Abstract
The long history of Cannabis sativa had its development stimulated and oriented for medicine after the discovery and chemical characterization of its main active ingredient, the 9-tetrahydrocannabinol (9-THC). Consequently, a binding site for 9-THC was identified in rat brains and the first cannabinoid receptor (CB1) was cloned, followed by the CB2 and by the discover of two endogenous agonists: anandamide and 2-arachidonoyl glycerol. Cannabinoid receptors, endocannabinoids and the enzymes that catalyze its synthesis and degradation constitute the endocannabinoid system (ECS), which plays an important role in the cardiovascular system. In vivo experiments with rats have demonstrated the action of anandamide and 2-AG on the development of atherosclerotic plaque, as well as an effect on heart rate, blood pressure, vasoactivity and energy metabolism (action in dyslipidemia and obesity). Recent studies with an antagonist of CB1 receptors showed that the modulation of ECS can play an important role in reducing cardiovascular risk in obese and dyslipidemic patients. Similarly, studies in rats have demonstrated the action of CB2 receptors in adhesion, migration, proliferation and function of immune cells involved in the atherosclerotic plaque formation process. The evidence so far gathered shows that the modulation of ECS (as agonism or antagonism of its receptors) is an enormous potential field for research and intervention in multiple areas of human pathophysiology. The development of selective drugs for the CB1 and CB2 receptors may open a door to new therapeutic regimens.This review article aims to address the key findings and evidences on the modulation of ECS, in order to prospect future forms of therapeutic intervention at the cardiovascular level. A recent, emerging, controversial and of undoubted scientific interest subject, which states as a potential therapeutic target to reach in the 21st century.
Keywords: Atherosclerosis, cannabinoids, cardiovascular system, endocannabinoids, hypertension, shock, ischemia/reperfusion, therapeutics
The recreational use of the plant Cannabis sativa and the attempt to exploit their potential therapeutic use have been described over the centuries. The popularity of marijuana, one of the most common forms of consumption as a recreational substance and as a drug, reflects its ability to later sensory perceptions and to reduce anxiety. Other non-psychoactive actions of marijuana, like pain relief, were also described in ancient texts. However, the biochemical and pharmacological study of this substance has a fairly recent start. The first attempts to isolate the cannabinoids (phytocannabinoids) of Cannabis sativa, conducted in mid-twentieth century, led to very rudimentary pharmacological investigations. In the sixties, the chemical structures of Δ-9-tetrahydrocannabinol (Δ 9-THC) and cannabidiol were described, their procedures for synthesis were found and THC was identified as the major phytocannabinoid among the 60 existing phytocannabinoids in Cannabis sativa.
Although the THC, cannabidiol and other phytocannabinoids present bioactivity with potential use (anti-inflammatory, anti-convulsive and antiemetic, for example), THC is the only psychotropic cannabinoid present in Cannabis sativa. Because of its psychoactivity and early availability in synthetic form as a research tool, THC quickly gained the status of cannabinoid prototype and became the target of numerous studies during the 70's and the 80's. Much of this research, in vivo, focused on the effects of THC in animal models using synthetic analogues, some of which radiolabeled as molecular probes for the interactions of the cannabinoids with the tissues. Given the psychotropic effects of THC, many biological investigations used brain and its plasma membranes, in order to describe the action of cannabinoids.[1–4]
Their connection with the plasma membranes of the brain is greedy, saturable and stereospecific, according to the responses in vivo and in vitro. These characteristics suggested that cannabinoid pharmacology could be mediated by receptors, which led to the research of THC binding sites in mammalian, to justify their psychotropic effects. These studies were the basis for the discovery and cloning of two G protein-coupled receptors (GPCR) for cannabinoids, called CB1 and CB2, which in humans share about 44% of homologous sequence.[5] The CB1 receptor has its preferential location in the central nervous system (CNS), reflecting his prevalence as the most abundant GPCR in the brain. CB1 receptors can be found in cortex, cerebellum, hippocampus and basal ganglia, which are brain regions that control motor functions and cognitive, emotional and sensory actions. Thus, activation of central CB1 receptors is responsible for most of the behavioral and psychotropic effects of cannabinoids.[6] These receptors are also present in high density in the brainstem, hypothalamus and pituitary gland, affecting the perception of pain, hormonal activity, thermoregulation and cardiovascular, gastrointestinal and respiratory physiology.[6] CB1 receptors are also located peripherally (eg. adipocytes, liver, uterus) where are able to regulate basic physiological processes, such as energy balance and reproduction.[6] Although they are detectable in trace concentrations in brain, CB2 receptors are expressed mainly in immune cells and hematopoietic cells, osteoblasts and osteoclasts, mediating immune responses, inflammation, neuropathic pain and bone remodeling.[5]
Endocannabinoids (EC) are involved in several physiological functions, among which, special attention has been given to the regulation of appetite by central mechanisms and its influence on obesity.[7,8] Considering these innovative findings, the research for new pharmacological agents has drastically increased and the discovery of rimonabant, a synthetic antagonist of CB1 receptors, has confirmed the important role of endocannabinoid system on the modulation of food ingestion and energetic balance.[9] These facts led to the first clinical studies using rimonabant as a new tool against obesity and its associated metabolic disorders. However, psychiatricside-effects, namely central, which include increased risk of depression and even suicide, US Food and Drug Administration declined permission for rimonabant, and in October 2008, rimonabant was also suspended across the EU. After rimonabant withdrawal, other CB1 antagonist drugs have also tested, including the taranabant, which was associated with weight loss in rats and in humans.[10,11] However, due also to central side effects, including anxiety and depression, the clinical trials were stopped in October 2008 (EMEA. The European Medicines Agency recommends suspension of the marketing authorisation of Acomplia: http://www.emea.europa.eu 2008).
Although several other different influences of endocannabinoids have been discussed during the last years, including in inflammation, diabetes, cancer, affective and neurodegenerative diseases, and epilepsy.[12] the most recent findings are related to their cardiovascular actions,[13] which seem to be very ample bust also complex. The endocannabinoid system (ECS), which includes the endocannabinoids and its receptors, have been implicated in hypotensive stages associated with hemorrhagic chock, both endotoxic and cardiogenic, and even to advanced liver cirrhosis; on the other hand, recent evidence suggests that ECS plays an important role in cardiovascular regulation associated with hypertension, as well as a protective role in ischemia grafting. The development of atherosclerotic plaque and the metabolic stages associated to obesity are also matter of study of possible ECS pharmacomodulation.
This article reviews the effects of endocannabinoids on the cardiovascular system, focusing on their role on cardiovascular pathophysiology and on new therapeutic opportunities in this field.
Endogen cannabinoids and the endocannabinoid signaling system
The apparent discrepancy between the presence of cannabinoid receptors in the brain of mammals and the absence of phytocannabinoids in tissues led to the demand of cannabinoid receptor ligand molecules that were produced and metabolized as endogenous bioactive constituents (“endocannabinoids”). In mild 1990, the first two endocannabinoids, N-arachidonoylethanolamine (anandamide) and 2-Arachidonoylglycerol (2-AG), were identified; both derived from arachidonic acid, a polyunsaturated fatty acid. Anandamide and 2-AG are members of a family of natural derivatives of amide and long chain fatty acids ester, which are found in humans and other mammals, acting as agonists of CB1 and/or CB2 receptors.[14] Anandamide is a partial agonist of CB1 receptors and a relatively weak ligand of CB2 receptors, with a low overall efficiency, while 2-AG, produced in much larger amounts than anandamide, is a full agonist of both receptors.[15] "Classical" transmission of CB1 and CB2 receptors involves inhibitory G proteins, which inhibits adenylcylase (thus, formation of cyclic AMP) and the calcium channels type L, N and P/Q, enabling potassium channels and mitogen activated protein kinase.[16] When coupled to other classes of G proteins, cannabinoid signaling mediated by receptors influences various signal transduction systems to induce, for example, the entry of calcium into the cells.[16] Moreover, endocannabinoids are able to make independent systems from signaling through G protein, involving messengers such as phospatidylinositol-3-phosphate or nitric oxide, and also effects independent from the receptors present in excitable cells.[17]
The endocannabinoid signaling system is composed by the endocannabinoids and their cellular receptors, together with the messengers and carriers that support the traffic of these hydrophobic mediators between cells and enzymes directly responsible for the biosynthesis and metabolism of endocannabinoids constitute. In line with the hydrophobic nature of the endocannabinoids and the selective provision of receptors in tissues, the metabolism and distribution of endocannabinoid metabolizing enzymes helps to define the broad influence that these lipid mediators can exert on mammals physiology. The balanced action of these enzymes of endocannabinoid biosynthesis and metabolism are essential for strict homeostatic regulation of endocannabinoid signaling. Contrary to many neurotransmitters that are stored in intracellular vesicles, awaiting their mobilization in the bioactive form, endocannabinoids are synthesized “on demand” in response to increased intracellular calcium induced by stimuli.
