Abstract
Objective:
This study was conducted to examine the relationship between the glycated hemoglobin level (HbA1c) and halitosis status among diabetic patients affected with periodontitis and to examine if there is a relationship between halitosis and different periodontal parameters.
Methods and Materials:
Consecutive type 2 diabetic patients were recruited from patients presented for treatment at a University hospital. Age, gender and smoking were recorded. A structured questionnaire on patients’ perception of their oral health, halitosis and diabetes severity and control was completed. Peripheral blood samples were obtained and analyzed for HbA1c levels. In addition, periodontal clinical parameters including probing depth, clinical attachment level, bleeding on probing and plaque scores were recorded.
Results:
A total of 38 type 2 diabetic patients were selected. The mean age was 52.1 (±8.8) years. Sixteen subjects (42.1%) reported halitosis. Of these, 62.5% were females, and only one subject was a current smoker. The mean levels of HbA1c were significantly different between those with and without halitosis, mean 9.6 (±2) and 8.2 (±1.6), respectively (p=0.03). No significant differences were found in the mean periodontal parameters between those with and without halitosis.
Conclusion:
The results of this study suggest an association between halitosis and increased levels of HbA1c. Further studies are needed to explain the nature of this association.
Keywords: Halitosis, Bad Breath, Glycated Hemoglobin, HbA1c. Diabetes, Periodontitis.
INTRODUCTION
Halitosis is a generic term defined as an unpleasant or offensive odor emanating from the oral cavity whether it originates from intra or extra oral sources, leading to personal discomfort and social isolation [1, 2]. Halitosis can also be referred to as bad or foul breath, breath malodor, oral malodor, and foetor oris. Halitosis is considered the third reason, after caries and periodontal disease, for patients to seek dental care [3]. The prevalence of halitosis in the general population was reported to range from 23% to 50% [4-8]. In about 80-90% of subjects suffering from halitosis, the condition was attributed to oral factors, hence the term oral malodor [9, 10].
Several factors play a role in oral halitosis including periodontal diseases, tongue coating, peri-implant diseases, deep carious lesions, exposed necrotic tooth pulp, pericoronitis, mucosal ulcerations, healing wounds, impacted food or debris, unclean dentures, and factors causing decrease salivary flow rate [5, 11].
Halitosis arises from microbial degradation of organic substrates present in saliva, crevicular fluids, oral soft tissues, and retained debris [12, 13]. This degradation leads to production of Volatile Sulfur Compounds, particularly Hydrogen sulphide (H2S), methyl mercaptan (CH3SH), and dimethyl sulphide [(CH3)2S] [2, 11, 12]. Hydrogen sulphide (H2S) and methyl mercaptan (CH3SH) mainly contribute to intra oral halitosis, while dimethyl sulphide [(CH3)2S] is mainly found in extraoral halitosis [14]. It has been reported that the Volatile Sulfur Compounds level in mouth breath of patients with periodontal disease is 8 times greater than control patients [15].
Extra-oral halitosis could be caused by disturbances in the upper and lower respiratory tract, metabolic diseases, medications, carcinoma, and other systemic diseases such as diabetes which gives rise to ketone bodies (ketoacidosis) in the breath [16, 17].
Diabetic patients have increased susceptibility to chronic infection and inflammation of the oral tissues especially when they are poorly controlled. Oral manifestations in diabetes include periodontal diseases, oral candidiasis and dry mouth [18, 19]. Diabetic patients are three times more prone to have periodontitis than non diabetics and the progression of periodontal disease in uncontrolled diabetic patients is more severe than in controlled patients [20]. Furthermore, periodontal disease has been shown to worsen the glycemic control [21, 22].
Therefore, the aims of this study were to examine the relationship between the glycated hemoglobin level (HbA1c) in the blood and halitosis status among diabetic patients affected with periodontitis and to examine if there is a relationship between severity of periodontal destruction and halitosis among the selected sample.
