reducible MnPs inhibit activation of any transcription factor thus far tested, HIF-1α, NF-κB, AP-1 and SP-1. Two explanations have been proposed. The older school of thoughts assume that MnPs remove signaling reactive species whereby preventing the activation of transcription by those species. This was evidenced by reduced oxidative stress in several cancer studies [100–103].
Pro-oxidative action
The more recent data imply, at least in the case of NF-κB, that its inhibition likely occurs via pro-oxidative mechanism. Such action has been proposed by Piganelli and Tse for the protection of isolated human islet cells and for the prevention of adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone [30,104–107]. The data suggest that MnTE-2-PyP5+ did not affect cytosolic pathways. Once inside nucleus it presumably oxidizes either cys 62 of p50 NF-κB subunit or AEP1/Ref-1 or thioredoxin, which all assure the reducing environment within nucleus critical for the transcription factor DNA binding. Such scenario rules out the antioxidative action of MnP which would have enhanced the reduced state of cysteine 62 of p50 and thus would have allowed its DNA binding and transcription to commence [30]. Based on MnP/glutathione aqueous chemistry, MnP could indeed oxidize SH-bearing proteins involved in the activation of major transcription factor, NF-κB [30]. Though of pro-oxidative nature, the antioxidative effects, i.e. the suppression of cellular inflammation were observed in diabetes studies [105–107]. This cautions us that we need to differentiate between the mechanism of action of MnPs involved and the nature of the effects observed.
Substantial evidence has recently been provided by Tome group that further supports the pro-oxidative action of MnPs [93–95]. In cytosol, in the presence of H2O2 and glutathione, MnTE-2-PyP5+ glutathionylates p65 subunit of NF-κB, whereby depriving the cells of reduced glutathione and enhancing the glucocorticoid-induced apoptosis of murine thymic lymphoma cells [93–95]. Glutathionylation and consequently inactivation of mitochondrial proteins has recently been reported by Enrique Cadenas as a major consequence of oxidative stress, facilitated by GSSG trapped in mitochondria [108].
The kcat(O2•−) as a measure of MnP ability to finely tune cellular redox-based pathways
While their in vivo mechanism awaits further consideration, in summary, a cationic Mn(III) N-substituted pyridylporphyrin with SOD-mimicing ability expressed as log kcat ≥ 6 (preferably in excess of 7), is able to efficiently eliminate other reactive species too, and suppress cellular transcriptional activity. Thus, we may safely view kcat (O2·−) as a measure of the MnP therapeutic potential in ameliorating diseases that have oxidative stress in common. We need to bear in mind that kcat describes both the electron-deficiency of the Mn center (thermodynamics) and the cationic charge of the whole molecule (electrostatics); the latter has large effect on Mn porphyrin bioavailability, i.e. tissue and subcellular localization.
Mn porphyrins and cellular reductants
The pro-oxidative action can be accomplished when (1) MnPs modulate cellular transcriptional activity (as described above); and (2) when they couple with cellular redox-active compounds, such as ascorbate, glutathione, flavoenzymes, proteins bearing signaling cysteines, etc.
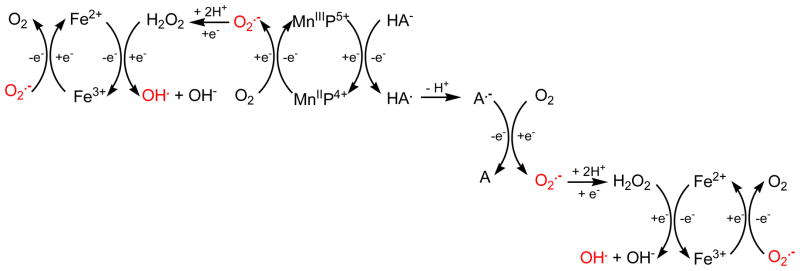
In vivo MnIIIP5+ could be reduced with endogenously abundant ascorbate rather than with superoxide, acting as superoxide reductase rather than superoxide dismutase [18]. In a subsequent step reduced MnIIP4+ may reoxidize to MnIIIP5+ with oxygen rather than with micro- or submicromolar levels of O2·−, whereby producing O2·−, and H2O2 in a subsequent step (Figure 10). Further, ascorbyl radical, HA·, formed in the reaction of MnIIIP5+ with ascorbate (HA−) [109], could be oxidized to dehydroascorbate (A), whereby transferring the electron to oxygen, and producing superoxide and eventually peroxide (Figure 10). In such scenario, MnP would function as a catalyst of ascorbate-driven peroxide production and oxygen consumption.
Figure 10.
The production of cytotoxic H2O2 when MnP was exposed to ascorbate in aerobic environment. Together, MnP and ascorbate can produce superoxide which can then undergo self-dismutation, or enzyme-catalyzed dismutation to H2O2. In the presence of Fe, the Fenton chemistry can lead to the production of hydroxyl radical ·OH. Reduction of Fe3+ to Fe2+ ·−. The abbreviations are: A, dehydroascorbic acid; HA−, monodeprotonated ascorbate; can happen with either ascorbate or O2 HA·, ascorbyl radical; A·− deprotonated ascorbyl radical.
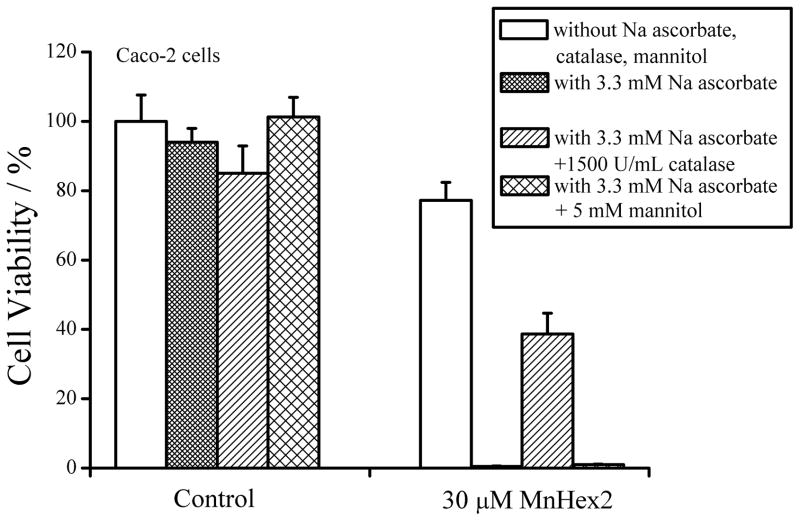
The cytotoxic effects produced in such scenario were witnessed by us and others. Four different cancer cell, Caco-2, 4T1, Hela and HCT116 were exposed to MnP and ascorbate. Neither alone was toxic under identical experimental conditions [110]. Together they act synergisticaly. The effect on Caco-2 cells is shown in Figure 11. Three different MnPs were tested, the easily reducible ortho MnTE-2-PyP5+ and MnTnHex-2-PyP5+, and the meta compound of weaker reducibility, MnTnHex-3-PyP5+ (Table 1). The data on MnTnHex-2-PyP5+ are shown only (Figure 11). MnPs are prone to oxidative degradation with H2O2. Compared to MnTE-2-PyP5+, and MnTnHex-3-PyP5+, MnTnHex-2-PyP5+was the most stable towards peroxide [53]. Thus highest synergistic effect was observed with MnTnHex-2-PyP5+. The effect was greatly diminished with catalase, but only marginally with hydroxyl radical scavenger, mannitol, indicating H2O2 as a main cytotoxic agent [68].
Figure 11.
Cytotoxicity of sodium ascorbate at 3.3 mM without or with 30 μM MnTnHex-2-PyP5+. 5 mM mannitol only weakly suppressed the cytotoxicity when MnP was combined with sodium ascorbate, while the viability of Caco-2 cells increased with the addition of 1500 U/mL catalase. This indicates that the cells were killed predominantly by hydrogen peroxide (WST-1-based cytotoxicity assay). The assay is based on the reduction of tetrazolium salt WST-1 to a water-soluble colored formazan by metabolically active cell. 10,000 viable cells were plated in each well of a 96-well plate in a complete medium. After 24 h in 5% CO2 at 37 oC, the medium was replaced with 100 μl of a fresh complete medium. A positive control containing the same number of cells in complete medium without any drug was used. A negative control without cells was used as blank. The plates were incubated for 96 h. At the end of the incubation, the cells were washed twice with PBS. Then 100 μl of Opti-Media (Invitrogen, USA) with 10 μl of proliferation reagent WST-1 was added to each well and the plates were incubated at 37 oC for another 2 h. The absorbance was measured in a microplate reader (BMG) at 450/595 nm and the percent viability was calculated from the following equation: (Asample−Ablank)/(Acontrol−Ablank) × 100. Adopted from [68].
Similar synergistic effects were reported by Tian et al, when hormone-dependent and hormone-independent prostatic, pancreatic and hepatic cell lines where exposed to the combination of MnTM-2-PyP5+ with ascorbate [111]. Cytotoxic effects were attributed to both H2O2OH radical, the latter formed in the reaction of H2O2 and Fe2+ (Figures 12 and 13). Levine et al data also support the cytotoxic effects of ascorbate [112]. An extensive panel of 43 tumor and 5 normal cell lines, as well as in vivo glioblastomas (9L), ovarian (Ovcar5) and pancreatic (Pan02) tumors in nude mice were studied. The effects reportedly result from the ascorbate-based peroxide formation catalyzed by endogenous metalloproteins [112]. Verrax et al also showed that redox-cycling system, ascorbate + quinone menadione, kills tumors via production of reactive species [113,114]. Finally, Jaramillo et al showed that MnTE-2-PyP5+ can improve treatment of hematologic malignancies with doxorubicin, cyclophosphamide and glucocorticoids in the presence of H2O2 and another major cellular reductant, glutathione (see under Mn porphyrins and transcription factors [93–95]).