The precursor for the synthesis of anandamide is the membrane phospholipid N-arachidonoyl phosphatidylethanolamine (NAPE). Acrylamids, such as anandamide, are synthesized by hydrolytic release of polyunsaturated fatty acids by a specific phospholipase D; by the formation of phosphorus-anandamide mediated by phospholipase C, being self-dephosphorylated by a specific phosphotase; or trough a sequence deacylation of NAPE by α,β-hydrolase 4 with the subsequent cleavage of glycerolphosphate.[18] NAPE is synthesized by an N-acetyltranferase that can be limiting for the biosynthesis of anandamide. The formation of 2-AG occurs mainly through the hydrolysis of 2-arachidonoyl-phosphatidylinositol to diacylglycerol by phospholipase C, that is hydrolysed to 2-AG by diacylglycerol lipase or, in certain tissues, by phospholipase A1 and subsequent lysophospholipase activity.[19]
Endocannabinoids are efficiently removed from their sites of action trough cellular uptake (a mechanism still to be entirely characterized), as well as by specific intracellular enzymes.[20] In most tissues, anandamide is metabolized by fatty acid amide hydrolase (FAAH), a membrane-bound amidase and, although FAAH can also act on 2-AG, this endocannabinoid is metabolized by soluble monoacylglycerol lipase (MAG). Several oxidative enzymes, including lipoxygenases, cytochrome P450 and cyclooxygenase-2, can transform the endocannabinoids in bioactive products related eicosanoids, whose physiological roles remain unclear.[21,22]
Cardiovascular effects of endocannabinoids in vivo
Cardiovascular effects of endocannabinoids in vivo are complex and may involve the modulation of autonomic flow of central and peripheral nervous systems, as well as the direct effects of myocardium and vasculature. However, its peripheral actions appear to play a dominant role, at least after systemic administration of doses used by most researchers. Besides that, the effects of endocannabinoids are complicated due to their rapid metabolism, which can release other vasoactive substances and their precursors.[23]
In humans, the acute effect of smoking Cannabis usually manifests by an increase in heart rate, without significant changes in blood pressure.[24] However, the chronic use of Cannabis in man, in addiction with acute and prolonged administration of THC to animals, causes a decrease in long-term blood pressure and heart rate.[25] Given the well known effects of cannabinoids in the CNS, the first studies focused on the ability of these substances to inhibit the sympathetic tone as the underlying mechanism. Indeed, experiments in dogs showed some evidence for a THC centrally mediated sympathetic inhibitory effect, although some peripheral sites of action could not be excluded.[26] In this way, in an early stage, the potential use of cannabinoids as anti-hypertensive drugs was considered,[27] hoping that the cardiovascular and the psychoactive effects could be separated. This hypothesis was first suggested in 1977, with the publication of the biological effects of “abnormal cannabidiol”, a synthetic analogue of cannabidiol, the non-psychoactive cannabinoid present in Cannabis sativa.[28] However, more than two decades passed before the development of this promising observation.
Role of CB1 receptors in the cardiovascular in vivo effects of cannabinoids
The discovery of the first endocannabinoid, anandamide, raised important questions about the potential cardiovascular activity similar to THC. The injection of an intravenous bolus of anandamide in anesthetized rats caused a triphasic blood pressure response and bradycardia,[29] in many ways similar to the one observed previously with THC. The first phase of the response is a sharp fall in heart rate and blood pressure, which persists for a few seconds. These effects are mediated by vagal responses, since they are absent in animals after bilateral transaction of the cervical vagus nerve or after pretreatment with methylatropine.[29] This vagal component is followed by a brief pressor response that persists in the presence of a α-adrenergic blockade, as well as in rats whose sympathetic tone was abolished by section of the spinal cord and it is not, therefore, mediated by sympathetic nervous system (SNS).[29] This pressor component is not affected by CB1 receptor antagonists and persists in mice lacking CB1 receptors,[30] indicating that they are not involved in the process. Recent observations suggest that this pressor component may be originated in vasoconstriction of certain vascular territories, such as the spleen.[31] The third and most prominent phase of the effect caused by anandamide is associated to hypotension, moderate bradycardia, persisting for 2-10 minutes. This phase is absent in normotensive conscious rats,[32] but is present and has prolonged duration in spontaneously hypertensive conscious rats.[33] Since the sympathetic tone is reduced in normotensive rats kept in a quiet environment,[34] these observations seem consistent with a sympathetic-inhibitory mechanism underlying the hypotension and bradycardia induced by anandamide. The discovery that a metabolically stable analog of anandamide, R-methanandamide,[35] causes hypotension and bradycardia similar but of longer duration,[36] eliminates the possibility that the hypotensive and bradycardia effects of anandamide are caused indirectly by a metabolite. Several evidences indicate that the hypotension induced by cannabinoids is mediated by CB1 receptors. At the outset, hypotension is antagonized by rimonabant, a CB1-selective antagonist.[29] This drug inhibits the hypotension induced by cannabinoids derived from plant or synthetic cannabinoids, as well as by anandamide.[37] However, in tests with anesthetized rats, the hypotensive effect of 2-AG resisted, unexpectedly, to inhibition by rimonabant, but was antagonized by indomethacin, suggesting an involvement of a cyclooxygenase metabolite.[38] In fact, it was shown that 2-AG is rapidly degraded in mouse blood (less than 30 seconds), generating arachidonic acid. Accordingly, the metabolically stable analog of 2-AG saw its hypotensive effect caused by rimonabant and was absent in mice deficient in CB1 receptors, indicating that, similar to anandamide, it is an effective agonist of CB1 hypotensive receptors.[38]
The second line of evidence for the involvement of CB1 receptors is a strong positive correlation between the concentrations of various cannabinoid agonists producing hypotensive and bradycardic responses and their affinity constants for binding to CB1 receptors in the brain.[37] The strongest evidence lies, however, in the absence of hypotension and bradycardia induced by cannabinoids in mice lacking CB1 receptors.[30] Interestingly, the isolated tachycardia in response to acute administration of THC in human volunteers, who are not chronic users of Cannabis sativa, was inhibited by rimonabant.[39] In healthy young adults, the heart is under dominant vagal tone, and the tachycardic effect of THC is, mainly, due to the inhibition of acetylcholine release from the efferent cardiac vagal, trough presynaptic CB1 receptors.[40]
The acute effects of drug on blood pressure result from changes in peripheral vascular resistance, cardiac output, or both. Although the exclusive role of CB1 receptors in the hypotensive effect of cannabinoids is strongly suggested, there is a growing evidence that vasodilatation induced by anandamide in the mesenteric vascular flow, and possibly elsewhere, is dependent of CB1 and CB2 receptors.
