Abstract
Background/Aim:
In patients with liver cirrhosis, the platelet count/spleen diameter ratio has been validated as a parameter for the noninvasive diagnosis of esophageal varices. Schistosoma infection is a frequent cause of portal hypertension in Middle Eastern countries, and is associated with the development of esophageal varices. In this study we aimed to evaluate the platelet count/spleen diameter ratio as a noninvasive tool for the prediction of the presence of esophageal varices in patients with schistosoma-related chronic liver disease.
Patients and Methods:
Forty-three patients with hepatosplenic schistosomiasis underwent upper digestive endoscopy to check for the presence of esophageal varices. Furthermore, all patients underwent abdominal ultrasonography, and maximum spleen diameter (in mm) was measured. The platelet count/spleen diameter ratio was calculated in all patients.
Results:
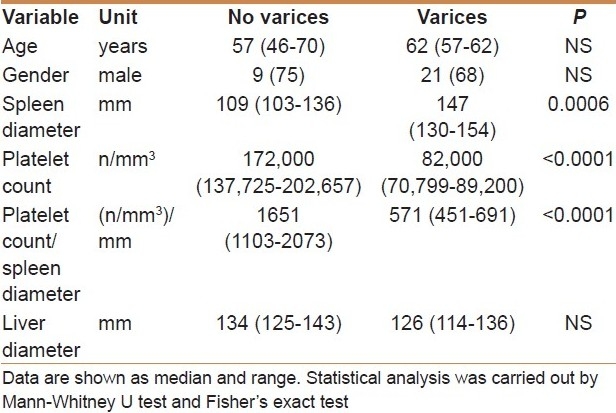
Esophageal varices were found in 31 patients (72%). Age and gender were not significantly different between patients with and without varices. In patients with varices, median platelet count (82,000/μL versus 172,000/μL, P < 0.0001) and platelet count/spleen diameter ratio (571 versus 1651, P < 0.0001) were significantly lower, while spleen diameter (147 mm versus 109 mm, P = 0.0006) was significantly larger. In multivariate analysis, the platelet count/spleen diameter ratio was the only parameter independently associated with the presence of varices (P < 0.0001).
Conclusions:
In this study we have validated the use of the platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices in patients with portal hypertension caused by schistosoma infection. In these patients, the platelet count/spleen diameter ratio might be used to allow better rationalization of medical resources and use of endoscopy.
Keywords: Esophageal varices, noninvasive, platelet count/spleen diameter ratio, portal hypertension, schistosomiasis
Schistosomiasis is a chronic parasitic disease caused by trematodes. Worldwide, about 200 million people are infected.[1] Liver involvement is frequent, especially with the S. mansoni and S. japonicum species. Humans become infected after contact with water containing the infective stage, cercaria. After skin penetration, cercariae become schistosomula and migrate to the lungs and to the liver. Adult flukes therefore migrate to the terminal branches of the mesenteric veins where each female produces 100-300 eggs per day. Part of these are swept by the portal blood to the liver, where they are deposited in the periportal spaces and along the liver capsule, causing periportal fibrosis that leads to presinusoidal blockage, portal hypertension, splenomegaly and gastroesophageal varices.[2,3]
Bleeding from esophago-gastric varices is the most important complication of portal hypertension.[4] An estimated 90% of the portal hypertension patients will develop esophageal varices at some time in their life and 30% of those will bleed.[5] Moreover 5-50% of the patients with acute variceal bleeding will die within a few days of the initial episode.[6] The parameters associated with variceal bleeding in patients with hepatosplenic schistosomiasis are endoscopic (variceal size, red signs, congestive gastropathy and fundic varices) and ultrasonographic (portal vein diameter and periportal thickness).[7] However, there are no consistent noninvasive parameters for the diagnosis of esophageal varices in this specific patient population.