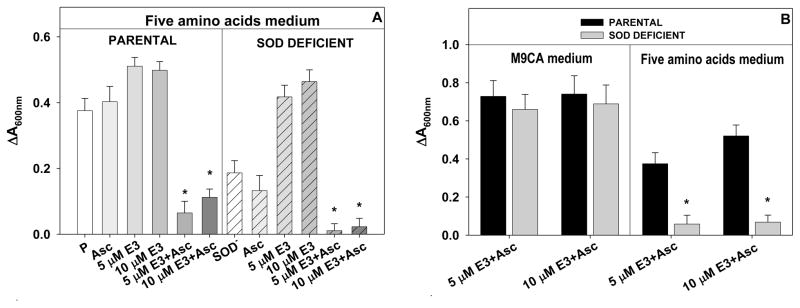
Figure 12.
(A) The growth of the wild type parental E. coli AB1157, and SOD-deficient JI132 E. coli with 5 and 10 μM MnP −/+ ascorbate (1 mM) in minimal, five amino acids medium at 14 h; (B) The growth of the wild type AB1157 and SOD-deficient E. coli JI132 with 5 and 10 μM MnP/ascorbate (1 mM) in M9CA medium at 18 h, and in minimal 5 amino acids medium at 24 h, respectively. P=parental, or SOD− = SOD-deficient E. coli, Asc=ascorbate, E3=MnTE-3-PyP5+. (*) represents statistical significance. The data are adopted from [44].
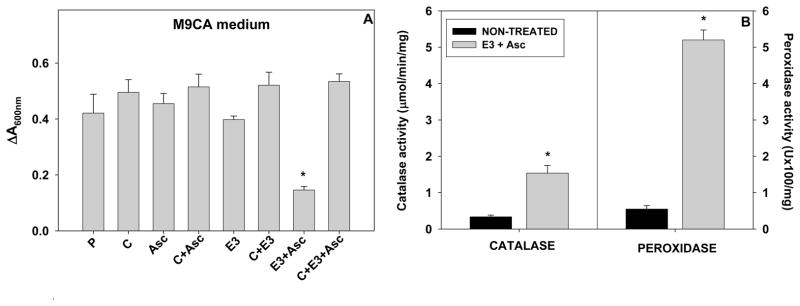
Figure 13.
(A) Effect of catalase on MnTE-3-PyP5+/ascorbate toxicity on the growth of E. coli at 9 hours in M9CA nutrient rich medium. The parental AB1157 E. coli (P) grew with or without 1 μM MnP and 1 mM ascorbate. Catalase (C) was added to the growth medium at 1,000 units/mL five minutes before the addition of MnP and ascorbate; (B) The effect of MnP/ascorbate on the upregulation of catalases and peroxidases. The parental (GC4468) cells were incubated for 2 hours in the presence of 1 μM MnTE-3-PyP5+ and 1 mM ascorbate. E. coli responds to the increased peroxide levels as a consequence of MnTE-3-PyP5+/ascorbate-based peroxide formation by upregulating catalases and peroxidases (E3+Asc bar vs nontreated bar). P=parental, C=catalase, Asc=ascorbate, E3=MnTE-3-PyP5+; (*) represents statistical significance. The data are adopted from [44].
The anticancer action of a variety of metal complexes (metal = Pt, Fe, Co, Cu, V, Ru, Rh, Au, As) appears redox-based and related to the increased production of cytotoxic reactive species; coupling with cellular reductants seems to be involved [115]. For example Ru bis(imidazole) complex operates at E1/2 ~ +30 mV vs NHE, which is similar to the one of meta Mn(III) N-alkylpyridylporphyrins. Thus, there may be analogous anticancer mechanisms operative with different metal complexes, regardless of the ligands coordinated to the Mn center, as long as the ligands form stable complexes with metals and allow metal site to couple with cellular redox-based systems in order to exert the anticancer effects [115].
The anticancer effects of MnTnHex-2-PyP5+ we observed in mouse brain tumor studies with intracranial and subcutaneous xenografts may be ascribed to the removal of signaling reactive species whereby suppressing HIF-1α activation and VEGF expression, as reported with 4T1 mouse study [103]. However, the body levels of ascorbate are the highest in brain and neuroendocrine glands [43]. Thus it may not be excluded that easily reducible and lipophilic MnTnHex-2-PyP5+ (E1/2 = +314 mV vs NHE) would act as a catalyst for ascorbate oxidation in the brain, whereby producing cytotoxic H2O2 and ·OH. Similar considerations on the cytotoxicity of H2O2 to glioblastomas (9L) [112], and human glioma cell line A172 were reported too [116].
To further substantiate the pro-oxidative action of MnPs, we recently explored a very simple and straightforward superoxide-specific SOD-deficient E. coli system, where MnTE-3-PyP5+ acted as a powerful SOD mimic [Figure 12A]. If ascorbate is added to the medium, SOD mimic becomes oxidant, causing oxidative damage to both wild and SOD-deficient strains growing in either rich (casamino acids M9CA medium) or minimal (instead of casamino acids only five amino acids were added to medium), and fully disabling their growth [44] (Figure 12A). Over time, and only in a rich medium both parental and SOD-deficient strains recover from oxidative insult (Figure 12B), presumbaly by upregulating the peroxide-removing enzymes, catalases and peroxidases via induction of oxyR regulon (Figure 13B). If the catalase is added to the medium, no damage was observed from MnP + ascorbate treatment (Figure 13A) [44].
The pro-oxidative action of metalloporphyrins in the presence of ascorbate or glutathione may be selective to cancer over normal cells due to their differential ability to remove peroxides. Normal cell has plenty of peroxide-removing enzymes, while cancer cell is already under oxidative stress and may have inadequate ratio of superoxide- to peroxide-removing systems. Any additional oxidative burden may signal tumor to die. Recently, Kuiper et al [117] showed that high grade tumors are ascorbate-deficient which drives HIF-1α production; low grade tumors with high ascorbate content have low HIF-1α levels. Thus, employing exogenous ascorbate in combination with redox-active catalyst seems a viable option for treating tumors.
In vivo systems are exceedingly complex, as is the biochemistry and biology of MnPs with four readily accessible oxidation states and high reactivity of Mn site [92]. Given the abundancy of cellular reductants and other redox-active compounds, it is very likely that MnPs can exert their actions via both anti- and pro-oxidative pathways; the effects observed could also be anti- and pro-oxidative. The observations on these SOD mimics parallel to a certain extent the actions and effects observed with MnSOD, in particular in the studies where the enzyme was overexpressed and increased levels of H2O2 frequently observed [39–44,118–121]. A brief article by Garry Buettner discussed the role of MnSOD as a central player in the redox biology of cells and tissues [121]. His report and reports by Irwin Fridovich [39] and Lee Ann MacMillan Crow and John Crow [40], as well as from Melendez [42] and St Clair [41] groups address the possible and still controversial origin of the increased peroxide as a direct or indirect consequence of MnSOD overexpression [39–44,121]. More work is needed to gain a profound insight into the in vivo destiny of MnPs, as is to fully understand the role of the MnSOD enzyme which those compounds mimic.
The experimental data teach us to distinguish between the pro- and antioxidative actions of MnPs and to understand which conditions would favor one over the other pathways; both actions could obviously produce beneficial therapeutic outcome. It is further important to distinguish between the effects we observe and the nature of actions that led to such effects. The pro-oxidative mechanism of action in diabetes studies resulted in the therapeutically beneficial antioxidative effect, while the pro-oxidative mechanism of action in lymphoma studies led to the therapeutically beneficial tumor killing effect. It is also possible that upon the oxidative insult, the cell would respond with adaptive response, via upregulation of endogenous antioxidative defenses, alike does E. coli when stressed with peroxide; obviously that would result in antioxidative effects [44]. Similar discussions on the consequences of overexpression of MnSOD was provided by Kim et al too [118].
Therapeutic effects and clinical development of MnPs
Therapeutic effects of MnPs have been described in refs [18, 29–31], and along with new data are summarized in Table 2. For more than two decades, the convincing and reproducible data have been provided by different groups from different Universities on the efficacy of three MnPs listed in Figure 1. Therefore, there is no doubt that they carry therapeutic potential. Significant efforts have been made recently to get them into the clinic as soon as possible.
Table 2.
Therapeutic effects of Mn porphyrins. Listed are the diseases where beneficial effects were observed, the porphyrin formula, the animal tested and the related references where the data on dosing and the study outcome could be found.