Central versus peripheral mechanisms in the cardiovascular in vivo effects of cannabinoids
The first studies with THC have suggested that the hypotensive effect of cannabinoids is mediated by a sympathetic-inhibitory mechanism.[26] However, hypotension induced by anandamide in anaesthetized rats is not associated with any alteration of sympathetic premotor neurons in medullary vasomotor center or to the activity of post ganglionic sympathetic nerve,[41] which excludes the centrally mediated sympathetic inhibition or the ganglionic blockade as a mechanism, at least with regard to anandamide. The intracerebroventricular administration in rabbits of the potent synthetic cannabinoid WIN55,212-2 induced an increase, rather than a decrease, in sympathetic tone, which is an argument against a central mechanism underlying the hypotension.[42] Furthermore, the pressor response by electrical stimulation of the vasomotor center was reversibly inhibited by anandamide, while the effect of phenylephrine was not affected, suggesting an inhibitory effect of presynaptic release of noradrenaline of peripheral sympathetic nerve terminals.[41] Indeed, stimulation of CB1 receptors inhibits the presynaptic release of noradrenaline, both in vitro[43] and in vivo.[42] However, when the sympathetic tone is eliminated by ganglionic blockade and vascular tone is restored by an infusion of vasopressin, the hypotensive response to potent cannabinoid HU-210 keeps unchanged, while the bradycardic effects are lost.[31] This suggests that the bradycardia induced by cannabinoids is due to the inhibition of sympathetic tone to the heart, while the hypotensive response is due to direct vasodilatation, and the same is suggested by its presence in rats, following chemical sympathetic chemical denervation.[44]
Cardiovascular effects of endocannabinoids in vitro
Direct vasodilatory effects of cannabinoids
It was soon demonstrated that the vasodilator response caused by anandamide in blood flow could be inhibited by indomethacin[45] and, thus, it was surmised that anandamide induces vasodilatation indirectly by producing arachindonic acid and its subsequent metabolism by cyclooxygenase. Although THC also produces a sensitive response to indomethacin, subsequent studies have not documented an effect of cyclooxygenase inhibition in vasodilatation induced by anandamide in other blood vessels, including mesenteric and coronary vasculature,[46] excluding the production of prostanoids as a major mechanism for the vasodilator effect.
The vasodilator effect of anandamide differs between tissues and species. Therefore, previous studies have demonstrated a vasodilator action of anandamide in the hepatic artery of the rat, guinea pig basilar artery[47] and bovine coronary arteries,[48] although the same was not observed in the carotid arteries and aorta of rats.[49] It was demonstrated that anandamide induces vasodilatation of afferent arterioles of the kidney by endothelial release of nitric oxide (NO).[50] The same is released in various human blood vessels and in the right atrium by anandamide action.[51] In contrast, other studies have shown the callousness of the vasodilatation induced by anandamide inhibition of NO synthase.[46]
New endothelial endocannabinoid receptor
The existence of non-CB1 and non-CB2 receptors have been hypothesized repeatedly during the last years. One of the most studied possibilities is the transient receptor potential vanillinoid (TRPV) receptors, in particular, but not only, the TRPV1, including on the vasculature.
The hypothesis of the existence of others cannabinoid receptors distinct from CB1 and CB2 was first raised after the discovery that potent synthetic cannabinoids, such as THC, did not induce vasodilatation in the same preparations of mesenteric vascular beds in which anandamide and methanandamide had a strong vasodilator action.[52] In these experiments, the effects of anandamide and methanandamide could be inhibited by rimonabant, but the concentration required was significantly higher than that required for inhibition of CB1 receptors.[30] Furthermore, the ability of rimonabant to inhibit vasodilatation induced by anandamide disappeared after endothelial denudation.[30] These data led to the postulation of a endothelial site, sensitive to inhibition by rimonabant, but distinct from CB1 and CB2 receptors, which will contribute to vasodilatation induced by anandamide in mesenteric circulation[31] and, possibly, in other vascular bedsteads, such as rats coronary circulation.[53] More recently, the existence of a local non-CB1/non-CB2 was also postulated in glutamatergic terminals in rat hippocampus, where activation by cannabinoids inhibits glutamatergic transmission and excitatory postsynaptic potentials.[54] Non-CB1/CB2 receptors have been also postulated for the immune cells, and some evidences suggest that for these cells, as well as for the brain, these cannabinoids receptor engaged by PEA could be the GPR55, which was first identified as an orphan G protein coupled receptor (GPCR) enriched in the brain, but is now known as part of a family of GPCRs that might play a role on endocannabinoids and cannabinoids actions.[55]
Direct cardiodepressant effects of cannabinoids
Unlike the vascular effects of cannabinoids, whose knowledge is increasing, not much is known about their direct cardiac effects. Anandamide, anandamide amidohidrolase and the signs of message to CB1 receptor[51,56] were detected in the human heart. In a more recent study, the existence of CB1 receptors was confirmed in human auricular myocytes by immunoblotting and immunohistochemistry.[57] In the same study, it was shown that anandamide, R-methanandamide and HU-210 all decreased, in a dose-dependent way, the contractility of isolated and electrically stimulated human atrial muscle. AM51 is a potent and selective CB1 receptor antagonist[58] which has blocked the negative ionotropic effect of three drugs, excluding the involvement of CB2 receptors activation, NO and prostanoids release.[57] Consistent with these in vitro results, it was shown that HU-210 decreases the cardiac output depending on CB1 receptors activity.[31] Previous studies had shown that anandamide induces coronary vasodilatation sensitive to rimonabant in isolated perfused rat hearts,[59] which meant cannabinoid receptors. These results were mimicked by arachidonic acid, indicating a vasodilator effect, not mediated by metabolites of arachidonic acid.
According to what is observed in isolated cardiac preparations, the use of Millar system of pressure-volume conductance to measure cardiac performance in vivo has strongly supported the idea of a crucial component in the cardiac hemodynamic effects of cannabinoids. Taken together, those studies suggest that CB1 receptors are present in cardiomyocytes and that they can induce a decrease of cardiac contractility trough mechanisms that can be dependent or independent from the CB1 receptors.