Upper digestive endoscopy is the only means to diagnose and grade esophageal varices.[8] However, endoscopy is an invasive procedure and its cost-effectiveness for screening is also questionable.[9,10] These limitations and the ever-increasing workload on endoscopy units has led many researchers to identify some parameters that can noninvasively predict the presence of esophageal varices.[11–14] The platelet count/spleen diameter ratio is a noninvasive parameter that proved to be a simple, reproducible, and cost-effective tool for predicting the presence of esophageal varices in patients with varying severity of cirrhosis and etiology of liver disease.[15–21]
The purpose of this study was to evaluate the platelet count/spleen diameter ratio as a noninvasive diagnostic tool to predict the presence of esophageal varices in patients with Schistosoma infection. This clinical setting was selected due to the fact that this disease is fairly frequent in Middle Eastern countries, where both accessibility to endoscopy and resources may be limited and the use of noninvasive parameters for the diagnosis of esophageal varices would help rationalize medical resources.
PATIENTS AND METHODS
Patients
This prospective study included 43 consecutive patients with evidence of schistosomal infection (based on seropositivity for Schistosoma mansonii) and periportal hepatic fibrosis confirmed at an abdominal ultrasound, referred to the Department of Medicine, King Fahad Hospital, Armed Forces Hospitals Southern Region, Khamis Mushayt, Kingdom of Saudi Arabia. Unstable patients, particularly those with acute variceal bleeding at admission, as well as patients with hepatorenal syndrome, non-controlled hepatic encephalopathy, hepatopulmonary syndrome or shock were excluded from this study. Patients with previous variceal bleeding, sclerosis or band ligation of esophageal varices, transjugular intrahepatic portosystemic stent shunt (TIPS) or surgery for portal hypertension, were also excluded. Other exclusion criteria included any active and past alcohol use, evidence of other etiology for liver disease (hepatitis B or C virus infection, autoimmunity, metabolic causes, and hereditary diseases such as hemochromatosis), and previously diagnosed esophageal varices on bleeding prophylaxis treatment. Informed consent was obtained for each patient and the study was approved by the Local Ethics Committee.
Methods
Each patient underwent a complete clinical and biochemical evaluation, and underwent upper digestive endoscopy. Endoscopic examinations were performed in the same endoscopy unit to check for the presence of varices and their findings, taken as gold standard, were classified into presence or absence of esophageal varices. Endoscopy was carried out by two experienced physicians (D.A and M.M.A.A.). Varices were further subdivided into small and large varices, where small varices were defined as varices that flatten with insufflations or minimally protrude into the esophageal lumen, while large varices were defined as varices that protrude into the esophageal lumen and touch each other (presence of confluence), or that fill at least 50% of the esophageal lumen.[22] This datum was reported for descriptive reasons alone. Furthermore, all patients underwent abdominal ultrasound to check for the presence of ascites, for measurement of the bipolar spleen diameter (in mm), and of the right lobe diameter in the midclavicular line.[15,23] We calculated the platelet count (n/mm 3)/spleen diameter (mm) ratio of each patient.
Statistical analysis
Quantitative values are shown as median and range and qualitative values are reported as absolute value and percentage. The Mann-Whitney U test was used for comparison of quantitative variables, while qualitative variables were compared using the Fisher's exact test. Univariate analysis was performed on age, gender, platelet count, spleen diameter, platelet count/spleen diameter ratio, and liver diameter. A multivariate analysis with logistic regression model was performed on parameters which were significantly different in the univariate analysis in patients with and without esophageal varices (platelet count, spleen diameter, platelet count/spleen diameter) in order to determine the variables independently associated with the presence of esophageal varices. Receiver operating characteristic (ROC) curves were used to identify the best sensitivity and specificity cutoff values of the significant variables for the presence of esophageal varices.[24] The validity of the model was measured by means of the concordance (c) statistic (equivalent to the area under the ROC curve). A model with a c value above 0.7 is considered useful while a c value between 0.8 and 0.9 indicates excellent diagnostic accuracy.[25] For all analyses a P value <0.05 was considered statistically significant. Data were analyzed using MedCalc software Version 9.2.1.0 (MedCalc Software bvba, Mariakerke, Belgium).