| Disorders/injuries | Porphyrins | Animal/cells | Refs | ||
|---|---|---|---|---|---|
| CNS injuries | Stroke | MnTE-2-PyP5+ MnTDE-2-ImP5+ MnTnHex-2-PyP5+ |
rodents | 85,122–124 | |
| Spinal cord injury | MnTE-2-PyP5+ MnTDE-2-ImP5+ |
mice | 30,125 | ||
| Subarachnoid hemorrhage | MnTnHex-2-PyP5+ | mice | 69,124 | ||
| Cerebral palsy | MnTnHex-2-PyP5+ | rabbit dams | 86 | ||
| Amyotrophic lateral sclerosis | MnTnHex-2-PyP5+ MnTDE-2-ImP5+ |
mice | 126–128 | ||
| Parkinson’s disease | MnTBAP3− | cells | 129 | ||
| AEOL11207 | mice | ||||
| Alzheimer’s disease | MnTE-2-PyP5+ | cells | 130 | ||
| Oxygen and glucose deprivation | MnTE-2-PyP5+, MnTDE-2-ImP5+, MnTnHex-2-PyP5+, MnTnOct-2-PyP5+ | cells | 80 | ||
| Staurosporine- induced neurotoxicity | MnTM-4-PyP5+, H2TM-4-PyP4+, ZnTM-4-PyP4+, MnTM-2- PyP5+, MnTE-2-PyP5+, MnTBAP3−, ZnTBAP4− | cells | 131 | ||
| Diabetes | MnTE-2-PyP5+ | mice | 105–107,132,133 | ||
| MnTDE-2-ImP5+ | cells | ||||
| MnTM-2-PyP5+ | rats | ||||
| MnTE-2-PyP5+ | mice | ||||
| Cancer | single agent | Skin | MnTE-2-PyP5+ | mice | 102 |
| Brain | MnTnHex-2-PyP5+ | mice | 43 | ||
| Breast | MnTE-2-PyP5+ | mice | 100 | ||
| combinatorial therapy | Chemotherapy | MnTE-2-PyP5+ MnTM-2-PyP5+ |
cells | 68,93, 111 | |
| MnTnHex-2-PyP5+ | mice | ||||
| Radiation | MnTE-2-PyP5+ | mice | 100,101, 134,135 | ||
| Hyperthermia | 136 | ||||
| Other ischemia/reperfusion conditions | MnTnHex-2-PyP5+ | rats | 83,137–141 | ||
| MnTM-4-PyP5+ | |||||
| MnTECP, MnTE-2- PyP5+, MnTM-2-PyP5+ | mice | ||||
| MnTE-2-PyP5+ | rats | ||||
| Lung injuries | MnTE-2-PyP5+ | fetal baboons | 142–144 | ||
| mice | |||||
| MnTDE-2-ImP5+ | rats | ||||
| Sickle-cell disease | MnTE-2-PyP5+ | mice | 145 | ||
| Pain management | MnTnHex-2-PyP5+ MnTE-2-PyP5+ |
mice | 146 | ||
| Radiation injury | Whole body | MnTE-2-PyP5+ | zebrafish | 147 | |
| radioprotection | MnTM-2-PyP5+ | mice | 148 | ||
| Hematopoietic stem cell radioprotection | MnTE-2-PyP5+ | 149,150 | |||
| Lung radioprotection | MnTE-2-PyP5+ MnTDE-2-ImP5+ |
rats | 151–154 | ||
| MnTnHex-2-PyP5+ | nonhuman primates | 155 | |||
| Eye radioprotection | MnTE-2-PyP5+ | rats | 156 | ||
| Brain radioprotection | MnTDE-2-ImP5+ | 157 | |||
| Cell radioprotection | EUK-451 | cells | 67,158 | ||
| MnTnHex-2-PyP5+ | |||||
| Sepsis | MnTE-2-PyP5+ | rats | 159 | ||
| Sperm motility | MnTM-2-PyP5+ | cells | 160 | ||
Due to clear patenting and licensing rights of imidazolium analogue, MnTDE-2-ImP5+ (AEOL10150), Aeolus Pharmaceuticals is developing it as a wide spectrum radioprotector. MnTDE-2-ImP5+ was successfully tested in Phase I Toxicity Clinical trials on amyotrophic lateral sclerosis patients. National Institute of Allergy and Infectious diseases, NIAID is heavily supporting the development of lipophilic MnTnHex-2-PyP5+ for pulmonary radioprotection; the first study was successfully performed on non-human primates. Support was also provided by Duke University Translational Research Institute, and the Wallace H. Coulter Translational Partners Grant Program for the translational studies of the lipophilic MnTnHex-2-PyP5+ and its analogues, primarily for the injuries of the central nervous system and for oncology applications. The Preston Robert Tisch Center at Duke is supporting the development of lipophilic analogues for treating brain tumors, motivated by their remarkable efficacy in suppressing growth of subcutaneous and intracranial brain tumor xenografts [43]. Importantly, the optimized generation of lipophilic analogues has clear patenting rights at Duke University Medical Center, and has not yet been licensed, which allows us to assure the much needed financial support to get them into clinics. The mishandling of IP rights has in past slowed the clinical developmentof MnPs for at least a decade.
The synthesis of the most in vivo studied cationic Mn porphyrin, MnTE-2-PyP5+, has been scaled up by Ricerca under the guidance and collaborative efforts of Duke University Medical Center (Ines Batinic-Haberle) and National Jewish Research and Medical Center (James Crapo). Master drug has been filed with Food and Drug Administration (FDA) for MnTE-2-PyP5+. The toxicity studies needed for Investigational New Drug (IND) filing with FDA are in progress.
Given the convincing and reproducible efficacy of those compounds in vivo, it is still hard to understand that much bigger progress has not yet been made in getting those compounds to clinic. Moreso, as there are essentially no efficacious drugs availbale for the stroke, cancer treatment and radioprotection of the normal tissue. Though scientific part in drug design and synthesis is difficult in its own right, it seems that the drug development is often limited by unresolved and/or mishandled IP rights.
Toxicity
Mn porphyrins, as well as any therapeutic, exert toxicity at high doses. The more lipophilic members, such as MnTnHex-2-PyP5+, are toxic at least in part via their enhanced micellar properties, which would allow them to incorporate into and damage membranes, thus compromising the cell integrity. The lack of dark toxicity of lipophilic Zn analogues [161] suggests that toxicity is not soilely due to surfactancy of these compounds but to redox-related and/or Mn axial coordination properties also. The most in vivo explored compounds (listed in Figure 1) are more toxic to rats than to mice: both exert blood pressure drop. No blood pressure drop was seen with non-human primates at comparable doses. All of the MnPs are more toxic if given ip than sc. Single 3 mg/kg ip injection of MnTnHex-2-PyP5+ is already toxic to mousee, and 5 mg/kg it would kill it [St. Clair et al, Sheng et al, unpublished]. 1.6 mg/kg/day, given b. i. d. sc to nu/nu genotype of Balb/c mouse background, caused no signs of toxicity during 6 weeks of treatment [43]. The PK study has been published with MnTE-2-PyP5+ [79]. Due to its plasma half-life of around 1 hour, the twice daily administration is preferred. The TD50 (the toxicity dose) of MnTE-2-PyP5+ given sc is 91.5 mg/kg, and for MnTnHex-2-PyP5+ is 12.5 mg/kg [67]. Toxicity was expressed as hypotonia, and shaking at higher doses. Given up to 120-fold higher in vivo potency of MnTnHex-2-PyP5+ (efficacious at as low as 0.05 mg/kg) than of MnTE-2-PyP5+ (efficacious at 6 mg/kg), the therapeutic window for the former is larger than for the latter [18,29-31].
MnTnHex-2-PyP5+ was given sc to macaque monkeys for 6 months at 0.05 mg/kg/day without any signs of organ pathology. At such low dose it was also protective in lung radiation macaque monkey model [155].
Identity and purity of redox-able compounds
The synthesis and purification of porphyrins is difficult alike of any other synthetic compound aimed at clinical use. Very low levels of impurities may have biological activities in their own rights, as we clearly showed with MnTBAP3− [55,96,162,163]. Therefore, the compounds with therapeutic potential must be of extreme purity in order to be used in clinics. Very often insufficient data related to the drug identity and purity have been reported. Mass spectra and elemental analyses are often missing. Frequently, the presence of molecular ion is stated only, which is meaningless unless the intensity of such ion, i. e. its contribution relative to other ions is clearly shown. The most obvious case is the anionic Mn porphyrin, MnTBAP3−, which has been incorrectly assigned as SOD mimic [96,163]. It was sold by different sources, each of different impurity. No commercial source produced batches of MnTBAP3− that are of consistent purity [96,163]. Finally, a particular batch of MnTDE-2-ImP5+ (made outside of our Lab), contained large amount of solid precipiatete. It was identified as MnO2, and is the result of the hydrolysis of excessive amounts of Mn aqua species which had not been removed after metallation. Inconsistency in the drug preparations is perpetuating damage done to the scientific audience and misleading them in understanding the magnitude of the drug efficacy and its mechanism of actions.
Conclusions
Mn porphyrins seem advantageous relative to other synthetic metal-based compounds (such as Mn salen compounds, Mn cyclic polyamines, Mn complexes with non-cyclic ligands) at least because they are of remarkable metal/ligand stability, which assures integrity of the metal site where all chemistry occurs; they are stable even in concentrated sulfuric and hydrochloric acids, at room temperature and under light. Further there are limitless possibilities of modifying their structure whereby improving redox ability, bioavailability and toxicity. Among MnP class of compounds, cationic analogues are superior to anionic and neutral compounds because: (1) they afford thermodynamic and kinetic facilitation for the approach of anionic reactive species, which gives raise to high rate constants for the removal of O2· − and ONOO−; and (2) they accumulate in mitochondria relative to cytosol.
Among the compounds that have been thus far heavily explored in vitro and in vivo (Figure 1), MnTnHex-2-PyP5+ is orders of magnitude more lipophilic than either MnTE-2-PyP5+ or MnTDE-2-ImP5+ and is thus superior for CNS injuries, as showed with animal models of cerebral palsy, pain, brain tumors, stroke, and subarachnoid hemorrhage (Table 2). MnTE-2-PyP5+ either failed (rabbit cerebral palsy model) or was much less effective than MnTnHex-2-PyP5+ (mouse pain model) in CNS injuries. There are essentially only E. coli studies where MnTE-2-PyP5+ and MnTDE-2-ImP5+ were directly compared in a single experiment. MnTDE-2-ImP5+ is a more bulkier molecule with ethyl groups below and above the porphyrin plane which limits its accumulation within E. coli cell and thus reduces its efficacy in protecting SOD-deficient E. coli to grow aerobically [54,61]. Such bulkiness further hinders positive charges which are delocalized over imidazole rings of MnTDE-2-ImP5+, and may reduce its accumulation within cellular compartments (in particular in mitochondria), as well as its interactions with negatively charged biological targets (proteins, enzymes, phospholipids, nucleic acids). The suppressed interactions with biomolecules presumably reduce the toxicity of MnTDE-2-ImP5+, allowing a larger therapeutic window in a stroke model than that of MnTE-2-PyP5+ [85]. More comparative studies are needed to compare the therapeutic potential of MnTDE-2-ImP5+ and MnTE-2-PyP5+. Due to the fully resolved IP rights, the MnTDE-2-ImP5+ is now pushed for the development as a wide spectrum radioprotector by Aeolus Pharmaceuticals, being supported in large part by USA Federal Government Contract. Additional translational aspects of the MnPs development are addressed above (Therapeutic effects and clinical development of MnPs).
The redox ability of MnPs, judged by the kcat(O2· −), is optimized, and for some compounds is nearly as high as the kcat(O2· −) for SOD enzymes. Mitochondria targeting has been achieved with lipophilic MnTnHex-2-PyP5+ [70]. Compounds that are able to cross the blood brain barrier and are efficcaious in stroke and hemorrhage models, such as MnTnHex-2-PyP5+, have been developed. More optimized drugs are in development. Most recent efforts were also aimed at decreasing toxicity while maintaining high lipophilicity and redox ability to modulate oxidative stress of a normal and cancer cell. Due to the very complex redox chemistry of Mn site, with 4 oxidation states available for the interaction with reactive species and signaling molecules and complex redox biology, further research is needed to fully comprehend the redox-based reactions of MnPs.