Effects of cannabinoids on cardiovascular diseases pathophysiology
Myocardial ischemia/reperfusion and preconditioning
Initial studies used isolated preparations of heart to study the role of endocannabinoid system in myocardial ischemia/reperfusion (I/R) and preconditioning. The involvement of ECS in preconditioning induced by the endotoxin (lipopolysaccharide: LPS) against the injury induced by I/R on myocardium has been implicated for the first time in 2001, based on the hypothesis that LPS could increase the production of endocannabinoids in inflammatory cells.[60] A 90 minutes of low flow ischemia followed by 60 minutes of reperfusion with normal flow in isolated rat hearts pretreated with LPS was compared with a saline solution. The pretreatment with LPS reduced the infarct size and improved functional recovery after reperfusion when compared with control group, which could be attenuated by SR144528 (CB2 antagonist), but not by rimonabant (CB1 antagonist), suggesting the involvement of myocardium CB2 in the cardioprotection induced by LPS.[61] In a subsequent study, in which the preconditioning was triggered by heat stress, the SR144528 also abolished the effect of reducing the infarct size, unlike rimonabant.[62]
These early studies suggested that the protection created by the preconditioning induced by heat stress or by LPS was mediated by the action of endocannabinoids in the CB2 receptors. In contrast, when preconditioning was induced by a brief period of ischemia (5 minutes), the blockade of CB1 and CB2 receptors did not raise the abolition of protection, and both receptors have been implicated in preserving endothelium-dependent vasodilatation induced by serotonin.[63] The palmitoylethanolamide or the 2-AG, but not anandamide, when added to perfused isolated rat hearts offer protection against ischemia by reducing myocardial damage and infarct size and by improving the functional recovery of myocardium.[64] The SR144528 completely blocked the cardioprotective effect of palmytoylethanolamide and 2-AG, whereas rimonabant only inhibited, partially, the effect of 2-AG.[64] Similarly, ACEA and JWH015 (CB1 and CB2 agonists) also reduced the size of the infarct in this model.[64] In contrast, it was found that anadamida's effect of reducing infarct area could also be antagonized by CB1 and CB2 antagonists; however, the same could not be mimicked by selective CB1 and CB2 agonists, suggesting an involvement of a different site of CB1 and CB2 receptors. Another recent study, which used a model of delayed preconditioning in rats, induced by transdermal treatment of nitroglycerin (as a NO donor) for 24 hours, suggested that the protective effect of nitroglycerin against myocardial infarction is mediated trough CB1 receptors. Nitroglycerin increased the concentration of 2-AG in myocardium, but did not increase anandamide.[65]
The major limitation of the studies mentioned above is the use of ex vivo models (eg, isolated preparations of heart) that could not clarify if endocannabinoids or the synthetic agonists can modulate the activation of immune or endothelial cells and their interactions, which constitute key events in the sequelae of reperfusion injury.[66] Despite those limitations, these pioneer studies implicated a possible contribution of CB2 functional receptors in cardiomyocytes and the endothelial cells responsibility, at least in part, on the protective effects of preconditioning. Indeed, subsequent studies showed the presence of CB2 receptors in myocardium,[67] in cardiomyoblast cells[67] and in endothelial cells with different origins.[68] Concurrently with the beneficial effect of the activation of CB2 receptors in cardiomyocytes, a recent study showed that THC protected cardiomyoblast cells H9c2 submitted to hypoxia in vitro, presumably trough the activation of CB2 receptors and increased NO production.[69]
In an ischemia/reperfusion injury model in rats, both anandamide and HU-210 decreased the incidence of ventricular arrhythmias and reduced the size of the infarct, presumably trough the activation of CB2 receptors but not CB1 receptors.[70] In an myocardic I/R injury model induced by ligation of coronary artery in rats, the reduction of the second myocardic injury depending on leucocytes subsequent to the initial I/R injury was attributed to the activation of CB2 receptors, since the protection given by WIN 55.212-2 could be prevented by AM630, but not by AM251 (a CB1 antagonist).[71] Two recent studies in myocardial infarct models, acute and chronic, in rats, showed that cannabinoids contribute to hypotension and cardiac depression associated to cardiogenic acute shock, which could be attenuated by antagonists of CB1 receptors.[72]
Overall, despite the role of CB1 receptors and of endocannabinoids in the protection given by the preconditioning against myocardic I/R, the issue remains controversial, recommending further investigation namely using mice with deletion of genes and more selective agonists of CB2 receptors. However, the findings that imply CB2 receptors’ importance, presumably in both endothelial and inflammatory cells and perhaps in cardiomyocytes, are quite encouraging.
Cerebral ischemia/reperfusion (cerebrovascular accident)
The endocannabinoid system may constitute an essential mechanism of neuroprotection, in both acute forms of neuronal injury, such as stroke or brain trauma, and in several chronic neurodegenative disorders, including multiple sclerosis, Parkinson's disease, Hungtington's disease, Alzheimer's disease and amyotrophic lateral sclerosis.[73] Although the exact mechanisms of this neuroprotection are not yet completely understood, several processes dependent and independent of CB receptors seem to be involved: 1) modulation of the immune responses and release of inflammatory mediators by CB1, CB2 and not CB1/not CB receptors in neurons, astrocytes, microglia, macrophages, neutrophils and lymphocytes;[74] 2) modulation of synaptic plasticity and excitatory glutamatergic transmissions via presynaptic CB1 receptors;[75] 3) activation of cytoprotective signaling pathways;[73] 4) modulation of calcium homeostasis and excitability trough interactions with calcium channels, potassium and sodium, gap junctions and intracellular calcium reserves, and with NMDA receptors;[75] 5) central hypothermia mediated by CB1 receptors, presumably by the reduction of the metabolic rate of needed oxygen; 6) antioxidant properties of cannabinoids;[76] 7) modulation of endothelial activity and inflammatory response, leucocytes mobilization, adhesions to the endothelium, transmigration and activation presumably through CB2 receptors.
The first evidence of a neuroprotective effect of cannabinoids has emerged in research studies on cerebrovascular accident, in which was used the non psychoactive cannabinoid dexanabinol/HU-211 that exerts its effect through CB1/CB2 independent mechanisms, in cerebral ischemia models in vivo in rats and gerbils.[73] Further studies also investigated the neuroprotective effects of CB1 receptors stimulation with synthetic agonists. The synthetic cannabinoid WIN 55.212-2 attenuated the neurological damage in the hypothalamus resulting from cerebral global and transient ischemia in rats and reduced infarct size after permanent focal cerebral ischemia induced by cerebral middle artery occlusion, when it was administrated 40 minutes before or 30 minutes after occlusion, in a dependent way from CB1 receptors, since the protective effect was prevented by rimonabant.[77] WIN 55.212-2, as well as anandamide and 2-AG, did also confer protection to cultured cortical neurons submitted to hypoxia and glucose deprivation in vitro, but these effects proved to be insensitive to antagonists of CB1 and CB2 receptors.[77]
In models of cerebral middle artery occlusion in rats, the agonist BAY38-7271 reduced the size of the infarct, even when administered intravenously 4 hours after the occlusion.[78] The pre-treatment with rimonabant partially attenuated the effect of HU-210, indicating the involvement of CB1 receptor. However, the protective effect of HU-210 could be completely abolished by warming the animals’ body until the controls temperature, showing that hypothermia mediated by CB1 receptors was responsible for the beneficial effects observed.[79] Similarly, hypothermia mediated by CB1 receptors was responsible for the neuroprotective effects of THC in a model of cerebral ischemic injury in rats,[80] and in a model of global cerebral ischemia injury in rats.[81] Concurrently with the neuroprotection mediated by CB1 receptors, mice without CB1 receptors showed an increased neurotoxicity to NMDA and high mortality levels in permanent focal cerebral ischemia, and an increased infarct area, with neurological deficits more severe after transient focal cerebral ischemia and decreased blood flow in brain in ischemic penumbra during reperfusion, when compared with controls under the same aggressions.[82]
In contrast, several recent studies do not support the neuroprotective role of endocannabinoids in the activation of CB1 receptors. In fact, rimonabant and LY320135 (CB1 receptors antagonist) reduced the size of the infarct and improved the neurological function in a cerebral ischemia model in rats, induced by brain middle artery occlusion,[83] while low doses of WIN 55,212-2 showed no protective effects.[83] Recent studies have evaluated the effect of selective CB2 agonists (O-3853, O-1966) in a model of cerebrovascular accidents. CB2 agonists significantly decreased cerebral infarct and improved motor function after cerebral middle artery occlusion for one hour, followed by 23 hours of reperfusion in rats, by attenuation of the increased mobilization of leucocytes and their adherence to vascular endothelial cells induced by transient ischemia.[84] The role of CB2 receptors in I/R injury was also supported by the increased accumulation of CB2-positive macrophages derived from resident microglia and/or from invading monocytes resulting from I/R cerebral injury.[85]
In general, it seems clear that both agonists and antagonists of CB1 receptors may play a neuroprotective effect on cerebral I/R injury. The reason for the contradictory effects of the pharmacological blockade versus genetic “knockout” of CB1 receptors is still unclear, but could be related with effects that are independent from CB1 antagonists, and that's the reason why this subject requires further clarification. In the case of CB2 agonists, the most likely protection mechanism is the reduction of increased leukocyte infiltration, mobilization and adhesion to vascular endothelial cells and consequent activation, in a process induced by I/R transient injury.
