Abstract
Objectives:
The present investigation was aimed to study the antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus peel and seed powder (AEPP and AESP) in streptozotocin (STZ)-induced diabetic rats.
Materials and Methods:
Acute toxicity of AEPP and AESP was studied in rats at 2000 mg/kg dose and diabetes was induced in rats by administration of STZ (60 mg/kg, i.p.). After 14 days of blood glucose stabilization, diabetic rats received AEPP, AESP, and glibenclamide up to 28 days. The blood samples were collected on day 28 to estimate the hemoglobin (Hb), glycosylated hemoglobin (HbA1c), serum glutamate-pyruvate transferase (SGPT), total protein (TP), and lipid profile levels.
Results:
In acute toxicity study, AESP and AESP did not show any toxicity or death up to a dose of 2000 mg/kg. Therefore, to assess the antidiabetic action, one by fifth and one by tenth dose of both powders were selected. Administration of AEPP and AESP at 100 and 200 mg/kg dose in diabetic rats showed significant (P < 0.001) reduction in blood glucose level and increase in body weight than diabetic control rats. A significant (P < 0.001) increased level of Hb, TP, and decreased level of HbA1c, SGPT were observed after the treatment of both doses of AEPP and AESP. Also, elevated lipid profile levels returned to near normal in diabetic rats after the administration of AEPP and AESP, 100 and 200 mg/kg dose, compared to diabetic control rats.
Conclusion:
The present study results, first time, support the antidiabetic and antihyperlipidemic potential of A. esculentus peel and seed powder in diabetic rats.
Keywords: Abelmoschus esculentus, antidiabetic, antihyperlipidemic, streptozotocin
Diabetes mellitus is a progressive metabolic disease and it has affected considerable percentage of population throughout the world. Epidemiologic data indicated that 2.8% of the world's population was diabetic in the year 2000 and it may progress to 4.4% of the world's population by 2030. It affects all age groups of people and ethnic groups.[1] In India, statistical analysis revealed that the number of diabetics will rise to 57 million in the year of 2025 compared to 15 million diabetics in 1995.[2] The pathogenesis of both type 1 and type 2 diabetes are different, but hyperglycemia and its associated complications are common in both conditions.[3] In diabetes, elevated level of lipid profile are commonly reported that increases the cardiovascular complications.[4] Presently, diabetes is managed or controlled using pharmacologic agents and nonpharmacologic methods, such as diet and exercise. However, all the pharmacologic agents are not devoid of adverse effects and it triggers scientific community to search for new drugs from all possible sources, including traditional medicines, which might be less toxic when compared to the available drug therapy.[5] Moreover, diabetic complications lead to morbidity and mortality due to multiple defects in its pathophysiology.[6] Presently, research is focused on traditional medicinal plants and herbs, which are used as potential alternative source to treat diabetes with its multiple pharmacologic actions.[7] Several phytoconstituents possessing antidiabetic activity were isolated and studied from many medicinal plants, but still scientists continue their research on medicinal plants to bring good antidiabetic lead or drugs to the healthcare community.
Abelmoschus esculentus (L.) Moench., synonym of okra, known in many English-speaking countries as lady's fingers or gumbo is a flowering plant in the mallow family.[8] It is valued for its edible green seed pods. It is an important vegetable and widely distributed from Africa to Asia, Southern Europe and America.[9] In Asia, okra is typically prepared as traditional medicine as a dietary meal in the treatment of gastric irritations.[10] The plant has a wide range of medicinal value and has been used to control various diseases and disorders. The fiber in okra helps to stabilize blood sugar by regulating the rate at which sugar is absorbed from the intestinal tract. It is a good vegetable for those feeling weak, exhausted, and suffering from depression and it is also used in ulcers, lung inflammation, sore throat as well as irritable bowel. Okra is good for asthma patients and it also normalizes blood sugar and cholesterol levels.[11,12] Previous studies reported that okra polysaccharide possesses anticomplementary and hypoglycemic activity in normal mice.[13] Also, okra polysaccharide lowers cholesterol level in blood and may prevent cancer by its ability to bind bile acids.[10,14] Based on the above scientific data, current literature research revealed that lowering of blood glucose and cholesterol levels by okra in diabetic condition is scientifically not yet documented. Therefore, the present study was aimed to investigate antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. peel and seed powders in streptozotocin-induced diabetic rats.
Materials and Methods
Plant materials
A. esculentus (L.) Moench was collected from the local farm in Coimbatore, Tamil Nadu, India. The plant material was identified and authenticated by Botanical Survey of India (BSI/SC/5/23/2010-11/Tech.1907), Coimbatore, and the certificate was deposited at our laboratory. The peel and seed was separated and dried under shade. The above plant materials were made as fine powder using mixer and it was stored in an airtight container up to the completion of the study.