Those MnPs which are easily coupled with cellular reductants are efficiently and catalytically scavenging reactive species, whereby affecting primary oxidative events, but also eliminating signaling species. Consequently, MnPs can modulate cellular transcriptional activity, and be effective not only at the point of a primary oxidative event, but even when given hours (stroke model) [122,123] or weeks (lung radiation) [152] afterwards, repairing damage done by cycling inflammation.
While considered predominantly antioxidants, the ability of Mn porphyrins to equally effectively oxidize and reduce O2· − indicates that they can be equally potent pro- and antioxidants. The effects at the level of cellular transcriptional activity is thus far predominantly viewed as antioxidative, though pro-oxidative action is involved in suppressing NF-κB activation. The pro-oxidative action reportedly occurs also when MnPs, other metalloporphyrins and metal complexes couple with cellular reductants, such as ascorbate and glutathione [68,93-95,111,112]. The pro-oxidative action was reported for metalloporphyrin-based activation (hydroxylation) of cyclophosphamide in a cyt P450 fashion [164]. The type of in vivo action of MnPs will predominantly depend upon the redox status of the cell, ratio of superoxide to peroxide removing systems, species they will encounter and compartments they will accumulate in.
Brief, yet very informative forum editorial by Paola Chiarugi [165] stressed the importance of the clinically relevant strategies in both degenerative diseases and cancer which could modulate cellular redox status either by pro-oxidants or antioxidants, or by affecting selected redox-based signaling pathways. The author describes “Redox Paradox” as the activation of a survival programme by the same molecules from which protection is needed. She further suggests that rational combination of pro-oxidants and compounds which are directed to known signaling redox-sensitive pathways, may give raise to prospective therapeutics. MnPs fulfil major requirements which make them perfectly fit as therapeutics. As already shown, their redox properties allow them to finely modulate redox status of the cell, and in turn apoptotic and proliferative events by either depleting the cell from reactive species or increasing their levels. The magnitude of the reactive species produced will direct cell to either upregulate its own endogenous antioxidative defenses in order to adapt and survive, or undergo apoptosis.
Though obvious, it needs to be stressed again that extreme attention must be paid to the identity and purity of compounds which are intended for therapeutic purposes; it is sine qua non. Even tiny impurity may have biological activity and mask or modify the action of the drug.
In summary, present data tell us that much is left to understand about chemistry, biology and pharmacology of Mn porphyrins. They also challenge our thinkings and ask for eyes wide open. Future explorations are justified as long as we continue to observe remarkable therapeutic effects of MnPs.
Figure 9.
The reactivity of MnPs towards reactive species and transcription factors thus far studied [18,30]. The log values of rate constants listed relate predominantly to ortho isomers. The rate constant of MnPs with HClO was estimated based on the reported data on the reaction of MnTM-4-PyP5+ and MnTBAP3− with HClO [89]. Data on the reaction of reduced Mn porphyrin, MnIITE-2-PyP4+ with ·NO is also listed. The highest log kcat(O2·−) ≥ 8.85 refers to MnIIBr8TM-3-PyP4+. Based on our present knowledge, the ability of MnPs to catalyze peroxide removal is weak. Based on the reactivity to other species listed, it is highly likely that potent SOD mimics would scavenge ·NO2 radical also.
Acknowledgments
Authors acknowledge financial help from IBH general research funds, NIH U19AI067798 (ZR, IBH), Duke University’s CTSA grant 1 UL 1 RR024128-01 from NCRR/NIH (AT, IBH, IS), NIH/NCI Duke Comprehensive Cancer Center Core Grant (5-P30-CA14236-29) (IS) and R01 CA40355. XDY is very grateful to the financial support from Hefei National Laboratory for Physical Sciences at the Microscale at University of Science and Technology of China during his stay in Professor Kam W. Leong group at Duke University.
Abbreviations
- MnPs
Mn(III) N-alkyl- and N-methoxyalkylpyridylporphyrins and N,N′-dialkylimidazolylporphyrins
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- ONOO−
peroxynitrite
- O2•−
superoxide
- ONOOCO2−
adduct of ONOO− with CO2
- •NO
nitric oxide
- CO3•−
carbonate radical
- ·OH
hydroxyl radical
- HClO
hypochlorous acid
- A
dehydroascorbic acid
- HA−
monodeprotonated ascorbate
- HA·
ascorbyl radical
- A· −
deprotonated ascorbyl radical
- MeCN
acetonitrile
- BBB
blood brain barrier
- H2TE-2-PyP4+
meso-tetrakis(N-ethylpyridinum-2-yl)porphyrin
- H2TE-3-PyP4+
meso-tetrakis(N-ethylpyridinum-3-yl)porphyrin
- H2TnHex-2-PyP4+
meso-tetrakis(N-n-hexylpyridinum-2-yl)porphyrin
- H2TnHex-3-PyP4+
meso-tetrakis(N-n-hexylpyridinum-3-yl)porphyrin
- H2TMOE-3-PyP4+
meso-tetrakis(N-(2′-methoxyethyl)pyridinum-3-yl)porphyrin
- H2TMOHex-3-PyP4+
meso-tetrakis(N-(6′-methoxyhexyl)pyridinum-3-yl)porphyrin
- MnTE-2-PyP5+
AEOL10113, FBC-007, Mn(III) meso-tetrakis(N-ethylpyridinum-2-yl)porphyrin
- MnTE-3-PyP5+
E3, Mn(III) meso-tetrakis(N-ethylpyridinum-3-yl)porphyrin
- MnTnBut-3-PyP5+
Mn(III) meso-tetrakis(N-n-butylpyridinum-2-yl)porphyrin
- MnTnHex-2-PyP5+
Mn(III) meso-tetrakis(N-n-hexylpyridinum-2-yl)porphyrin
- MnTnHex-3-PyP5+
Mn(III) meso-tetrakis(N-n-hexylpyridinum-3-yl)porphyrin
- MnTnHep-2-PyP5+
Mn(III) meso-tetrakis(N-n-heptylpyridinum-3-yl)porphyrin
- MnBr8TM-3-PyP4+
Mn(II) β-octabromo-meso-tetrakis(N-methylpyridinum-3-yl)porphyrin
- MnTMOE-3-PyP5+
Mn(III) meso-tetrakis(N-(2′-methoxyethyl)pyridinum-3-yl)porphyrin
- MnTMOHex-3-PyP5+
Mn(III) meso-tetrakis(N-(6′-methoxyhexyl)pyridinum-3-yl)porphyrin
- MnTnBuOE-2-PyP5+
Mn(III) meso-tetrakis(N-2′-butoxyethylpyridinium-2-yl)porphyrin
- MnTDE-2-ImP5+ (AEOL10150)
Mn(III) meso-tetrakis(N, N′-diethylimidazolium-2-yl)porphyrin
- MnTBAP3− (also MnTCPP3− and AEOL10201)
Mn(III) meso-tetrakis(4-carboxylatophenyl)porphyrin
- MnTM-4-PyP5+
mangenese(III) tetrakis (1-methyl-4-pyridyl) porphyrin
- MnTECP
manganese tetrakis-(ethoxycarbonyl) porphyrin
- EUK-451
manganese 5,15-di(4-tetra-hydro-pyrano) porphyrin
- Rf
thin-layer chromatographic retention factor that presents the ratio between the solvent and compound path in 1:1:8 = KNO3(sat):H2O:CH3CN solvent system
- POW
partition between n-octanol and water
- E1/2
half-wave reduction potential
- SOD
superoxide dismutase
- NHE
normal hydrogen electrode
- EDTA
ethylenediaminetetraacetic acid
- CNS
central nervous system
- DMBA
7,12-dimethylbenz-(a)-anthracene
- TPA
12-O-tetradecanoylphorbol-13-acetate
- CEES
2-chloroethyl ethyl sulfide
- MPTP - 1-methyl-4-phenyl-1
2,3,6-tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium, 6-OHDA, 6-hydroxydopamine
- PK
pharmacokinetics
- ip
intraperitoneal
- icv
intracerebroventricular
- sc
subcutaneous
- iv
intravenous
- HIF-1α
hypoxia inducible factor-1
- NF-κB
nuclear factor κB
- AP-1
activator protein-1
- SP-1
specificity protein 1
- TF
transcription factor
- TD50
dose at which 50% of animals exhibit toxicity, determined as described in ref [67]
- LPS
lipopolysaccharides
- FDA
Food and Drug Administration
- IND
Investigational New Drug
- DMF
drug master file
- IP
intellectual rights
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilbert DL. O2 and Living Processes: an Inter-disciplinary Approach. New York: Springer; 1981. [Google Scholar]
- 2.McCord JM, Fridovich I. Superoxide dismutase: An enzymatic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 3.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curnutte JT, Whitten DM, Babior BM. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974;290:593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- 6.Takeya R, Sumimoto H. Regulation of novel superoxide-producing NADP(H) oxidases. Antioxid Redox Signal. 2006;8:1523–1532. doi: 10.1089/ars.2006.8.1523. [DOI] [PubMed] [Google Scholar]
- 7.Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by NOX family NADPH oxidases. Antioxid Redox Signal. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petry A, Weitnauer M, Görlach A. Receptor activation of NADPH oxidases. Antioxid Redox Signal. 2010;13:467–487. doi: 10.1089/ars.2009.3026. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trachootham D, Lu W, Ogasawara MA, Rivera-Del Valle N. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groeger AL, Freeman BA. Signaling actions of electrophiles: anti-inflammatory therapeutic candidates. Mol Interv. 2010;10:39–50. doi: 10.1124/mi.10.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha V, Wijewickrama GT, Chandrasena RE, Xu H, Edirisinghe PD, Schiefer IT, Thatcher GR. Proteomic and mass spectroscopic quantitation of protein S-nitrosation differentiates NO-donors. ACS Chem Biol. 2010;5:667–680. doi: 10.1021/cb100054m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Garcia D, Wood CD, Castro-Obregon S, Covarrubias L. Reactive oxygen species: a radical role in development? Free Radic Biol Med. 2010;49:130–143. doi: 10.1016/j.freeradbiomed.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Liou G-Y, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo M, He H, Kelley MR, Georgiadis MM. Redox regulation of DNA repair: Implications for human health and cancer therapeutic development. Antioxid Redox Signal. 2010;12:1247–1268. doi: 10.1089/ars.2009.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iles KE, Forman HJ. Macrophage signaling and respiratory burst. Immun Res. 2002;26:95–105. doi: 10.1385/IR:26:1-3:095. [DOI] [PubMed] [Google Scholar]