Circulatory shock (organ/body ischemia and/or ischemia/reperfusion)
In addition to its well-known immunologic and neurobehavioral actions, cannabinoids and their synthetic endogenous analogs exert complex cardiopressant and vasodilator effects, which were implicated in the mechanisms underlying hypotension associated to hemorrhagic shock, cardiogenic and septic, advanced liver cirrhosis, cirrhotic cardiomyopathy, heart failure induced by doxorubicin and shock associated to necrotizing pancreatitis.[66,86–89] These depressant effects of the cardiovascular system could be prevented or reversed by the pretreatment with CB1 receptor antagonists, and they have been analyzed in many recent studies. CB receptors antagonists (eg. rimonabant, AM281, AM251 and SR144528) prolonged the survival in septic shock or in necrotizing pancreatitis,[60] increasing mortality in hemorrhagic[90] and cardiogenic[91] shock, despite the increase in blood pressure. One possible explanation for this intriguing controversy is the hypothesis that vasodilatation mediated by endocannabinoids can provide a survival value by increasing tissues oxygenation, neutralizing the excessive sympathetic vasoconstriction triggered by hemorrhage or by myocardium infarct, which could be avoided by blocking CB1 receptors. In contrast, the blockade of CB1 receptors could increase survival in endotoxic shock by preventing the primary hypotensive response to LPS.[73] Even more complicated is the fact that, in hemorrhagic shock, both cardiogenic and septic, UH-210, WIN 55,212-2 and THC (CB agonists) are able to improve endothelial function and/or survival.[60] Since cardiovascular failure and dysfunction in many of the cited studies are triggered by I/R injury and/or ischemia, and consequently oxidative/nitrosative stress and inflammatory response associated to the activation of several cell death pathways downstream,[92] another explanation for the different beneficial effects of agonists and antagonists in circulatory shock could reside in their various anti-inflammatory and/or antioxidant properties.[74] These could be attributed to their inverse agonist properties or to mechanisms independent from CB1 and CB2 receptors.[15]
In global terms, it seems clear that both cannabinoids and antagonists of CB receptors may exert several beneficial effects in shock models in rats; however, the specificity of these effects and their importance for the circulatory shock in humans requires further investigation.
Role of endocannabinoid system in hypertension
The potential use of cannabinoid ligands as antihypertensive agents was even considered since 1970,[93,94] and were further reviewed.[89,95] Cannabinoids decrease blood pressure in hypertensive rodents primarily because of decrease cardiac contractility, suggesting that could have a therapeutic role on hypertension and cardiac hypertrophy. Rimonabant, the CB(1) receptor blocker induced a significant increase in cardiac contractility and blood pressure in hypertensive rats but, on the contrary, contributed to decrease blood pressure in weight-loss clinical trials especially in obese patients with hypertension, which suggests that the overactivation of the ECS in intra-abdominal obesity could be a deleterious effect, in particular from a cardiometabolic opinion.[89]
In addition to the studies in animal models that were already mentioned, it was found that inhalation of THC causes a greater and more lasting fall in blood pressure in hypotensive subjects when compared with normotensive subjects.[96] Although the mechanism underlying this increased sensitivity is not cleared yet, it suggests a role of endocannabinoid system in the regulation of cardiovascular functions in hypertension. In a recent study, using three different experimental models of hypertension to explore this possibility, the authors found a significant endocannabinergic tone in hypertension that limits the blood pressure rise and cardiac contractility through the activation of cardiac and vascular CB1 receptors.[97] It was also found, that over-regulation of these same receptors contributes to potentiate of this tone, maybe trough the inhibition of the activation of endogenous anandamide, stabilizing blood pressure and the contractility of the heart in hypertension. These findings contribute to the interesting possibility of using inhibitors of fatty acid amide hydrolase in the treatment of hypertension. More clinical studies will be needed to clarify this interesting possibility in a near future.
Role of endocannabinoid system in atherosclerosis
Cannabinoids, endogenous and synthetic, have complex cardiovascular actions through the activation of CB1 receptors (vascular and myocardial).[98] The decline of cardiac function associated with age and the changes in inflammation genes expression, nitrative stress and apoptosis in rats FAAH-/- compared with wild type rats was analyzed.[99,100] The authors found that increased levels of anandamide in FAAH-/- rats have a protective effect, which is consistent with the protective role of cannabinoids in inflammatory disorders, such as atherosclerosis. Besides that, anandamide demonstrated its capacity to attenuate, in a dose-dependent manner, the expression of ICAM-1, induced by TNF-α, and of VCAM-1 in endothelial cells of human coronary arteries, and also THP-1 monocytes adhesion in a process dependent on CB1 and CB2 receptors.[99]
Contrary to the potential beneficial effect in cardiovascular disease, the endocannabinoids may exhibit some prothrombotic effects. In fact, both anandamide and 2-AG were described as activators of human and rodent platelets. The platelets are cellular anucleated fragments that circulate on blood stream. Besides their recognized role in homeostasis and in thrombus formation, platelets may also have proinflammatory properties and be growth regulators, contributing to the progression of atherosclerosis.[101] Endothelial cells, macrophages and platelets may, by itself, increase their synthesis of endocannabinoids during the formation of atherosclerotic plaque, leading to the activation of platelets. Alternatively, these cells are able to metabolize 2-AG and anandamide, which can offset the increased levels of cannabinoids.
CB1 blockade with rimonabant, besides reduce weight and abdominal adiposity, improves cardiometabolic profile, due to multiple influences, including increased levels of high density lipoprotein (HDL)-cholesterol and reduced triglycerides.[102,103]
A possible role for CB2 receptors on the progression of atherosclerosis was suggested in an experimental model. The authors found that oral low-doses THC treatment could inhibit the development of atherosclerostic plaque, which was reversed using SR144528, an antagonist of CB2 receptors.[104] The progression of atherosclerosis was associated with a reduced infiltration of macrophages in the atherosclerotic lesions. The mobilization, adhesion and trans-endothelial migration of leukocytes are triggered by the local production of chemokines, its receptors and adhesion molecules.[105] Cannabinoids, endogenous or synthetic, have shown to modulate the migration of several cell types, including immune cells trough activation of CB2 receptors.[106]
In overall, despite some interesting findings, a specific role of endocannabinoid signaling during atherosclerosis remains to be better elucidated.
New therapeutic opportunities of ECS in cardiovascular disorders
Obesity remains a continuous healthy problem and research issue, which is explained by the serious consequences associated with it, as well as by the increasing incidence of type 2 diabetes and associated obesity, including in younger individuals. In this way, the EC, due to its well known properties of weight control and energy balance, appeared as a promising target for the treatment of obesity, namely by blocking its receptors. The blockade of these receptors was effectively done by rimonabant, which was viewed as a promising drug for the treatment of obesity. Besides its action on obesity, rimonabant has also proved to be efficient in controlling vascular diseases in several clinical trials and, therefore, this drug was presented as an effective therapeutic approach for treating obesity and cardiovascular disease. However, despite the proven effectiveness in weight loss, rimonabant clinical use was associated with several side effects, which mainly includes the following three groups: the first one includes psychiatric disorders such as depression and anxiety; the second one is associated with gastrointestinal disturbances such as nausea; and finally the third group with regard to neurological changes that are reflected in headaches and vertigo. Despite these adverse effects, which originated its removal from the marker, since the blockade of CB1 receptors continues to prove an asset in the management of obesity and its associated risks (such as reduction of lipogenesis, decreased waist circumference, insulin resistance and dyslipidemia), research in the modulation of the ECS has continued.
Although CB1 receptors seem to be the main target for new therapeutic interventions, CB2 receptors are also involved in several mechanisms and, since they are present in immune cells and are, apparently, involved in modulating immune responses, they are extremely important and may be seen as a therapeutic target as well. Several line of evidences have been clearly suggesting that cannabinoids and their endogenous and synthetic analogs can promote important cardiac effects, which includes hypotension and cardiodepression. The actions seem to be mediated by complex mechanisms, including both direct and indirect effects both on the vasculature and on the myocardium. Furthermore, the ECS, including endocannabinoids and cannabinoid receptors, have been implicated in the myocardial and cerebral ischemia/reperfusion, in hypotensive state associated with hemorrhagic, endotoxic and cardiogenic shock, and in advanced liver cirrhosis. There is also promising evidences hypothesizing a key role for the endocannabinergic system in the cardiovascular regulation in hypertension, as well as a beneficial action on atherosclerotic plaque.