RESULTS
A total of 43 patients were enrolled in this study, with a median age of 61 years (range 27-82). Gender distribution showed a male preponderance in the study population (30 patients, 70%). Thirty-one patients had endoscopic evidence of esophageal varices (72%), and among these patients 19 had large esophageal varices (44%). Ascites was present in 17 patients (40%).
Clinical, laboratory, and ultrasonographic characteristics of the patients subdivided according to the presence of esophageal varices are shown in Table 1. Age and gender were not significantly different between patients with and without esophageal varices. Median platelet count (82,000/μL versus 172,000/μL, P < 0.0001), and platelet count/spleen diameter ratio (571 versus 1651, P < 0.0001) were significantly lower in patients with varices. Lastly, larger mean spleen diameter was observed in patients with varices as compared to patients without varices (147 mm versus 109 mm, P = 0.0006).
Table 1.
Main characteristics of the 43 study patients subdivided according to the presence of esophageal varices
Multivariate logistic regression analysis was then performed on parameters that were significantly different in the univariate analysis (platelet count, spleen diameter, platelet count/spleen diameter ratio) to identify variables independently associated with the presence of varices. The only parameter independently associated with the presence of varices was the platelet count/spleen diameter ratio (P < 0.0001).
ROC curves were used in order to assess the platelet count/spleen diameter ratio cutoff value with the best sensitivity and specificity for a diagnosis of varices. We found that a platelet count/spleen diameter ratio ≤885 had 100% sensitivity (89-100, 95% confidence interval (CI)), 92% specificity (62-99, 95% CI), 12.0 positive likelihood ratio, and 0.01 negative likelihood ratio for the diagnosis of esophageal varices. Accuracy of the platelet count/spleen diameter ratio cutoff value as evaluated by the c index was 0.989 (95% CI 0.897-0.992) [Figure 1].
Figure 1.
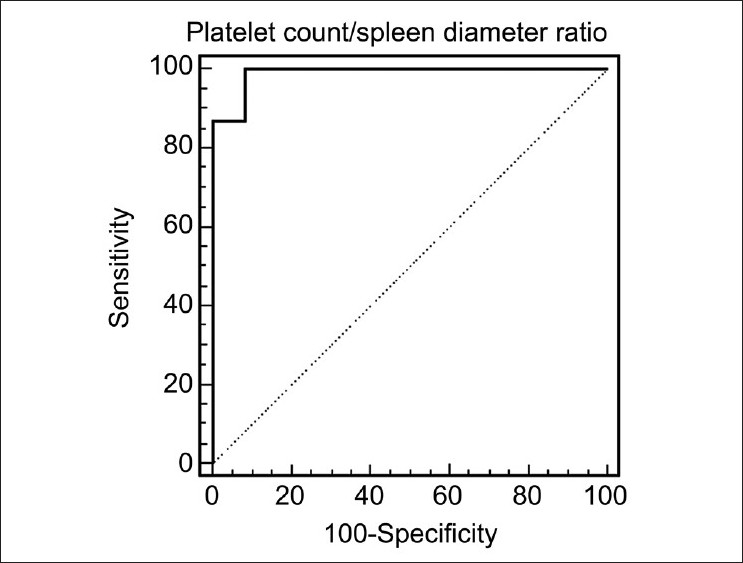
Receiver operating characteristics’ curves showing the platelet count/spleen diameter ratio cutoff with the best diagnostic accuracy on the basis of the presence of esophageal varices
DISCUSSION
The development of gastro-esophageal varices is a common complication of portal hypertension and bleeding from varices is a frequent cause of mortality and morbidity.[4] In patients affected by hepatosplenic schistosomiasis there is predominance of the clinical manifestations of portal hypertension, (e.g., esophageal varices) while other complications of chronic liver disease are less common.[26] Upper digestive endoscopy is nowadays the gold standard to diagnose and grade esophageal varices.[8] However, endoscopy is an invasive procedure, not always available, and its cost-effectiveness for screening is also questionable. Moreover, schistosomiasis is endemic in tropical and subtropical countries where resources are limited and there is a particular need for noninvasive predictors of esophageal varices. In these countries, Schistosoma infection can be responsible for approximately 30% of patients diagnosed with esophageal varices.[27]
Many previous studies have shown good predictive value of different non-endoscopic variables for the presence or absence of varices, but to our knowledge no studies are available in this particular setting of patients.[4,11] In particular, ultrasound grading of periportal fibrosis, which is commonly used in patients with Schistosoma infection, has shown contrasting results when correlated with the grade of esophageal varices.[28,29] Furthermore, the use of other biochemical and clinical parameters that can be altered by chronic liver disease is unlikely to be useful in these patients as Schistosoma infection leads to mild hepatocellular dysfunction.[11,30] In order to assess the presence/absence of esophageal varices, in this prospective study we used simple, reproducible tools usually performed in the normal management of patients with chronic liver disease. This is important in developing countries where financial resources are limited, so as to decide which patients should undergo endoscopy for screening of gastro-esophageal varices.