- 17.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nature Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 18.Batinic-Haberle I, Rebouças JS, Spasojevic I. Superoxide dismitase mimics: Chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasternack RF, Halliwell B. Superoxide dismutase activities of an iron porphyrin and other iron complexes. J Am Chem Soc. 1979;101:1026–1031. [Google Scholar]
- 20.Pasternack RF, Skowronek WR., Jr Catalysis of the disproportionation of superoxide by metalloporphyrins. J Inorg Biochem. 1979;11:261–267. doi: 10.1016/s0162-0134(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 21.Pasternack RF, Banth A, Pasternack JM, Johnson CS. Catalysis of the disproportionation of superoxide by metalloporphyrins. III. J Inorg Biochem. 1981;15:261–267. doi: 10.1016/s0162-0134(00)80161-0. [DOI] [PubMed] [Google Scholar]
- 22.Weinraub D, Peretz P, Faraggi M. Chemical properties of water-soluble porphyrins. 1. Equilibria between some ligands and iron(III) tetrakis(4-N-methylpyridyl)porphyrin. J Phys Chem. 1982;86:1839–1842. [Google Scholar]
- 23.Solomon D, Peretz P, Faraggi M. Chemical properties of water-soluble porphyrins. 2. The reaction of iron(III) tetrakis(4-N-methylpyridyl)porphyrin with superoxide radical dioxygen couple. J Phys Chem. 1982;86:1842–1849. [Google Scholar]
- 24.Peretz P, Solomon D, Weinraub D, Faraggi M. Chemical properties of water-soluble porphyrins: 3. The reaction of superoxide radicals with some metalloporphyrins. Int J Radiat Biol. 1982;42:449–456. doi: 10.1080/09553008214551361. [DOI] [PubMed] [Google Scholar]
- 25.Faraggi M, Peretz P, Weinraub D. Chemical properties of water-soluble porphyrins: 4. The reduction of a “picket-fence-like” iron(III) complex with superoxide oxygen couple. Int J Radiat Biol. 1986;49:951–968. doi: 10.1080/09553008514553181. [DOI] [PubMed] [Google Scholar]
- 26.Weinraub D, Levy P, Faraggi M. Chemical properties of water-soluble porphyrins. 5. Reactions of some manganese (III) porphyrins with the superoxide and other reducing radicals. Int J Radiat Biol. 1986;50:649–658. doi: 10.1080/09553008614551051. [DOI] [PubMed] [Google Scholar]
- 27.Ilan Y, Rabani J, Fridovich I, Pasternack RF. Superoxide dismuting activity of iron porphyrin. Inorg Nucl Chem Lett. 1981;17:93–96. [Google Scholar]
- 28.Faulkner KM, Liochev SI, Fridovich I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- 29.Batinic-Haberle I, Reboucas JS, Benov L, Spasojevic I. In: Chemistry, biology and medical effects of water soluble metalloporphyrins. Kadish KM, Smith KM, Guillard R, editors. Vol. 11. Singapore: World Scientific Handbook of Porphyrin Science; 2011. pp. 291–393. [Google Scholar]
- 30.Batinic-Haberle I, Spasojevic I, Tse HM, Tovmasyan A, Rajic Z, StClair DK, Vujaskovic Z, Dewhirst MW, Piganelli JD. Design of Mn porhyrins for treating oxidative stress injuries and their redox-based regulation of cellular transcriptional activities. Amino Acids. 2010 doi: 10.1007/s726-010-0603-06. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spasojevic I, Batinic-Haberle I. SOD mimics. In: Pantopoulos K, Schipper H, editors. Principles of Free Radical Biomedicine. Nova Science Publishers, Inc; Hauppauge NY: 2011. in press. [Google Scholar]
- 32.Korsvik C, Patil S, Seal S, Self WT. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun. 2007:1056–1058. doi: 10.1039/b615134e. [DOI] [PubMed] [Google Scholar]
- 33.Colon J, Herrera L, Smith J, Patil S, Komanski C, Kupelian P, Seal S, Jenkins DW, Baker CH. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine. 2009;5:225–231. doi: 10.1016/j.nano.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein S, Czapski G, Heller A. Osmium tetraoxide, used in the treatment of arthritic joints, is a fast mimic of superoxide dismutase. Free Radic Biol Med. 2005;38:839–845. doi: 10.1016/j.freeradbiomed.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Takahashi M, Shimizu T, Shirasawa T, Kajita M, Kanayama A, Miyamoto Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech Ageing Dev. 2008;129:322–331. doi: 10.1016/j.mad.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Para AE, Bezjak A, Yeung IW, Van Dyk J, Hill RP. Effects of genistein following fractionated lung irradiation in mice. Radiother Oncol. 2009;92:500–510. doi: 10.1016/j.radonc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 38.Pang X, Yi Z, Zhang J, Lu B, Sung B, Qu W, Aggarwat BB, Liu M. Celastrol suppresses angiogenesis-mediated tumor growth inhibition of AKT/mammalian target of rapamycin pathway. Cancer Res. 2010;70:1951–1959. doi: 10.1158/0008-5472.CAN-09-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fridovich I. Superoxide dismutases: anti-versus pro-oxidants? Anticancer Agents Med Chem. 2011;11:175–177. doi: 10.2174/187152011795255966. [DOI] [PubMed] [Google Scholar]
- 40.MacMillan-Crow LA, Crow JP. Does more MnSOD mean more hydrogen peroxide? Anticancer Agents Med Chem. 2011;11:178–180. doi: 10.2174/187152011795255939. [DOI] [PubMed] [Google Scholar]
- 41.Miriyali S, Holley AK, St Clair DK. Mitochondrial superoxide dismutase – signals of distinction. Anticancer Agents Med Chem. 2011;11:181–190. doi: 10.2174/187152011795255920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hempel N, Carrico PM, Melendez JA. Manganese superoxide dismutate (Sod2) and redox-control of signaling events that drive metastasis. Anticancer Agents Med Chem. 2011;11:191–201. doi: 10.2174/187152011795255911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keir ST, Dewhirst MW, Kirkpatrick JP, Bigner DD, Batinic-Haberle I. Cellular redox modulator, ortho Mn(III) meso-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin, MnTnHex-2-PyP5+ in the treatment of brain tumors. Anticancer Agents Med Chem. 2011;11:202–212. doi: 10.2174/187152011795255957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batinic-Haberle R, Rajic Z, Benov L. A combination of two antioxidants (an SOD mimic and ascorbate) produces pro-oxidative effect forcing Escherichia coli to adapt via induction of oxyR regulon. Anticancer Agents Med Chem. 2011 doi: 10.2174/187152011795677562. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vance CK, Miller AF. A simple proposal that can explain the inactivity of metal-substituted superoxide dismutases. J Am Chem Soc. 1998;120:461–467. [Google Scholar]
- 46.Goldstein S, Fridovich I, Czapski G. Kinetic properties of Cu,Zn-superoxide dismutase as a function of metal content - order restored. Free Radic Biol Med. 2006;41:937–941. doi: 10.1016/j.freeradbiomed.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 47.Ellerby RM, Cabelli DE, Graden JA, Valentine JS. Copper-zinc superoxide dismutase: why not pH-dependent? J Am Chem Soc. 1996;118:6556 –6561. [Google Scholar]
- 48.Batinic-Haberle I, Benov L, Spasojevic I, Fridovich I. The ortho effect makes manganese (III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin (MnTM-2-PyP) a powerful and potentially useful superoxide dismutase mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 49.Batinic-Haberle I, Benov L, Spasojevic I, Hambright P, Crumbliss AL, Fridovich I. The relationship between redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vitro and in vivo superoxide dismutase activities of manganese(III) and iron(III) cationic and anionic porphyrins. Inorg Chem. 1999;38:4011–4022. [Google Scholar]
- 50.Batinic-Haberle I, Spasojevic I, Stevens RD, Hambright P, Fridovich I. Manganese(III) meso tetrakis ortho N-alkylpyridylporphyrins. synthesis, characterization and catalysis of O2· − dismutation. J Chem Soc Dalton Trans. 2002:2689–2696. [Google Scholar]
- 51.Kos I, Rebouças JS, DeFreitas-Silva G, Salvemini D, Vujaskovic Z, Dewhirst MW, Spasojevic I, Batinic-Haberle I. The effect of lipophilicity of porphyrin-based antioxidants. Comparison of ortho and meta isomers of Mn(III) N-alkylpyridylporphyrins. Free Radic Biol Med. 2009;47:72–78. doi: 10.1016/j.freeradbiomed.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kos I, Benov L, Spasojevic I, Rebouças JS, Batinic-Haberle I. High lipophilicity of meta Mn(III) N-alkylpyridylporphyrin-based SOD mimics compensates for their lower antioxidant potency and makes them equally effective as ortho analogues in protecting E. coli. J Med Chem. 2009;52:7868–7872. doi: 10.1021/jm900576g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tovmasyan A, Rajic Z, Spasojevic I, Reboucas JS, Chen X, Sheng H, Warner DS, Benov L, Batinic-Haberle I. Methoxy-derivatization of alkyl chains increases the efficacy of cationic Mn porphyrins. Synthesis, characterization, SOD-like activity, and SOD-deficient E. coli study of meta Mn(III) N-methoxyalkylpyridylporphyrins. Dalton Trans. 2011 doi: 10.1039/C0DT01321H. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batinic-Haberle I, Spasojević I, Stevens RD, Hambright P, Neta P, Okado-Matsumoto A, Fridovich I. New class of potent catalysts of O2· −dismutation. Mn(III) methoxyethylpyridyl- and methoxyethylimidazolylporphyrins. J Chem Soc Dalton Trans. 2004:1696–1702. doi: 10.1039/b400818a. [DOI] [PubMed] [Google Scholar]