METHODS AND MATERIALS
This study was a cross sectional study conducted in complete accordance with the Helsinki declared ethical principles. Consecutive type 2 diabetic patients were recruited from patients presented for treatment at King Abdulaziz University hospital. Informed consent was obtained from each participant prior to enrollment in the study. The inclusion criteria were age ≥ 35 years, confirmed diagnosis of type 2 diabetes, generalized moderate to severe chronic periodontitis and at least 20 remaining teeth. Pregnant women and patients who received periodontal treatment or antibiotic therapy three months prior to the study were excluded.
A complete medical and dental history was gathered from all participants. Participants thencompleted a structured questionnaire on self perceived halitosis, oral health, and diabetes severity and control. Full periodontal examination was performed by two calibrated examiners. The inter-examiner reliability for detecting probing depth within 1mm was 86% and the intra-examiners reliability for both examiners was more than 92%. The following periodontal parameters were measured at six sites per each tooth: probing depth (PD), gingival recession (GR), clinical attachment loss (CAL) and plaque (PI) and bleeding (BI) scores. Peripheral blood samples were also obtained and the levels of glycated hemoglobin (HbA1c) were measured. Samples were coded to insure blindness during the laboratory analysis. HbA1c assay (The dimension® and Flex® HA1C kit, Dade Behring Limited, Kent, UK) was used. All data were collected before 12 noon prior to any oral hygiene instruction or periodontal treatment.
Chi square (χ2) test was used to examine if there was a relationship between halitosis and smoking and gender. Student’s t-tests were used to evaluate mean differences in age, periodontal parameter and HbA1c levels between subject with and without halitosis. The association between halitosis and HbA1c levels, after controlling for periodontal condition was examined using multivariable logistic regression analysis.
Statistical analyses were conducted using the Statistical Package for Social Science (SPSS, version 16, Chicago, IL).
RESULTS
A total of 38 diabetic patients were enrolled in the study. The mean age of the study sample was 52.1(±8.8). Halitosis was reported by 16 subjects (42.1%). Of those, 62.5% were females, and only one subject was a current smoker. Chi square test showed that there is no significant relationship between halitosis, and smoking and gender, p>0.05 (Table 1). Mean age was also similar among those reporting and not reporting halitosis (51±8.3 and 53±9.1 years, respectively).
Table 1.
Relationship between Gender, Smoking and Halitosis
| Total (n=38) | Halitosis (n [%]) | |||||
|---|---|---|---|---|---|---|
| n | % | Yes | No | p-Value | ||
| Gender | Male | 19 | 50 | 6 (31.6) | 13 (68.4) | 0.19* |
| Female | 19 | 50 | 10 (52.6) | 9 (47.4) | ||
| Smoking | Ever | 10 | 26.3 | 3 (30) | 7 (70) | 0.64* |
| Never | 28 | 73.7 | 13 (46.4) | 15 (53.6) | ||
Not significant, based on Chi square (χ2) test
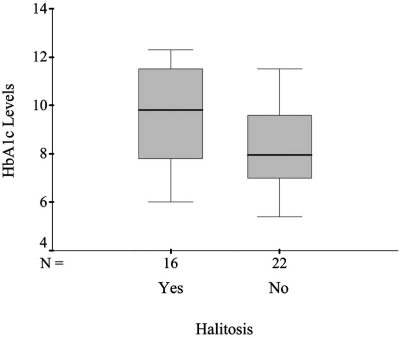
The mean HbA1c level in the blood was 8.8 % (±1.9) in the total sample. The mean levels of HbA1c for subjects with and without halitosis, were 9.6 (±2) and 8.2 (±1.6), respectively. The minimum, first quartile, median and third quartile for HbA1c among those with and without halitosis are shown in Fig. (1). A significant difference was found in the mean levels of HbA1c between subjects with and without halitosis, t df=36 = 2.3, p = 0.03 (Table 2).
Fig. (1).
Boxplot graph showing the glycated hemoglobin level by halitosis status.
Table 2.