The prevalence of esophageal varices observed in our study is in keeping with previous prospective studies carried out in similar patient populations.[31] In univariate analysis, we found that median platelet count (82,000/μL versus 172,000/μL, P < 0.0001), and platelet count/spleen diameter ratio (571 versus 1651, P < 0.0001) were significantly lower, while spleen diameter (147 mm versus 109 mm, P = 0.0006) was significantly larger in patients with varices. However, in multivariate analysis only the platelet count/spleen diameter ratio was significantly associated with the presence of esophageal varices. The cutoff value that can best predict the presence of esophageal varices, evaluated using the area under the ROC curve, was 885 and presented a high accuracy (c-index: 0.989). It is noteworthy that this cutoff has a very high sensitivity in ruling out the presence of esophageal varices and can be used to safely avoid unnecessary endoscopy in patients who should be free from varices. As platelet count is a commonly available parameter and measurement of the spleen bipolar diameter has the highest reproducibility in abdominal ultrasound studies,[32,33] we feel that its use might be of help for the clinical management of patients with Schistosoma infection and suspected esophageal varices. In fact, portable ultrasonography devices can be carried even to remote areas, and therefore the platelet count/spleen diameter ratio might be used as an inexpensive and easily obtainable screening tool for the identification of high-risk patients during community surveys. These considerations are particularly relevant in this patient population as timely diagnosis of esophageal varices may allow appropriate pharmacological prophylaxis of first variceal bleeding,[34] that can be especially catastrophic due to the presence of complex haemostatic abnormalities.[35]
In conclusion, in this study we have demonstrated that the platelet count/spleen diameter ratio is a noninvasive parameter with a very high accuracy for the diagnosis of esophageal varices in a population of patients with Schistosoma-related portal hypertension. In particular, the platelet count/spleen diameter ratio could be used to allow better rationalization of medical resources and use of endoscopy, especially in financially deprived developing countries. However, we studied only a small sample of the population and further validation of this parameter in independent cohorts of patients is needed before using this tool routinely as a screening method in clinical practice.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Da Silva LC, Chieffi PP, Carrilho FJ. Schistosomiasis mansoni- Clinical features. Gastroenterol Hepatol. 2005;28:30–9. doi: 10.1157/13070382. [DOI] [PubMed] [Google Scholar]
- 3.Manzella A, Ohtomo K, Monzawa S, Lim JH. Schistosomiasis of the liver. Abdom Imaging. 2008;33:144–50. doi: 10.1007/s00261-007-9329-7. [DOI] [PubMed] [Google Scholar]
- 4.De Franchis R, Dell’Era A, Primignani M. Diagnosis and monitoring of portal hypertension. Dig Liver Dis. 2008;40:312–7. doi: 10.1016/j.dld.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 1997;92:1081–91. [PubMed] [Google Scholar]
- 6.D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: A meta-analytic review. Hepatology. 1995;22:332–54. doi: 10.1002/hep.1840220145. [DOI] [PubMed] [Google Scholar]