- 55.Batinic-Haberle I, Cuzzocrea S, Rebouças JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojevic I, Benov L, Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: Comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic Biol Med. 2009;46:192–201. doi: 10.1016/j.freeradbiomed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spasojevic I, Menzeleev R, White PS, Fridovich I. Rotational isomers of N-alkylpyridylporphyrins and their metal complexes. HPLC separation, 1H NMR and X-ray structural characterization, electrochemistry, and catalysis of O2· − disproportionation. Inorg Chem. 2002;41:5874–5881. doi: 10.1021/ic025556x. [DOI] [PubMed] [Google Scholar]
- 57.Spasojevic I, Batinic-Haberle I, Rebouças JS, Idemori YM, Fridovich I. Electrostatic contribution in the catalysis of O2· − dismutation by superoxide dismutase mimics. J Biol Chem. 2003;278:6831–6837. doi: 10.1074/jbc.M211346200. [DOI] [PubMed] [Google Scholar]
- 58.Rebouças JS, DeFreitas-Silva G, Idemori YM, Spasojevic I, Benov L, Batinic-Haberle I. Impact of electrostatics in redox modulation of oxidative stress by Mn porphyrins: Protection of SOD-deficient Escherichia coli via alternative mechanism where Mn porphyrin acts as a Mn carrier. Free Radic Biol Med. 2008;45:201–210. doi: 10.1016/j.freeradbiomed.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rebouças JS, Spasojevic I, Tjahjono DH, Richaud A, Méndez F, Benov L, Batinic-Haberle I. Redox modulation of oxidative stress by Mn porphyrin-based therapeutics: The effect of charge distribution. Dalton Trans. 2008:1233–1242. doi: 10.1039/b716517j. [DOI] [PubMed] [Google Scholar]
- 60.Kachadourian R, Johnson CA, Min E, Spasojevic I, Day BJ. Flavin-dependent antioxidant properties of a new series of meso-N,N′-dialkyl-imidazolium substituted manganese(III) porphyrins. Biochem Pharmacol. 2004;67:77–85. doi: 10.1016/j.bcp.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 61.Okado-Matsumoto A, Batinić-Haberle I, Fridovich I. Complementation of SOD deficient Escherichia coli by manganese porphyrin mimics of superoxide dismutase. Free Radic Biol Med. 2004;37:401–410. doi: 10.1016/j.freeradbiomed.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 62.Batinic-Haberle I, Spasojevic I, Fridovich I. Tetrahydrobiopterin rapidly reduces the SOD mimic Mn(III) ortho-tetrakis(N-ethylpyridinium-2-yl)porphyrin. Free Radic Biol Med. 2004;37:367–374. doi: 10.1016/j.freeradbiomed.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 63.Ferrer-Sueta G, Hannibal L, Batinic-Haberle I, Radi R. Reduction of manganese porphyrins by flavoenzymes and submitochondrial particles and the catalytic redox cycle of peroxynitrite. Free Radic Biol Med. 2006;41:503–512. doi: 10.1016/j.freeradbiomed.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 64.Benov L, Fridovich I. Why superoxide imposes an aromatic amino acid auxotrophy on Escherichia coli. The transketolase connection. J Biol Chem. 1999;274:4202–4206. doi: 10.1074/jbc.274.7.4202. [DOI] [PubMed] [Google Scholar]
- 65.Benov L, Kredich NM, Fridovich I. The mechanism of the auxotrophy for sulfur-containing amino acids imposed upon Escherichia coli by superoxide. J Biol Chem. 1996;271:21037–21040. doi: 10.1074/jbc.271.35.21037. [DOI] [PubMed] [Google Scholar]
- 66.Benov L, Fridovich I. Superoxide imposes leakage of sulfite from Escherichia coli. Arch Biochem Biophys. 1997;347:271–274. doi: 10.1006/abbi.1997.0343. [DOI] [PubMed] [Google Scholar]
- 67.Pollard JM, Rebouças JS, Durazo A, Kos I, Fike F, Panni M, Gralla EB, Valentine JS, Batinic-Haberle I, Gatti RA. Radioprotective effects of manganese-containing superoxide dismutase mimics on ataxia telangiectasia cells. Free Radic Biol Med. 2009;47:250–260. doi: 10.1016/j.freeradbiomed.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye X, Fels D, Dedeugd C, Dewhirst MW, Leong K, Batinic-Haberle I. The in vitro cytotoxic effects of Mn(III) alkylpyridylporphyrin/ascorbate system on four tumor cell lines. Free Radic Biol Med. 2009;47:S136. [Google Scholar]
- 69.Sheng H, Tse HM, Jung JY, Zhang Z, Spasojevic I, Piganelli J, Batinic-Haberle I, Warner DS. Neuroprotective efficacy from a lipophilic MnPorphyrin, MnTHex-2-PyP: Rodent models of ischemic stroke and subarachnoid hemorrhage. J Pharmacol Exp Ther. 2011 doi: 10.1124/jpet.110.176701. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spasojevic I, Li AM, Tovmasyan A, Rajic Z, Salvemini D, StClair D, Valentine JS, Vujaskovic Z, Gralla EB, Batinic-Haberle I. Accumulation of porphyrin-based SOD mimics in mitochondria is proportional to their lipophilicityS. cerevisiae study of ortho Mn(III) N-alkylpyridylporphyrins. Free Radic Biol Med. 2010;49:S199. [Google Scholar]
- 71.Benatar M. Lost in translation: Treatment trials in the SOD1 mouse and in human ALS. Neurobiol Dis. 2007;26:1–13. doi: 10.1016/j.nbd.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 72.Orell RW. AEOL-10150 Aeolus. Expert Opin Invest Drugs. 2006;7:70–80. [PubMed] [Google Scholar]
- 73.Batinic-Haberle I, Fridovich I, Spasojevic I. Substituted porphyrins. 7807825. US. 2010 October 5;
- 74.Batinic--octabromo-meso-tetrakis-(N-methylpyridinium-4-yl) porphyrin. Arch Biochem Biophys. 1997;343:225–233. doi: 10.1006/abbi.1997.0157. [DOI] [PubMed] [Google Scholar]
- 75.Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature. 1969;222:1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- 76.Murphy MP, Smith RAJ. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 77.Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta. 2008;177:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 78.Spasojevic I, Yumin C, Noel T, Yu I, Pole MP, Zhang L, Zhao Y, StClair DK, Batinic-Haberle I. Mn porphyrin-based SOD mimic, MnTE-2-PyP5+ targets mouse heart mitochondria. Free Radic Biol Med. 2007;42:1193–1200. doi: 10.1016/j.freeradbiomed.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spasojevic I, Chen Y, Noel TJ, Fan P, Zhang L, Rebouças JS, St Clair DK, Batinic-Haberle I. Pharmacokinetics of the potent redox modulating manganese porphyrin, MnTE-2-PyP5+ in plasma and major organs of B6C3F1 mice. Free Radic Biol Med. 2008;45:943–949. doi: 10.1016/j.freeradbiomed.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wise-Faberowski L, Warner DS, Spasojevic I, Batinic-Haberle I. The effect of lipophilicity of Mn (III) ortho N-alkylpyridyl- and diortho N, N′-imidazolylporphyrins in two in vitro models of oxygen and glucose deprivation -induced neuronal death. Free Radic Res. 2009;43:329–339. doi: 10.1080/10715760902736283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spasojevic I, Kos I, Benov LT, Rajic Z, Fels D, Dedeugd C, Ye X, Vujaskovic Z, Reboucas JS, Leong KW, Dewhirst MW, Batinic-Haberle I. Bioavailability of metalloporphyrin-based SOD mimics is greatly influenced by a single charge residing on a Mn site. Free Radic Res. 2011;45:188–200. doi: 10.3109/10715762.2010.522575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doyle T, Bryant L, Batinic-Haberle I, Little J, Cuzzocrea S, Masini E, Spasojevic I, Salvemini D. Supraspinal inactivation of mitochondrial superoxide dismutase is a source of peroxynitrite in the development of morphine antinociceptive tolerance. Neuroscience. 2009;164:702–710. doi: 10.1016/j.neuroscience.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saba H, Batinic-Haberle I, Munusamy S, Mitchell T, Lichti C, Megyesi J, MacMillan-Crow LA. Manganese porphyrin reduces renal injury and mitochondrial damage during ischemia/reperfusion. Free Radic Biol Med. 2007;42:1571–1578. doi: 10.1016/j.freeradbiomed.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kos I, Rebouças JS, Sheng H, Warner DS, Spasojevic I, Batinic-Haberle I. Oral availability of MnTE-2-PyP5+, a potent antioxidant and cellular redox modulator. Free Radic Biol Med. 2008;45:S86. [Google Scholar]
- 85.Sheng H, Enghild J, Bowler R, Patel M, Calvi CL, Batinic-Haberle I, Day BJ, Pearlstein RD, Crapo JD, Warner DS. Effects of metalloporphyrin catalytic antioxidants in experimental brain ischemia. Free Radic Biol Med. 2002;33:947–961. doi: 10.1016/s0891-5849(02)00979-6. [DOI] [PubMed] [Google Scholar]
- 86.Yu L, Jia X, Derricka M, Drobyshevsky A, Liu T, Batinic-Haberle I, Tan S. Testing new porphyrins in in vivo model systems: effect of Mn porphyrins in animal model of cerebral palsy. 6th International Conference on Porphyrins and Phthalocyanines; Albuquerque, USA. 2010. [Google Scholar]
- 87.Kosswig K. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim; Wiley-VCH: 2005. Surfactants. [Google Scholar]
- 88.Robinson VC, Bergfeld WF, Belsito DV, Hil RA, Klaassen CD, Marks JG, Jr, Shank RC, Slaga TI, Snyder PW, Andersen A. Final report of the amended safety assessment of sodium laureth sulfate and related salts of sulfated ethoxylated alcohols. Int J Toxicol. 2010;29:151S–161S. doi: 10.1177/1091581810373151. [DOI] [PubMed] [Google Scholar]
- 89.Carnieri N, Harriman A, Porter G. Photochemistry of manganese porphyrinsPart 6. Oxidation-reduction equilibria of manganese(III) porphyrins in aqueous solution. J Chem Soc Dalton Trans. 1982:931–938. [Google Scholar]
- 90.Ferrer-Sueta G, Vitturi D, Batinic-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J Biol Chem. 2003;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 91.Weitner T, Budimir A, Kos I, Batinic-Haberle I, Birus M. Acid-base and electrochemical properties of manganese meso(ortho- and meta-ethylpyridyl)porphyrins: potentiometric, spectrophotometric and spectroelectrochemical study of protolytic and redox equilibria. Dalton Trans. 2010;39:11568–11576. doi: 10.1039/c0dt00585a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Budimir A, Smuc T, Weitner T, Batinic-Haberle I, Birus M. Thermodynamics and electrochemistry of manganese tetra(ortho-n-butylpyridyl)porphyrin in aqueous solutions. J Coord Chem. 2010;63:2750–2765. [Google Scholar]