Chemicals
Streptozotocin and all other chemicals used in this study are analytical grade and were procured from Himedia Laboratories, Mumbai, India. For the estimation of biochemical parameters, kits were procured from Primal Healthcare Limited, Lab Diagnostic Division, Mumbai, India. The glibenclamide received as gift sample from Orchid Chemicals and Pharmaceuticals Ltd, Chennai, India.
Experimental animals
Male Wistar albino rats (150–200 g) were used to assess antidiabetic activity. Female Wistar rats (150–180 g) were used for the acute toxicity study. The animals were kept and maintained under standard laboratory conditions [temperature (22°C ± 2°C) and humidity (45°C ± 5°C)] with 12:12 h day:night cycle. The animals were fed with standard laboratory diet and allowed to drink water ad libitum. Studies were carried out in accordance with institutional ethical guidelines for the care of laboratory animals of KMCH College of Pharmacy, Coimbatore, India, after the approval (KMCRET/Ph.D/02/2010).
Acute toxicity study
Acute oral toxicity of A. esculentus (L.) Moench. peel and seed powders were determined as per Organization for Economic Cooperation and Development guidelines 423.[15] After the oral administration of AEPP and AESP, animals are observed individually at least once during the first 30 min, periodically during the first 24 h, with special attention given during the first 4 h, and 14 days regularly observed for toxicity determination of AEPP and AESP.
Induction of diabetes
Diabetes was induced in overnight fasted rats by intraperitoneal injection of STZ at a dose of 60 mg/kg body weight in 0.1 M cold citrate buffer (pH 4.5). To prevent the STZ-induced hypoglycemia, rats received 10% dextrose solution after 6 h of STZ administration for next 24 h. Induction of diabetes was verified after 72 h by measuring blood glucose level with strips using glucometer (Accu-Chek® Active, Roche Diagnostic Corporation, Mannheim, Germany) and the animals were allowed 14 days for the stabilization of blood glucose level.[16] On day 14, animals having a blood glucose level higher than 250 mg/dL were considered diabetic and included in the experiments.
Experimental design for antidiabetic activity
Animals were divided into 7 groups and each group consisted of 6 rats. The grouping details are follows:
Group I served as normal control received 0.2% carboxy methyl cellulose (CMC) (5 mL/kg).
Group II served as diabetic control received 0.2% CMC (5 mL/kg).
Groups III and IV served as AEPP 100 and 200 mg/kg treated diabetic rats, respectively.
Groups V and VI served as AESP 100 and 200 mg/kg treated diabetic rats, respectively.
Group VII served as standard drug, glibenclamide (5 mg/kg) treated diabetic rats.
The vehicle (0.2% CMC), AEPP, AESP, and glibenclamide were administered orally to the respective group animals for 28 days. AEPP and AESP were triturated with distilled water and glibenclamide with vehicle just before the oral administration. The fasting body weight, blood glucose level (Accu-Chek® Active, Roche Diagnostic Corporation, Mannheim, Germany) was estimated at the end of every week.[16] On 28th day, overnight fasted animals received respective treatment and after 1 h all animals were anesthetized with ketamine (100 mg/kg, i.p.); blood sample was collected through retro-orbital plexus puncture and stored in containers with and without disodium ethylene diamine tetra acetate (EDTA) for the biochemical parameters estimation.
Biochemical parameters estimation
The EDTA blood was used to estimate the Hb and HbA1c levels.[17,18] The serum was separated and its high density lipoprotein (HDL),[19] total cholesterol (TC),[20] triglycerides (TG),[21] glutamate-pyruvate transferase,[22] and total protein[23] were estimated by commercially available kits using semi-autoanalyzer (Photometer 5010V5+, Germany). The low density lipoprotein (LDL) and very low density lipoprotein (VLDL) levels was calculated by the following equation:[24]
VLDL = Triglycerides/5
LDL = TC – (HDL + VLDL)
Statistical analysis
All the data are expressed as mean ± SEM were evaluated by one-way analysis of variance (ANOVA), followed by Dunnett's test for multiple comparisons using prism Graphpad version 5.0 and values of P < 0.05 were considered as statistically significant.