Summary of the Means and Standard Deviations of Glycated Hemoglobin and Periodontal Parameters in Patients with and without Halitosis
| Total Sample | Halitosis | ||||||
|---|---|---|---|---|---|---|---|
| Yes | No | p-Value | |||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | ||
| HbA1c | 8.79 | (1.89) | 9.57 | (1.99) | 8.22 | (1.62) | 0.03* |
| Mean PD (mm) | 3.10 | (0.48) | 3.22 | (0.45) | 3.01 | (0.48) | 0.18 |
| Mean clinical attachment (mm) | 4.20 | (1.08) | 4.25 | (0.98) | 4.14 | (1.18) | 0.77 |
| Percentage of sites with: | |||||||
| - PD > 4mm | 25% | (19) | 29% | (20) | 22% | (18) | 0.22 |
| - Bleeding on probing | 79% | (20) | 83% | (15) | 73% | (23) | 0.21 |
| - Plaque deposits | 87% | (17) | 89% | (14) | 86% | (20) | 0.70 |
SD = standard deviation,
= significant p<0.05
The relation between HbA1c and halitosis remained significant even after controlling for the mean probing depth in the multivariable logistic regression as shown in Table 3. There was also a trend toward worse periodontal health among those with than those without halitosis. However, the difference was not statistically significant between the two groups in any of the measured periodontal parameters (Table 2). Also, no difference was found between those with and without halitosis in regard to self reported diabetes control and severity, gum condition, oral and periodontal health, gingival bleeding, and frequency of recurrent oral infection, pain, and abscess formation, (p > 0.05).
Table 3.
Odds Ratio (OR) and 95% Confidence Interval (CI) for Glycated Hemoglobin and Probing Depth in Relation to Halitosis
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Mean glycated hemoglobin* | 1.51 | 1.01-2.27 | 0.045 |
| Mean probing depth* | 2.45 | 0.53-11.43 | 0.25 |
Variables were modeled as continuous variables.
DISCUSSION
Diabetes mellitus is a chronic metabolic disease characterized by elevated level of glucose in the blood. Glucose elevation is caused by an increase in cellular resistance to the insulin action or insulin secretion deficiency. The elevation of glucose level may lead to many pathological mechanisms, such as activation of the sorbitol pathway, formation of advanced glycation end products and lipid metabolism alteration. Common clinical complications caused by diabetes include nephropathy, macrovascular disease, neuropathy, retinopathy and poor wound healing. Periodontitis is considered the sixth complication of diabetes mellitus [23, 24].
In this study, the relationship of self reported halitosis, levels of glycated hemoglobin and different periodontal parameters were studied. Almost half of the subjects reported suffering from halitosis. The results showed a significant difference in the mean levels of glycated hemoglobin in subjects reporting and not reporting halitosis. Furthermore, the association between the level of glycated hemoglobin and self reported halitosis was significant even after controlling for probing depth. A one percent increase in the HbA1c was associated with a 50% increase in the likelihood of having halitosis. Although there was a trend toward having a worse periodontal condition among those reporting halitosis, this was not statistically significant. This is in contrast to previous findings where periodontal disease had been found to be related to halitosis when subjects with and without periodontal disease were compared [5, 11, 15]. A possible reason for this finding is that all patients in the present study were affected with moderate to severe periodontitis which could have prejudiced the ability to discriminate between the groups.
Previous studies focused mainly on the relationship between halitosis and oral conditions [25]. This is the first study that evaluated the relationship between self-reported halitosis and HbA1c level among type 2 diabetic subjects. The findings of this study suggest that the risk of halitosis increases with elevated levels of HbA1c. Thus, poorly controlled diabetics may be more susceptible to having halitosis. There are plausible reasons that can explain the association between halitosis and elevated levels of HbA1c. A possible reason could be related to the ketoacidosis phenomenon associated with poorly controlled diabetes. In fact, several hundred volatile compounds are detected in exhaled breath and many of which represent byproducts of endogenous biological process [26]. Recent studies suggest that integrated analysis of exhaled air in diabetic patients has the potential to serve as a marker of blood glucose level [27, 28]. This could be a potential subject for further studies. Another reason could be attributed to a dry mouth which is a common manifestation among diabetic subjects and a contributing factor to halitosis [18, 20, 29]. An additional reason would be the increased probability of infections among diabetic patients which lead to oral ulcerations [21, 22]. Also, it has been reported that anaerobic microbiota covering the tongue is involved in the occurrence of halitosis by releasing of sulfur-containing compounds (VSC) [30].