- 93.Jaramillo MC, Frye JB, Crapo JD, Briehl MM, Tome ME. Increased manganese superoxide dismutase expression or treatment with manganese porphyrin potentiates dexamethasone-induced apoptosis in lymphoma cells. Cancer Res. 2009;69:5450–5457. doi: 10.1158/0008-5472.CAN-08-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jaramillo MC, Briehl MM, Tome ME. Manganese porphyrin glutathionylates the p65 subunit of NF-κB to potentiate glucocorticoid-induced apoptosis in lymphoma. Free Radic Biol Med. 2010;49:S63. doi: 10.1016/j.freeradbiomed.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jaramillo MC, Briehl MM, Crapo JD, Batinic Haberle I, Tome ME. Manganese porphyrins acts as a pro-oxidant to glutathionylate p65 NF-κB and potentiate glucocorticoid-induced apoptosys in lymphoma cells. doi: 10.1016/j.freeradbiomed.2012.02.001. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rebouças JS, Spasojevic I, Batinic-Haberle I. Pure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: a case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. J Inorg Biol Chem. 2008;13:289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 97.Esposito E, Cuzzocrea S. Role of nitroso radicals as drug targets in circulatory shock. Brit J Pharmacol. 2009;157:494–508. doi: 10.1111/j.1476-5381.2009.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arambula JF, Prihs C, Borthwick D, Magda D, Sessler JL. Texaphyrins: tumor localizing redox active expanded porphyrins. Anticancer Agents Med Chem. 2011;11:222–232. doi: 10.2174/187152011795255894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okun Z, Kuperschmidt L, Youdim MBH, Gross Z. Cellular uptake and organ accumulation of bipolar metallcorroles with cytoprotective and cytotoxic properties. Anticancer Agents Med Chem. 2011 doi: 10.2174/187152011795677535. in press. [DOI] [PubMed] [Google Scholar]
- 100.Moeller BJ, Batinic-Haberle I, Spasojevic I, Rabbani ZN, Anscher MS, Vujaskovic Z, Dewhirst MW. A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int J Radiat Oncol Biol Phys. 2005;63:545–552. doi: 10.1016/j.ijrobp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 101.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of oxygenation, free radicals and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 102.Zhao Y, Chaiswing L, Oberley TD, Batinic-Haberle I, StClair W, Epstein CJ, St Clair D. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;65:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 103.Rabbani ZN, Spasojevic I, Zhang X, Moeller BJ, Haberle S, Vasquez-Vivar J, Dewhirst MW, Vujaskovic Z, Batinic-Haberle I. Antiangiogenic action of redox-modulating Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin, MnTE-2-PyP5+, via suppression of oxidative stress in a mouse model of breast tumor. Free Radic Biol Med. 2009;47:992–1004. doi: 10.1016/j.freeradbiomed.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tse H, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: Implication for their use in targeting oxidation/reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–47. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 105.Piganelli JD, Flores SC, Cruz C, Koepp J, Young R, Bradley B, Kachadourian R, Batinic-Haberle I, Haskins K. A metalloporphyrin superoxide dismutase mimetic (SOD mimetic) inhibits autoimune diabetes. Diabetes. 2002;51:347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 106.Bottino R, Balamurugan AN, Bertera S, Pietropaolo M, Trucco M, Piganelli JD. Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes. 2002;51:2561–1567. doi: 10.2337/diabetes.51.8.2561. [DOI] [PubMed] [Google Scholar]
- 107.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53:2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 108.Garcia J, Han, Sancheti H, Yap LP, Kaplowitz N, Cadenas E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J Biol Chem. 2010;285:39646–39654. doi: 10.1074/jbc.M110.164160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williams NH, Yandell JK. Outer-sphere electron-transfer reactions of ascorbate anions. Aust J Chem. 1982;35:1133–1144. [Google Scholar]
- 110.Gülden M, Jess A, Kammann J, Maser E, Seibert H. Cytotoxic potency of H2O2 in cell cultures: Impact of cell concentration and exposure time. Free Radic Biol Med. 2010;49:1298–1305. doi: 10.1016/j.freeradbiomed.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 111.Tian J, Peehl DM, Knox SJ. Metalloporphyrin synergizes with ascorbic acid to inhibit cancer cell growth through Fenton chemistry. Cancer Biother Radiopharm. 2010;25:439–447. doi: 10.1089/cbr.2009.0756. [DOI] [PubMed] [Google Scholar]
- 112.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, Levine M. Pharmacologic doses of ascorbate act as a pro-oxidant and desrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verrax J, Pedrosa RC, Beck R, Dejeans N, Taper H, Calderon PB. In situ modulation of oxidative stress: A novel and efficient strategy to kill cancer cells. Curr Med Chem. 2009;16:1821–1830. doi: 10.2174/092986709788186057. [DOI] [PubMed] [Google Scholar]
- 114.Verrax J, Beck R, Dejeans N, Glorieux C, Sid B, Curi Pedrosa R, Benites J, Vasquez D, Valderrama JA, Buc Calderon P. Redox-active quinones and ascorbate: an innovative cancer therapy that exploits the vulnerability of cancer cells to oxidative stress. Anti Canc Agents Med Chem. 2011;11:213–221. doi: 10.2174/187152011795255902. [DOI] [PubMed] [Google Scholar]
- 115.Jungwirth U, Kowol C, Hartinger C, Keppler B, Berger W, Heffeter P. Anticancer activity of metal complexes: involvement of redox processes. Antioxid Redox Signal. 2011 doi: 10.1089/jwh.2010.2233. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee YW, Ha MS, Kim YK. H2O2-induced cell death in human glioma cells: role of lipid peroxidation and PARP activation. Neurochem Res. 2001;26:337–343. doi: 10.1023/a:1010993428770. [DOI] [PubMed] [Google Scholar]
- 117.Kuiper C, Molenaar IGM, Dachs GU, Currie MJ, Sykes PH, Vissers MCM. Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res. 2010;70:5749–5758. doi: 10.1158/0008-5472.CAN-10-0263. [DOI] [PubMed] [Google Scholar]
- 118.Kim A, Joseph S, Khan A, Epstein CJ, Sobel R, Huang T-T. Enhanced expression of mitochondrial superoxide dismutase leads to prolonged in vivo cell cycle progression and up-regulation of mitochondrial thioredoxin. Free Radic Biol Med. 2010;48:1501–1512. doi: 10.1016/j.freeradbiomed.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ridnour LA, Oberley TD, Oberley LW. Tumor supressive effects of MnSOD overexpression may involve imbalance in peroxide generation versus peroxide removal. Antioxid Redox Signal. 2004;6:501–512. doi: 10.1089/152308604773934260. [DOI] [PubMed] [Google Scholar]
- 120.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. New York: Oxford University Press; 2007. [Google Scholar]
- 121.Buettner GR. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anticancer Agents Med Chem. 2011 doi: 10.2174/187152011795677544. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sheng H, Yang W, Fukuda S, Tse HM, Paschen W, Johnson K, Batinic-Haberle I, Crapo JD, Pearlstein RD, Piganelli J, Warner DS. Long-term neuroprotection from a potent redox-modulating metalloporphyrin in the rat. Free Radic Biol Med. 2009;47:917–923. doi: 10.1016/j.freeradbiomed.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mackensen GB, Patel M, Sheng H, Calvi CL, Batinic-Haberle I, Day BJ, Liang LP, Fridovich I, Crapo JD, Pearlstein RD, Warner DS. Neuroprotection from delayed postischemic administration of a metalloporphyrin catalytic antioxidant in the rat. J Neurosci. 2001;21:4582–4592. doi: 10.1523/JNEUROSCI.21-13-04582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Warner DP, Batinic-Haberle I, Sheng H. Mn porphyrins and the injured brain. 6th International Conference on Porphyrins and Phthalocyanines; Albuquerque, USA. 2010. [Google Scholar]
- 125.Sheng H, Spasojevic I, Warner DS, Batinic-Haberle I. Mouse spinal cord compression injury is ameliorated by intrathecal manganese(III) porphyrin. Neurosci Lett. 2004;366:220–225. doi: 10.1016/j.neulet.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 126.Crow J. Catalytic antioxidants to treat amyotrophic lateral sclerosis. Expert Opin Invest Drugs. 2006;15:1383–1392. doi: 10.1517/13543784.15.11.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Crow JP, Calinasan NY, Chen J, Hill JL, Beal MF. Manganese porphyrin given at symptom onset markedly extends survival of ALS mice. Ann Neurol. 2005;58:258–265. doi: 10.1002/ana.20552. [DOI] [PubMed] [Google Scholar]
- 128.Crow JP. Administration of Mn porphyrin and Mn texaphyrin at symptom onset extends survival of ALS mice. In: Doctrow SR, McMurry TJ, Sessler SJ, editors. Medicinal Inorganic Chemistry. Washington, DC: American Chemical Society; 2005. [Google Scholar]