Resuming, cannabinoids are able to modulate a countless number of physiologic functions, demonstration a pleiotropic protective action on the cardiovascular physiology [Figure 1] and therefore, endocannabinoid system is a potential target for the treatment of several diseases and the research about this subject still have a long way to go.
Figure 1.
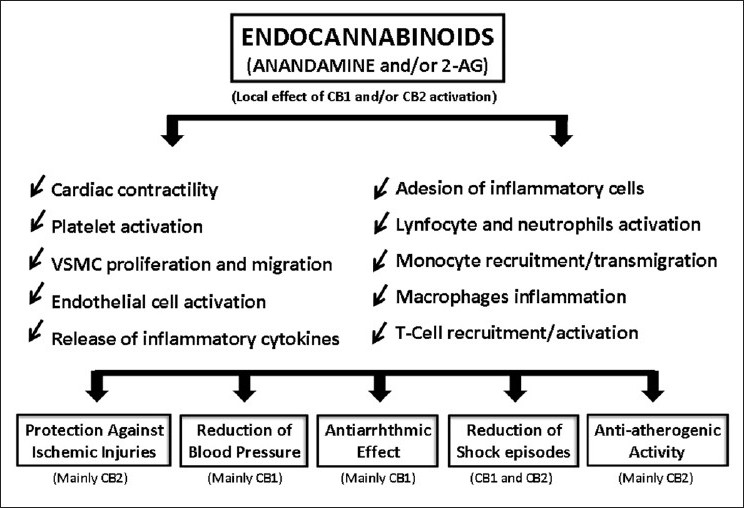
Endocannabinoids exhibit pleiotropic effects on cardiovascular physiology, demonstrating protective activity on several tissues, including the cardiac and the vascular, together with positive impact on various other cells that contribute to cardiovascular/atherosclerotic pathologies, such as the monocytes, macrophages, lymfocyttes, leucocytes, neutrophils and other inflammatory cells
Conclusions
The continued approach of biophysics and molecular characterization of ligands for the cannabinoid receptor will contribute decisively to the success of cross-level research of ECS. Those advances will be pivotal for the development and definition of the profile of new chemical entities as therapeutic endocannabinoid modulators. They may also facilitate the identification of new dynamics of the ECS to be used as predictive and/or diagnostic orientation biomarkers for the patients, as well as therapeutic based on ECS pharmacomodulation.
The therapeutic approach of cardiovascular system starting from the modulation of ECS appears to be a promising and multidisciplinary issue of study that is still in its early stages but that could be a field for better therapeutic intervention in several disorders, including of cardiovascular and cardiometabolic nature.
Authors’ contributions
PC, AMR, FMM, HT and FR drafted the manuscript. HT and FR critically reviewed the manuscript. All authors read and approved the final manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Irving AJ, Rae MG, Coutts AA. Cannabinoids on the brain. ScientificWorldJournal. 2002;2:632–48. doi: 10.1100/tsw.2002.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–48. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 3.Biegon A. Cannabinoids as neuroprotective agents in traumatic brain injury. Curr Pharm Des. 2004;10:2177–83. doi: 10.2174/1381612043384196. [DOI] [PubMed] [Google Scholar]
- 4.Hanus LO. Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med Res Rev. 2009;29:213–71. doi: 10.1002/med.20135. [DOI] [PubMed] [Google Scholar]
- 5.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 6.Patel KD, Davison JS, Pittman QJ, Sharkey KA. Cannabinoid CB(2) receptors in health and disease. Curr Med Chem. 2010;17:1393–410. doi: 10.2174/092986710790980041. [DOI] [PubMed] [Google Scholar]
- 7.van Thuijl H, Kola B, Korbonits M. Appetite and metabolic effects of ghrelin and cannabinoids: involvement of AMP-activated protein kinase. Vitam Horm. 2008;77:121–48. doi: 10.1016/S0083-6729(06)77006-6. [DOI] [PubMed] [Google Scholar]
- 8.Kirkham TC. Cannabinoids and appetite: food craving and food pleasure. Int Rev Psychiatry. 2009;21:163–71. doi: 10.1080/09540260902782810. [DOI] [PubMed] [Google Scholar]
- 9.Butler H, Korbonits M. Cannabinoids for clinicians: the rise and fall of the cannabinoid antagonists. Eur J Endocrinol. 2009;161:655–62. doi: 10.1530/EJE-09-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong TM, Guan XM, Marsh DJ, Shen CP, Stribling DS, Rosko KM, et al. Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1S,2S)-3-(4-chlorophenyl)-2-(3-cyanophenyl)- 1-methylpropyl]-2-methyl-2-[[5-(trifluoromethyl)pyridin-2- yl]oxy]propanamide (MK-0364), in rodents. J Pharmacol Exp Ther. 2007;321:1013–22. doi: 10.1124/jpet.106.118737. [DOI] [PubMed] [Google Scholar]
- 11.Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, et al. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 2008;7:68–78. doi: 10.1016/j.cmet.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–27. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Durst R, Lotan C. The potential for clinical use of cannabinoids in treatment of cardiovascular diseases. Cardiovasc Ther. 2011;29:17–22. doi: 10.1111/j.1755-5922.2010.00233.x. [DOI] [PubMed] [Google Scholar]
- 14.Di Marzo V, Petrosino S. Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol. 2007;18:129–40. doi: 10.1097/MOL.0b013e32803dbdec. [DOI] [PubMed] [Google Scholar]
- 15.Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond) 2006;30(Suppl 1):S13–8. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- 16.Demuth DG, Molleman A. Cannabinoid signaling. Life Sci. 2006;78:549–63. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 17.Oz M. Receptor-independent actions of cannabinoids on cell membranes: focus on endocannabinoids. Pharmacol Ther. 2006;111:114–44. doi: 10.1016/j.pharmthera.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54:1–7. doi: 10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45:405–46. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Matias I, Di Marzo V. Endocannabinoid synthesis and degradation, and their regulation in the framework of energy balance. J Endocrinol Invest. 2006;29(3 Suppl):15–26. [PubMed] [Google Scholar]
- 21.Ho WS, Randall MD. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br J Pharmacol. 2007;150:641–51. doi: 10.1038/sj.bjp.0707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bisogno T. Endogenous cannabinoids: structure and metabolism. J Neuroendocrinol. 2008;20(Suppl 1):1–9. doi: 10.1111/j.1365-2826.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- 23.Mechoulam R, Fride E, Ben-Shabat S, Meiri U, Horowitz M. Carbachol, an acetylcholine receptor agonist, enhances production in rat aorta of 2-arachidonoyl glycerol, a hypotensive endocannabinoid. Eur J Pharmacol. 1998;362:R1–3. doi: 10.1016/s0014-2999(98)00777-8. [DOI] [PubMed] [Google Scholar]
- 24.Kanakis C, Pouget JM, Rosen KM. The effects of 9-THC (cannabis) on cardiac performance with or without beta blockade. Circulation. 1976;53:703–9. doi: 10.1161/01.cir.53.4.703. [DOI] [PubMed] [Google Scholar]
- 25.Rosenkrantz H, Braude M. Acute, Subacute and 23-day chronic marihuana inhalation toxicities in the rat. Toxicol Appl Pharmacol. 1974;28:428–41. doi: 10.1016/0041-008x(74)90228-2. [DOI] [PubMed] [Google Scholar]
- 26.Vollmer RR, Cavero I, Ertel RJ, Solomon TA, Buckley JP. Role of the central autonomic nervous system in the hypotension and bradycardia induced by (-)-delta 9-trans-tetra-hydrocannabinol. J Pharmacol. 1974;26:186–92. doi: 10.1111/j.2042-7158.1974.tb09252.x. [DOI] [PubMed] [Google Scholar]
- 27.Archer RA. The cannabinoids: therapeutic potentials. Annu Rep Med Chem. 1974;9:253–9. doi: 10.1016/s0065-7743(08)61448-7. [DOI] [PubMed] [Google Scholar]
- 28.Adams MD, Earnhardt JT, Martin BR, Harris LS, Dewey WL, Razdan RK. A cannabinoid with cardiovascular activity but no overt behavioral effects. Experientia. 1977;33:1204–5. doi: 10.1007/BF01922330. [DOI] [PubMed] [Google Scholar]
- 29.Varga K, Lake K, Martin BR, Kunos G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol. 1995;278:279–83. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- 30.Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–41. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner JA, Járai Z, Bátkai S, Kunos G. Hemodynamic effects of cannabinoids: coronary and cerebral vasodilation mediated by cannabinoid CB(1) receptors. Eur J Pharmacol. 2001;423:203–10. doi: 10.1016/s0014-2999(01)01112-8. [DOI] [PubMed] [Google Scholar]