In the present study halitosis was measured using self reports of halitosis. This has been argued in the literature where one study showed a weak correlation between objective and self-reported halitosis [31]. In contrast, Iwanicka-Grzegorek et al., [32] reported an agreement between self-perceived and objective evaluation of halitosis. Objective methods for measuring halitosis are organoleptic measurement, gas chromatography, and sulfide monitoring. Even though organoleptic is the most common simple method used by examiners, it lacks reliability and reproducibility [33]. When precise measurements are needed, gas chromatography is the method of choice. The main draw back of sulfide monitoring method is that odorants other than volatile sulfur compounds are not detected which may result in false negative results [33]. Future studies to assess possible correlation between objective and self-reported halitosis and glycated hemoglobin levels are warranted.
CONCLUSION
The findings of this study suggest a positive relationship between HbA1c levels and halitosis among diabetic patients. However, no association was found between halitosis and periodontal parameters among the study sample. Further studies to investigate the mechanism underlying the association between HbA1c and halitosis are suggested.
CONFLICT OF INTEREST
None declare.
REFERENCES
- 1.Tonzetich J. Production and origin of oral malodor: a review of mechanisms and methods of analysis. J Periodontol. 1977;48:13–20. doi: 10.1902/jop.1977.48.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Tonzetich J, Ng SK. Reduction of malodor by oral cleansing procedures. Oral Surg Oral Med Oral Pathol. 1976;42:172–81. doi: 10.1016/0030-4220(76)90121-3. [DOI] [PubMed] [Google Scholar]
- 3.Rayman S, Almas K. Halitosis among racially diverse populations: an update. Int J Dent Hyg. 2008;6:2–7. doi: 10.1111/j.1601-5037.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ansari JM, Boodai H, Al-Sumait N, Al-Khabbaz AK, Al-Shammari KF, Salako N. Factors associated with self-reported halitosis in Kuwaiti patients. J Dent. 2006;34:444–9. doi: 10.1016/j.jdent.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Liu XN, Shinada K, Chen XC, Zhang BX, Yaegaki K, Kawaguchi Y. Oral malodor-related parameters in the Chinese general population. J Clin Periodontol. 2006;33:31–6. doi: 10.1111/j.1600-051X.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 6.Outhouse TL, Al-Alawi R, Fedorowicz Z, Keenan JV. Tongue scraping for treating halitosis. Cochrane Database Syst Rev. 2006;(Issue 2):CD005519. doi: 10.1002/14651858.CD005519.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Sanz M, Roldan S, Herrera D. Fundamentals of breath malodour. J Contemp Dent Pract. 2001;2:1–17. [PubMed] [Google Scholar]
- 8.Tessier JF, Kulkarni GV. Bad breath: etiology, diagnosis and treatment. Oral Health. 1991;81:19–22, 24. [PubMed] [Google Scholar]
- 9.Delanghe G, Ghyselen J, Feenstra L, van Steenberghe D. Experiences of a Belgian multidisciplinary breath odour clinic. Acta Otorhinolaryngol Belg. 1997;51:43–8. [PubMed] [Google Scholar]
- 10.Miyazaki H, Sakao S, Katoh Y, Takehara T. Correlation between volatile sulphur compounds and certain oral health measurements in the general population. J Periodontol. 1995;66:679–84. doi: 10.1902/jop.1995.66.8.679. [DOI] [PubMed] [Google Scholar]
- 11.Yaegaki K, Sanada K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J Periodontal Res. 1992;27:233–8. doi: 10.1111/j.1600-0765.1992.tb01673.x. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt NF, Missan SR, Tarbet WJ. The correlation between organoleptic mouth-odor ratings and levels of volatile sulfur compounds. Oral Surg Oral Med Oral Pathol. 1978;45:560–7. doi: 10.1016/0030-4220(78)90037-3. [DOI] [PubMed] [Google Scholar]