- 7.Martin RD, Szejnfeld J, Lima FG, Ferrari AP. Endoscopic, ultrasonographic, and US-Doppler parameters as indicators of variceal bleeding in patients with schistosomiasis. Dig Dis Sci. 2000;45:1013–8. doi: 10.1023/a:1005501930808. [DOI] [PubMed] [Google Scholar]
- 8.De Franchis R. Evolving consensus in portal hypertension report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–76. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel BM, Targownik L, Dulai GS, Karsan HA, Gralnek IM. Endoscopic screening for esophageal varices in cirrhosis. Is it ever cost effective? Hepatology. 2003;37:366–77. doi: 10.1053/jhep.2003.50050. [DOI] [PubMed] [Google Scholar]
- 10.Arguedas MR, Heudebert GR, Eloubeidi MA, Abrams GA, Fallon MB. Cost-effectiveness of screening, surveillance and primary prophylaxis strategies for esophageal varices. Am J Gastroenterol. 2002;97:2441–52. doi: 10.1111/j.1572-0241.2002.06000.x. [DOI] [PubMed] [Google Scholar]
- 11.Amico GD, Morabito A. Noninvasive markers of esophageal varices: Another round, not the last. Hepatology. 2004;39:30–4. doi: 10.1002/hep.20018. [DOI] [PubMed] [Google Scholar]
- 12.Pilette C, Oberti F, Aubè C, Rousselet MC, Bedossa P, Gallois Y, et al. Non-invasive diagnosis of esophageal varices in chronic liver disease. J Hepatol. 1999;31:867–73. doi: 10.1016/s0168-8278(99)80288-8. [DOI] [PubMed] [Google Scholar]
- 13.Madhotra R, Mulcahy HE, Willner I, Reuben A. Prediction of esophageal varices in patients with cirrhosis. J Clin Gastroenterol. 2002;34:81–5. doi: 10.1097/00004836-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Thomopoulos KC, Labropoulou KC, Mimidis KP, Katsakoulis EC, Iconomou G, Nikolopoulou VN. Non-invasive predictors of the presence of large esophageal varices in patients with cirrhosis. Dig Liver Dis. 2003;35:473–8. doi: 10.1016/s1590-8658(03)00219-6. [DOI] [PubMed] [Google Scholar]
- 15.Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, et al. Platelet count/spleen diameter ratio: Proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200–5. doi: 10.1136/gut.52.8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannini E, Botta F, Borro P, Dulbecco P, Testa E, Mansi C, et al. Application of the platelet count/spleen diameter ratio to rule out the presence of oesophageal varices in patients with cirrhosis: A validation study based on follow-up. Dig Liver Dis. 2005;37:779–85. doi: 10.1016/j.dld.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Zimbwa TA, Blanshard C, Subramaniam A. Platelet count/spleen diameter ratio as a predictor of oesophageal varices in alcoholic cirrhosis. Gut. 2004;53:1055. [PMC free article] [PubMed] [Google Scholar]
- 18.Baig WW, Nagaraja MV, Varma M, Prabhu R. Platelet count to spleen diameter ratio for the diagnosis of esophageal varices: Is it feasible? Can J Gastroenterol. 2008;22:825–8. doi: 10.1155/2008/287274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agha A, Anwar A, Bashir K, Savarino E, Giannini EG. External Validation of the platelet count/spleen diameter ratio for the diagnosis of esophageal varices in hepatitis C virus-related cirrhosis. Dig Dis Sci. 2009;54:654–60. doi: 10.1007/s10620-008-0367-y. [DOI] [PubMed] [Google Scholar]
- 20.Cammà C, Petta S, Di Marco V, Bronte F, Ciminnisi S, Licata G, et al. Insulin resistance is a risk factor for esophageal varices in hepatitis C virus cirrhosis. Hepatology. 2009;49:195–203. doi: 10.1002/hep.22655. [DOI] [PubMed] [Google Scholar]