- 129.Golden TR, Patel M. Catalytic antioxidants and neurodegeneration. Antioxid Redox Signal. 2009;11:555–569. doi: 10.1089/ars.2008.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sompol P, Ittarat W, Tangpong J, Chen Y, Doubinskaia I, Batinic-Haberle I, Mohammad Abdul H, Butterfield A, StClair DK. Alzheimer’s disease: an insight into the mechanisms of oxidative stress-mediated mitochondrial injury. Neuroscience. 2008;153:120–130. doi: 10.1016/j.neuroscience.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tauskela JS, Brunette E. Neuroprotection against staurosporine by metalloporphyrins independent of antioxidant capability. Neurosci Lett. 2009;466:41–46. doi: 10.1016/j.neulet.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 132.Khan I, Batinic-Haberle I, Benov LT. Effect of potent redox-modulating manganese porphyrin, MnTM-2-PyP, on the Na(+)/H(+) exchangers NHE-1 and NHE-3 in the diabetic rat. Redox Rep. 2009;14:236–242. doi: 10.1179/135100009X12525712409698. [DOI] [PubMed] [Google Scholar]
- 133.Sklavos MM, Bertera S, Tse HM, Bottino R, He J, Beilke JN, Coulombe MG, Gill RG, Crapo JD, Trucco M, Piganelli JD. Redox modulation protects islets from transplant-related injury. Diabetes. 2010;59:1731–1738. doi: 10.2337/db09-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Makinde AY, Luo-Owen X, Rizvi A, Crapo JD, Pearlstein RD, Slater JM, Gridley DS. Effect of a metalloporphyrin antioxidant (MnTE-2-PyP) on the response of a mouse prostate cancer model to radiation. Anticancer Res. 2009;29:107–118. [PubMed] [Google Scholar]
- 135.Makinde AY, Rizvi A, Crapo JD, Pearlstein RD, Slater JM, Gridley DS. A metalloporphyrin antioxidant alters cytokine responses after irradiation in a prostate tumor model. Radiat Res. 2010;173:441–452. doi: 10.1667/RR1765.1. [DOI] [PubMed] [Google Scholar]
- 136.Jackson IL, Gaunter-Fleckenstein BM, Batinic-Haberle I, Poulton S, Zhao Y, Dewhirst MW, Vujaskovic Z. Hyperthermia enhances the anti-angiogenic effect of metalloporphyrin mimetic of superoxide dismutase (abstract). 24th annual meeting of the European society for hyperthermic oncology; Prague, Czech Republic. 2007. [Google Scholar]
- 137.Wu T-J, Khoo NH, Zhou F, Day BJ, Parks DA. Decreased hepatic ischemia-reperfusion injury by manganese-porphyrin complexes. Free Radic Res. 2007;41:127–134. doi: 10.1080/10715760600801298. [DOI] [PubMed] [Google Scholar]
- 138.Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, Conrads TP, Veenstra TD, Song BJ, Pacher P. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Csont T, Viappiani S, Sawicka J, Slee S, Altarejos JY, Batinic-Haberle I, Schulz R. The involvement of superoxide and iNOS-derived NO in cardiac dysfunction induced by pro-inflammatory cytokines. J Mol Cell Cardiol. 2005;39:833–840. doi: 10.1016/j.yjmcc.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 140.Lubbers NL, Polakowski JS, Crapo JD, Wegner CD, Cox BF. Preischemic and postischemic administration of AEOL10113 reduces infarct size in a rat model of myocardial ischemia and reperfusion. J Cardiovasc Pharmacol. 2003;41:714–719. doi: 10.1097/00005344-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 141.Nilakantan V, Liang HL, Rajesh S, Mortensen J, Chandran K. Time-dependant protective effects of mangenese(III) tetrakis (1-methyl-4-pyridyl) porphyrin on mitochondrial function following renal ischemia-reperfusion injury. Free Radic Res. 2010;44:773–782. doi: 10.3109/10715761003786164. [DOI] [PubMed] [Google Scholar]
- 142.Chang L-Y, Subramaniam M, Yoder BA, Day BJ, Ellison MC, Sunday ME, Crapo JD. A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2003;167:57–64. doi: 10.1164/rccm.200203-232OC. [DOI] [PubMed] [Google Scholar]
- 143.Chang L-Y, Crapo JD. Inhibition of airway inflammation and hyperreactivity by an antioxidant mimetic. Free Radic Biol Med. 2002;33:379–386. doi: 10.1016/s0891-5849(02)00919-x. [DOI] [PubMed] [Google Scholar]
- 144.O’Neill HC, White CW, Veress LA, Hendry-Hofer TB, Loader JE, Min E, Huang J, Rancourt RC, Day BJ. Treatment with the catalytic metalloporphyrin AEOL 10150 reduces inflammation and oxidative stress due to inhalation of the sulfur mustard analog 2-chloroethyl ethyl sulfide. Free Radic Biol Med. 2010;48:1188–1196. doi: 10.1016/j.freeradbiomed.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Tarpey MM, Patel RP, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric-oxide dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Batinic-Haberle I, Ndengele MM, Cuzzocrea S, Rebouças JS, Masini E, Spasojevic I, Salvemini D. Lipophilicity is a critical parameter that dominates the efficacy of metalloporphyrins in blocking the development of morphine antinociceptive tolerance through peroxynitrite-mediated pathways. Free Radic Biol Med. 2009;46:212–219. doi: 10.1016/j.freeradbiomed.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Daroczi B, Kari G, Zengin AY, Chinnaiyan P, Batinic-Haberle I, Rodeck U, Dicker AP. Radioprotective effects of two superoxide dismutase (SOD) mimetics and the nanoparticle DF-1 in a vertebrate zebrafish model (abstract). 48th ASTRO, Annual Meeting of the American Society for Radiation Oncology; 2006. [Google Scholar]
- 148.Lee JH, Park JW. A manganese porphyrin complex is a novel radiation protector. Free Radic Biol Med. 2004;37:272–283. doi: 10.1016/j.freeradbiomed.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 149.Pazhanisamy SK, Li H, Wang Y, Batinic-Haberle I, Zhou D. NADPH oxidase inhibition attenuates total body irradiation-induced hematopoietic genomic instability. Mutagenesis. 2011 doi: 10.1093/mutage/ger001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li H, Wang Y, Pazhanisamy SK, Shao L, Batinic-Haberle I, Meng A, Zhou D. MnTE mitigates total body irradiation-induced long-term bone marrow suppression. Free Radic Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2011.04.016. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jian C, Batinic-Haberle I, Vujaskovic Z. Comparison of two Mn porphyrin-based mimics of superoxide-dismutase (SOD) in pulmonary radioprotection. Free Radic Biol Med. 2008;44:982–989. doi: 10.1016/j.freeradbiomed.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, Vujaskovic Z. Early and late administration of antioxidant mimic MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48:1034–1043. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rabbani Z, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, Dewhirst MW, Vujaskovic Z. Long term administration of a small molecular weight catalytic metalloporphyrin antioxidant AEOL10150 protects lungs from radiation-induced injury. Int J Radic Oncol Biol Phys. 2007;67:573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rabbani Z, Salahuddin FK, Yarmolenko P, Batinic-Haberle I, Trasher BA, Gauter-Fleckenstein B, Dewhirst MW, Anscher MS, Vujaskovic Z. Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radic Res. 2007;41:1273–1282. doi: 10.1080/10715760701689550. [DOI] [PubMed] [Google Scholar]
- 155.Cline M, Dugan G, Perry D, Batinic-Haberle I, Vujaskovic Z. MnTnHex-2-PyP5+ provides protection in nonhuman primate lungs after whole-thorax exposure to ionizing irradiation. 56th Radiation Research Society Meeting; Hawaii, USA. 2010. p. 54. Book of Abstracts, PS1.05. [Google Scholar]
- 156.Mao XW, Crapo JD, Mekonnen T, Lindsey N, Martinez P, Gridley DS, Slater JM. Radioprotective effect of a metalloporphyrin compound in rat eye model. Curr Eye Res. 2009;34:62–72. doi: 10.1080/02713680802546948. [DOI] [PubMed] [Google Scholar]
- 157.Pearlstein RD, Higuchi Y, Moldovan M, Johnson K, Fukuda S, Gridley DS, Crapo JD, Warner DS, Slater JM. Metalloporphyrin antioxidants ameliorate normal tissue radiation damage in rat brain. Int J Radiat Biol. 2010;86:145–163. doi: 10.3109/09553000903419965. [DOI] [PubMed] [Google Scholar]
- 158.Vorotnikova E, Rosenthal RA, Tries M, Doctrow SR, Braunhut SJ. Novel synthetic SOD/catalase mimetics can mitigate capillary endothelial cell apoptosis caused by ionizing radiation. Radiat Res. 2010;173:748–759. doi: 10.1667/RR1948.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nin N, Cassina A, Boggia J, Alfonso E, Botti H, Peluffo G, Trostchansky A, Batthyány C, Radi R, Rubbo H, Hurtado FJ. Septic diaphragmatic dysfunction is prevented by Mn(III)porphyrin therapy and inducible nitric oxide synthase inhibition. Intensive Care Med. 2004;30:2271–2278. doi: 10.1007/s00134-004-2427-x. [DOI] [PubMed] [Google Scholar]
- 160.Aboua YG, Du Plessis SS, Reichgelt P, Brooks N. The in vitro effects of superoxide, some commercially available antioxidants and red palm oil on sperm motility. Asian J Androl. 2009;11:695–702. doi: 10.1038/aja.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Benov L, Craik J, Batinic-Haberle I. The potential of Zn(II) N-alkylpyridylporphyrins for anticancer therapy. Anti Canc Agents Med Chem. 2011;11:233–241. doi: 10.2174/187152011795255975. [DOI] [PubMed] [Google Scholar]
- 162.Reboucas JS, Kos I, Vujaskovic Z, Batinic-Haberle I. Determination of residual manganese in Mn porphyrin-based superoxide dismutase (SOD) and peroxynitrite reductase mimics. J Pharm Biomed Anal. 2009;50:1088–1091. doi: 10.1016/j.jpba.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reboucas JS, Spasojevic I, Batinic-Haberle I. Quality of potent Mn porphyrin-based SOD mimics and peroxynitrite scavengers for pre-clinical mechanistic/therapeutic purposes. J Pharm Biomed Anal. 2008;48:1046–1049. doi: 10.1016/j.jpba.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Spasojevic I, Colvin OM, Warshany KR, Batinic-Haberle I. New approach to the activation of anti-cancer pro-drug by metalloporphyrin-based cytochrome P450 mimics in all-aqueous biologically relevant system. J Inorg Biochem. 2006;100:1897–1902. doi: 10.1016/j.jinorgbio.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 165.Chiarugi P. Survival or Death: The Redox Paradox. Antioxid Redox Signal. 2009;11:2651–2654. doi: 10.1089/ars.2009.2770. [DOI] [PubMed] [Google Scholar]