- 13.Waler SM. On the transformation of sulfur-containing amino acids and peptides to volatile sulfur compounds (VSC) in the human mouth. Eur J Oral Sci. 1997;105:534–7. doi: 10.1111/j.1600-0722.1997.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 14.Tangerman A, Winkel EG. Intra- and extra-oral halitosis: finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J Clin Periodontol. 2007;34:748–55. doi: 10.1111/j.1600-051X.2007.01116.x. [DOI] [PubMed] [Google Scholar]
- 15.Yaegaki K, Sanada K. Biochemical and clinical factors influencing oral malodor in periodontal patients. J Periodontol. 1992;63:783–9. doi: 10.1902/jop.1992.63.9.783. [DOI] [PubMed] [Google Scholar]
- 16.Attia EL, Marshall KG. Halitosis. Can Med Assoc J. 1982;126:1281–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Tangerman A. Halitosis in medicine: a review. Int Dent J. 2002;52(Suppl 3):201–6. doi: 10.1002/j.1875-595x.2002.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 18.Taylor GW, Manz MC, Borgnakke WS. Diabetes, periodontal diseases, dental caries, and tooth loss: a review of the literature. Compend Contin Educ Dent. 2004;25:179–84. 186-8, 190: quiz 92. [PubMed] [Google Scholar]
- 19.Soell M, Hassan M, Miliauskaite A, Haikel Y, Selimovic D. The oral cavity of elderly patients in diabetes. Diabetes Metab. 2007;33(Suppl 1):S10–8. doi: 10.1016/s1262-3636(07)80053-x. [DOI] [PubMed] [Google Scholar]
- 20.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 21.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 22.Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001;6:99–112. doi: 10.1902/annals.2001.6.1.99. [DOI] [PubMed] [Google Scholar]
- 23.Lamster IB, Lalla E, Borgnakke WS, Taylor GW. The relationship between oral health and diabetes mellitus. J Am Dent Assoc. 2008;139(Suppl):19S–24S. doi: 10.14219/jada.archive.2008.0363. [DOI] [PubMed] [Google Scholar]
- 24.Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 25.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 26.Greiter MB, Keck L, Siegmund T, Hoeschen C, Oeh U, Paretzke HG. Differences in exhaled gas profiles between patients with type 2 diabetes and healthy controls. Diabetes Technol Ther. 2010;12:455–63. doi: 10.1089/dia.2009.0181. [DOI] [PubMed] [Google Scholar]
- 27.Galassetti PR, Novak B, Nemet D, et al. Breath ethanol and acetone as indicators of serum glucose levels: an initial report. Diabetes Technol Ther. 2005;7:115–23. doi: 10.1089/dia.2005.7.115. [DOI] [PubMed] [Google Scholar]
- 28.Novak BJ, Blake DR, Meinardi S, et al. Exhaled methyl nitrate as a noninvasive marker of hyperglycemia in type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104:15613–8. doi: 10.1073/pnas.0706533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visvanathan V, Nix P. Managing the patient presenting with xerostomia: a review. Int J Clin Pract. 2010;64:404–7. doi: 10.1111/j.1742-1241.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 30.Casemiro LA, Martins CH, de Carvalho TC, Panzeri H, Lavrador MA, Pires-de-Souza Fde C. Effectiveness of a new toothbrush design versus a conventional tongue scraper in improving breath odor and reducing tongue microbiota. J Appl Oral Sci. 2008;16:271–4. doi: 10.1590/S1678-77572008000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg M, Kozlovsky A, Gelernter I, et al. Self-estimation of oral malodor. J Dent Res. 1995;74:1577–82. doi: 10.1177/00220345950740091201. [DOI] [PubMed] [Google Scholar]
- 32.Iwanicka-Grzegorek E, Michalik J, Kepa J, Wierzbicka M, Aleksinski M, Pierzynowska E. Subjective patients' opinion and evaluation of halitosis using halimeter and organoleptic scores. Oral Dis. 2005;11(Suppl 1):86–8. doi: 10.1111/j.1601-0825.2005.01101.x. [DOI] [PubMed] [Google Scholar]
- 33.van den Broek AM, Feenstra L, de Baat C. A review of the current literature on aetiology and measurement methods of halitosis. J Dent. 2007;35:627–35. doi: 10.1016/j.jdent.2007.04.009. [DOI] [PubMed] [Google Scholar]