- 21.Sarangapani A, Shanmugam C, Kayanasundaram M, Rangachari B, Thangavelu P, Subbarayan JK. Noninvasive prediction of large esophageal varices in chronic liver disease patients. Saudi J Gastroenterol. 2010;16:38–42. doi: 10.4103/1319-3767.58767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Franchis R, Pascal JP, Ancona E, Burroughs AK, Henderson M, Fleig W, et al. Definitions, methodology and therapeutic strategies in portal hypertension. A Consensus Development Workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. J Hepatol. 1992;15:256–61. doi: 10.1016/0168-8278(92)90044-p. [DOI] [PubMed] [Google Scholar]
- 23.Alempijevic T, Bulat V, Djuranovic S, Kovacevic N, Jesic R, Tomic D, et al. Right liver lobe/albumin ratio: Contribution to non-invasive assessment of portal hypertension. World J Gastroenterol. 2007;13:5331–5. doi: 10.3748/wjg.v13.i40.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrell FE, Jr, Lee KL, Mark BD. Tutorial in biostatistics.Multivariable prognostic models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Hanley JA, McNeil BJ. The meaning and use of the area under receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 26.Rebouças G. Clinical aspects of hepatosplenic schistosomiasis: A contrast with cirrhosis. Yale J Biol Med. 1975;48:369–76. [PMC free article] [PubMed] [Google Scholar]
- 27.De Cock KM, Awadh S, Raja RS, Wankya BM, Lucas SB. Esophageal varices in Nairobi, Kenya: A study of 68 cases. Am J Trop Med Hyg. 1982;31:579–88. doi: 10.4269/ajtmh.1982.31.579. [DOI] [PubMed] [Google Scholar]
- 28.Davidson RN, Houston S, Kiire CF. Schistosomal periportal fibrosis in Zimbabwe: Use of ultrasound in patients with oesophageal varices. Trans R Soc Trop Med Hyg. 1991;85:380–2. doi: 10.1016/0035-9203(91)90298-d. [DOI] [PubMed] [Google Scholar]
- 29.Domingues AL, Lima AR, Dias HS, Leao GC, Coutinho A. An ultrasonographic study of liver fibrosis in patients infected with Schistosoma mansoni in north-east Brazil. Trans R Soc Trop Med Hyg. 1993;87:555–8. doi: 10.1016/0035-9203(93)90087-7. [DOI] [PubMed] [Google Scholar]
- 30.Nash TE, Cheever AW, Ottesen EA, Cook JA. Schistosome infections in humans: Perspectives and recent findings.NIH conference. Ann Intern Med. 1982;97:740–54. doi: 10.7326/0003-4819-97-5-740. [DOI] [PubMed] [Google Scholar]
- 31.Saad AM, Homeida M, Eltom I, Nash T, Bennett JL, Hassan MA. Oesophageal varices in a region of the Sudan endemic for Schistosoma mansoni. Br J Surg. 1991;78:1252–3. doi: 10.1002/bjs.1800781033. [DOI] [PubMed] [Google Scholar]
- 32.O’Donohue J, Ng C, Catnach S, Farrant P, Williams R. Diagnostic value of Doppler assessment of the hepatic and portal vessels and ultrasound of the spleen in liver disease. Eur J Gastroenterol Hepatol. 2004;16:147–55. doi: 10.1097/00042737-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Winkfield B, Aubé C, Burtin P, Calès P. Inter-observer and intra-observer variability in hepatology. Eur J Gastroenterol Hepatol. 2003;15:959–66. doi: 10.1097/00042737-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Farias AQ, Kassab F, da Rocha EC, Dos Santos Bomfim V, Vezozzo DC, Bittencourt PL, et al. Propranolol reduces variceal pressure and wall tension in schistosomiasis presinusoidal portal hypertension. J Gastroenterol Hepatol. 2009;24:1852–6. doi: 10.1111/j.1440-1746.2009.05912.x. [DOI] [PubMed] [Google Scholar]
- 35.Tanabe M. Haemostatic abnormalities in hepatosplenic schistosomiasis mansoni. Parasitol Int. 2003;52:351–9. doi: 10.1016/s1383-5769(03)00051-5. [DOI] [PubMed] [Google Scholar]