- 32.Stein EA, Fuller SA, Edgemond WS, Campbell WB. Physiological and behavioural effects of the endogenous cannabinoid, arachidonylethanolamide (anandamide), in the rat. Br J Pharmacol. 1996;119:107–14. doi: 10.1111/j.1476-5381.1996.tb15683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997;29:1204–10. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- 34.Carruba MO, Bondiolotti G, Picotti GB, Catteruccia N, Da Prada M. Effects of diethyl ether, halothane, ketamine and urethane on sympathetic activity in the rat. Eur J Pharmacol. 1987;134:15–24. doi: 10.1016/0014-2999(87)90126-9. [DOI] [PubMed] [Google Scholar]
- 35.Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, et al. (R)-Methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–93. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- 36.Kunos G, Járai Z, Bátkai S, Goparaju SK, Ishac EJ, Liu J, et al. Endocannabinoids as cardiovascular modulators. Chem Phys Lipids. 2000;108:159–68. doi: 10.1016/s0009-3084(00)00194-8. [DOI] [PubMed] [Google Scholar]
- 37.Lake KD, Compton DR, Varga K, Martin BR, Kunos G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther. 1997;281:1030–7. [PubMed] [Google Scholar]
- 38.Járai Z, Wagner JA, Goparaju SK, Wang L, Razdan RK, Sugiura T, et al. Cardiovascular effects of 2-arachidonoyl glycerol in anesthetized mice. Hypertension. 2000;35:679–84. doi: 10.1161/01.hyp.35.2.679. [DOI] [PubMed] [Google Scholar]
- 39.Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–8. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- 40.Szabo B, Nordheim U, Niederhoffer N. Effects of cannabinoids on sympathetic and parasympathetic neuroeffector transmission in the rabbit heart. J Pharmacol Exp Ther. 2001;297:819–26. [PubMed] [Google Scholar]
- 41.Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–6. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- 42.Niederhoffer N, Szabo B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J Pharmacol Exp Ther. 2000;294:707–13. [PubMed] [Google Scholar]
- 43.Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023–8. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vidrio H, Sanchez-Salvatori MA, Medina M. Cardiovascular effects of (-)-11-OH-delta 8-tetrahydrocannabinol-dimethylheptyl in rats. J Cardiovasc Pharmacol. 1996;28:332–6. doi: 10.1097/00005344-199608000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Ellis EF, Moore SF, Willoughby KA. Anandamide and delta 9-THC dilation of cerebral arterioles is blocked by indomethacin. Am J Physiol. 1995;269:H1859–64. doi: 10.1152/ajpheart.1995.269.6.H1859. [DOI] [PubMed] [Google Scholar]
- 46.Randall MD, Alexander SP, Bennett T, Boyd EA, Fry JR, Gardiner SM, et al. An endogenous cannabinoid as an endotheliumderived vasorelaxant. Biochem Biophys Res Commun. 1996;229:114–20. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- 47.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–7. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 48.Pratt PF, Hillard CJ, Edgemond WS, Campbell WB. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am J Physiol. 1998;274:H375–81. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- 49.Holland M, John Challiss RA, Standen NB, Boyle JP. Cannabinoid CB1 receptors fail to cause relaxation, but couple via Gi/Go to the inhibition of adenylyl cyclase in carotid artery smooth muscle. Br J Pharmacol. 1999;128:597–604. doi: 10.1038/sj.bjp.0702842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–46. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilfinger TV, Salzet M, Fimiani C, Deutsch DG, Tramu G, Stefano GB. Pharmacological evidence for anandamide amidase in human cardiac and vascular tissues. Int J Cardiol. 1998;64(Suppl 1):S15–22. doi: 10.1016/s0167-5273(98)00031-x. [DOI] [PubMed] [Google Scholar]
- 52.Wagner JA, Varga K, Járai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–34. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- 53.Ford WR, Honan SA, White R, Hiley CR. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilator responses in rat isolated hearts. Br J Pharmacol. 2002;135:1191–8. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 55.De Petrocellis L, Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. 2010;5:103–21. doi: 10.1007/s11481-009-9177-z. [DOI] [PubMed] [Google Scholar]
- 56.Felder CC, Nielsen A, Briley EM, Palkovits M, Priller J, Axelrod J, et al. Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Lett. 1996;393:231–5. doi: 10.1016/0014-5793(96)00891-5. [DOI] [PubMed] [Google Scholar]
- 57.Bonz A, Laser M, Küllmer S, Kniesch S, Babin-Ebell J, Popp V, et al. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol. 2003;41:657–64. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 58.Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci. 1997;61:PL191–7. doi: 10.1016/s0024-3205(97)00690-5. [DOI] [PubMed] [Google Scholar]
- 59.Randall MD, Kendall DA. Involvement of a cannabinoid in endotheliumderived hyperpolarizing factor-mediated coronary vasorelaxation. Eur J Pharmacol. 1997;335:205–9. doi: 10.1016/s0014-2999(97)01237-5. [DOI] [PubMed] [Google Scholar]
- 60.Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–44. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- 61.Lagneux C, Lamontagne D. Involvement of cannabinoids in the cardioprotection induced by lipopolysaccharide. Br J Pharmacol. 2001;132:793–6. doi: 10.1038/sj.bjp.0703902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joyeux M, Arnaud C, Godin-Ribuot D, Demenge P, Lamontagne D, Ribuot C. Endocannabinoids are implicated in the infarct size-reducing effect conferred by heat stress preconditioning in isolated rat hearts. Cardiovasc Res. 2002;55:619–25. doi: 10.1016/s0008-6363(02)00268-7. [DOI] [PubMed] [Google Scholar]
- 63.Bouchard JF, Lepicier P, Lamontagne D. Contribution of endocannabinoids in the endothelial protection afforded by ischemic preconditioning in the isolated rat heart. Life Sci. 2003;72:1859–70. doi: 10.1016/s0024-3205(02)02474-8. [DOI] [PubMed] [Google Scholar]
- 64.Lepicier P, Bouchard JF, Lagneux C, Lamontagne D. Endocannabinoids protect the rat isolated heart against ischaemia. Br J Pharmacol. 2003;139:805–15. doi: 10.1038/sj.bjp.0705313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner JA, Abesser M, Harvey-White J, Ertl G. 2-Arachidonylglycerol acting on CB1 cannabinoid receptors mediates delayed cardioprotection induced by nitric oxide in rat isolated hearts. J Cardiovasc Pharmacol. 2006;47:650–5. doi: 10.1097/01.fjc.0000211752.08949.eb. [DOI] [PubMed] [Google Scholar]
- 66.Lamontagne D, Lepicier P, Lagneux C, Bouchard JF. The endogenous cardiac cannabinoid system: a new protective mechanism against myocardial ischemia. Arch Mal Coeur Vaiss. 2006;99:242–6. [PubMed] [Google Scholar]
- 67.Mukhopadhyay P, Bátkai S, Rajesh M, Czifra N, Harvey-White J, Haskó G, et al. Pharmacological inhibition of cannabinoid receptor-1 protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. 2007;50:528–36. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blázquez C, Casanova ML, Planas A, Gómez Del Pulgar T, Villanueva C, Fernández-Aceñero MJ, et al. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 2003;17:529–31. doi: 10.1096/fj.02-0795fje. [DOI] [PubMed] [Google Scholar]
- 69.Shmist YA, Goncharov I, Eichler M, Shneyvays V, Isaac A, Vogel Z, et al. Delta-9-tetrahydrocannabinol protects cardiac cells from hypoxia via CB2 receptor activation and nitric oxide production. Mol Cell Biochem. 2006;283:75–83. doi: 10.1007/s11010-006-2346-y. [DOI] [PubMed] [Google Scholar]
- 70.Krylatov AV, Ugdyzhekova DS, Bernatskaya NA, Maslov LN, Mekhoulam R, Pertwee RG, et al. Activation of type II cannabinoid receptors improves myocardial tolerance to arrhythmogenic effects of coronary occlusion and reperfusion. Bull Exp Biol Med. 2001;131:523–5. doi: 10.1023/a:1012381914518. [DOI] [PubMed] [Google Scholar]
- 71.Di Filippo C, Rossi F, Rossi S, D’Amico M. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: involvement of cytokine/chemokines and PMN. J Leukoc Biol. 2004;75:453–9. doi: 10.1189/jlb.0703303. [DOI] [PubMed] [Google Scholar]
- 72.Wagner JA, Hu K, Karcher J, Bauersachs J, Schäfer A, Laser M, et al. CB(1) cannabinoid receptor antagonism promotes remodelling and cannabinoid treatment prevents endothelial dysfunction and hypotension in rats with myocardial infarction. Br J Pharmacol. 2003;138:1251–8. doi: 10.1038/sj.bjp.0705156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–11. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- 75.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signalling. Physiol Rev. 2003;83:1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 76.Hampson AJ, Grimaldi M, Lolic M, Wink D, Rosenthal R, Axelrod J. Neuroprotective antioxidants from marijuana. Ann NY Acad Sci. 2000;899:274–82. [PubMed] [Google Scholar]
- 77.Nagayama T, Sinor AD, Simon RP, Chen J, Graham SH, Jin K, et al. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci. 1999;19:2987–95. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mauler F, Mittendorf J, Horvath E, De Vry J. Characterization of the diarylether sulfonylester (-)-(R)-3-(2-hydroxymethylindanyl-4-oxy)phenyl-4,4,4-trifluoro-1-sulfonate (BAY 38-7271) as a potent cannabinoid receptor agonist with neuroprotective properties. J Pharml Exp Ther. 2002;302:359–68. doi: 10.1124/jpet.302.1.359. [DOI] [PubMed] [Google Scholar]
- 79.Leker RR, Gai N, Mechoulam R, Ovadia H. Drug-induced hypothermia reduces ischemic damage: effects of the cannabinoid HU-210. Stroke. 2003;34:2000–6. doi: 10.1161/01.STR.0000079817.68944.1E. [DOI] [PubMed] [Google Scholar]
- 80.Hayakawa K, Mishima K, Abe K, Hasebe N, Takamatsu F, Yasuda H, et al. Cannabidiol prevents infarction via the non-CB1 cannabinoid receptor mechanism. Neuroreport. 2004;15:2381–5. doi: 10.1097/00001756-200410250-00016. [DOI] [PubMed] [Google Scholar]
- 81.Louw DF, Yang FW, Sutherland GR. The effect of delta-9-tetrahydrocannabinol on forebrain ischemia in rat. Brain Res. 2000;857:183–7. doi: 10.1016/s0006-8993(99)02422-1. [DOI] [PubMed] [Google Scholar]
- 82.Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA. Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci. 2002;22:9771–5. doi: 10.1523/JNEUROSCI.22-22-09771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muthian S, Rademacher DJ, Roelke CT, Gross GJ, Hillard CJ. Anandamide content is increased and CB1 cannabinoid receptor blockade is protective during transient, focal cerebral ischemia. Neuroscience. 2004;129:743–50. doi: 10.1016/j.neuroscience.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 84.Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, Tuma RF. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab. 2007;27:1387–96. doi: 10.1038/sj.jcbfm.9600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett. 2007;412:114–7. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 86.Ashton JC, Smith PF. Cannabinoids and cardiovascular disease: the outlook for clinical treatments. Curr Vasc Pharmacol. 2007;5:175–85. doi: 10.2174/157016107781024109. [DOI] [PubMed] [Google Scholar]
- 87.Ribuot C, Lamontagne D, Godin-Ribuot D. Cardiac and vascular effects of cannabinoids: toward a therapeutic use? Ann Cardiol Angeiol (Paris) 2005;54:89–96. doi: 10.1016/j.ancard.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 88.Moezi L, Gaskari SA, Lee SS. Endocannabinoids and liver disease. V. endocannabinoids as mediators of vascular and cardiac abnormalities in cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G649–53. doi: 10.1152/ajpgi.90352.2008. [DOI] [PubMed] [Google Scholar]
- 89.Sarzani R. Endocannabinoids, blood pressure and the human heart. J Neuroendocrinol. 2008;20(Suppl 1):58–62. doi: 10.1111/j.1365-2826.2008.01677.x. [DOI] [PubMed] [Google Scholar]
- 90.Wagner JA, Varga K, Ellis EF, Rzigalinski BA, Martin BR, Kunos G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–21. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- 91.Wagner JA, Hu K, Bauersachs J, Karcher J, Wiesler M, Goparaju SK, et al. Endogenous cannabinoids mediate hypotension after experimental myocardial infarction. J Am Coll Cardiol. 2001;38:2048–54. doi: 10.1016/s0735-1097(01)01671-0. [DOI] [PubMed] [Google Scholar]
- 92.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Archer RA. The cannabinoids: therapeutic potentials. Annu Rev Med Chem. 1974;9:253–9. doi: 10.1016/s0065-7743(08)61448-7. [DOI] [PubMed] [Google Scholar]
- 94.Birmingham MK. Reduction by 9-tetrahydrocannabinol in the blood pressure of hypertensive rats bearing regenerated adrenal glands. Br J Pharmacol. 1973;48:169–71. doi: 10.1111/j.1476-5381.1973.tb08236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pacher P, Bátkai S, Kunos G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology. 2005;48:1130–8. doi: 10.1016/j.neuropharm.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crawford WJ, Merritt JC. Effects of tetrahydrocannabinol on arterial and intraocular hypertension. Int J Clin Pharmacol Biopharm. 1979;17:191–6. [PubMed] [Google Scholar]
- 97.Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, et al. Endocannabinoids acting at CB1 receptors regulates cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steinberg B, Cannon C. Cannabinoid-1 Receptor Blockade in Cardiometaolic Risk Reduction; Safety, Tolerability, and Therapeutic Potential. Am J Cardiol. 2007;100:27P–32. doi: 10.1016/j.amjcard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 99.Bátkai S, Rajesh M, Mukhopadhyay P, Haskó G, Liaudet L, Cravatt BF, et al. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol. 2007;293:H909–18. doi: 10.1152/ajpheart.00373.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mach F, Steffens S. The Role of the Endocannabinoid System in Atherosclerosis. J Neuroendocrinol. 2008;20(Suppl 1):53–7. doi: 10.1111/j.1365-2826.2008.01685.x. [DOI] [PubMed] [Google Scholar]
- 101.Leite CE, Mocelin CA, Petersen GO, Leal MB, Thiesen FV. Rimonabant: an antagonist drug of the endocannabinoid system for the treatment of obesity. Pharmacol Rep. 2009;61:217–24. doi: 10.1016/s1734-1140(09)70025-8. [DOI] [PubMed] [Google Scholar]
- 102.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–97. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 103.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–96. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 104.Braunersreuther V, Mach F. Leukocyte recruitment in atherosclerosis: potential targets for therapeutic approaches? Cell Mol Life Sci. 2006;63:2079–88. doi: 10.1007/s00018-006-6127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miller AM, Stella N. CB2 receptor-mediated migration of immune cells: it can go either way. Br J Pharmacol. 2008;153:299–308. doi: 10.1038/sj.bjp.0707523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–34. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]