Results and Discussion
The oral administration of AEPP and AESP did not show any toxicity signs and mortality up to 14 days during acute toxicity study. Both the powders were found to be safe at a dose level of 2000 mg/kg body weight. Therefore, to study the antidiabetic potential of AEPP and AESP, the dose of 100 and 200 mg/kg was selected. In animals, diabetes was induced after the administration of STZ due to its cytotoxicity on pancreatic islet β-cells.[25] The selective toxicity on β-cell, by alkylation of DNA after the STZ injection, produces reduction in insulin level, which leads to alteration of glucose metabolism and utilization thereby causing hyperglycemia.[26] Moreover, intracellular metabolism of STZ produces nitric oxide (NO) free radical and it further initiates the alkylation of β-cells DNA strands and its breaks.[27] In our study, administration of AEPP (100 and 200 mg/kg) and AESP (100 and 200 mg/kg) decreased elevated blood glucose levels significantly (P < 0.001) from first to fourth week compared to diabetic control rats. The AEPP and AESP at a dose 200 mg/kg showed significantly (P < 0.001) more blood glucose reduction than its 100 mg/kg dose. Also, treatment of both the doses of AESP significantly (P < 0.001) produced greater blood glucose reduction when compared to AEPP 100 and 200 mg/kg dose [Table 1]. There are many reports available to support the multiple mechanisms of antidiabetic plants to exert their blood glucose lowering effect, such as inhibition of carbohydrate metabolizing enzymes, enhancement of insulin sensitivity, regeneration of damaged pancreatic islet β-cells, and enhancement of insulin secretion and release.[28] The AEPP and AESP may exert blood glucose lowering activity possibly above mechanism(s) and the antidiabetic activity of both powders was comparable to that of glibenclamide. A significant (P < 0.001) body weight loss was observed after the administration of STZ and it may be due to the degradation of structural proteins.[29] In our study, AEPP and ASEP treated animals showed significant (P < 0.05, P < 0.01, P < 0.001) increase in body weight compared to diabetic control [Figure 1]. This action may be due to the preventive effect of AEPP and AESP on structural protein degradation.
Table 1.
Effect of AEPP and AESP on blood glucose level in STZ-induced diabetic rats
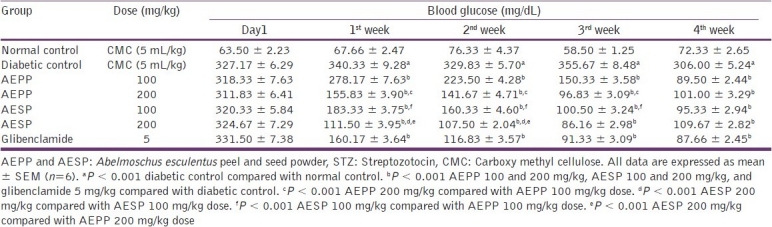
Figure 1.
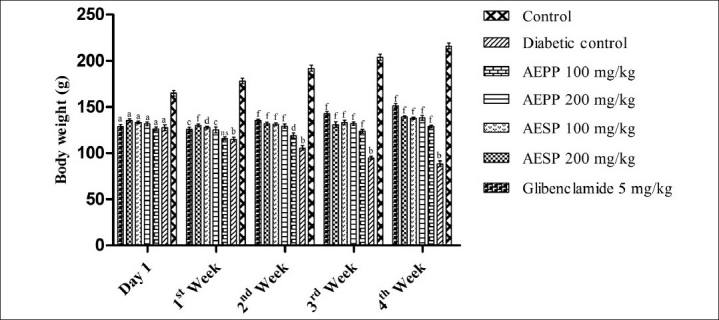
Effect of AEPP and AESP on body weight in STZ-induced diabetic rats.(All data are expressed as mean ± SEM (n=6). aP < 0.001 All groups compared with normal control. bP < 0.001 Diabetic control compared to normal control. cP < 0.05 AEPP 200 mg/kg and glibenclamide 5 mg/kg compared to diabetic control. dP < 0.01 AEPP and AESP 100 mg/kg compared to diabetic control. fP < 0.001 AEPP, AESP (100 and 200 mg/kg) and glibenclamide 5 mg/kg compared to diabetic control)
Glycosylated hemoglobin is formed through the nonenzymatic binding of circulating glucose to hemoglobin. Higher levels of glucose in the blood contribute to more binding and consequent increased levels of glycosylated hemoglobin.[30] In diabetic condition, decrease in protein synthesis in all tissues and thus the synthesis of hemoglobin is also reduced due to relative deficiency of insulin.[31] HbA1c concentration is associated with diabetic micro, macrovascular complications and risk of death.[30,32] In the present study, increased level of HbA1c and decreased level of Hb and total protein was observed in diabetic control rats than normal control rats. Total protein level was significantly (P < 0.001) increased after the administration of both doses of AEPP and AESP compared with diabetic control rats. Also, administration of AEPP and AESP significantly (P < 0.001) reduced elevated HbA1c levels and increased the Hb level in diabetic rats [Table 2]. The above actions indicate that AEPP and AESP have potential to prevent the diabetic associated complications.
Table 2.
Effect of AEPP and AESP on Hb, HbA1c, total protein, and SGPT in STZ-induced diabetic rats
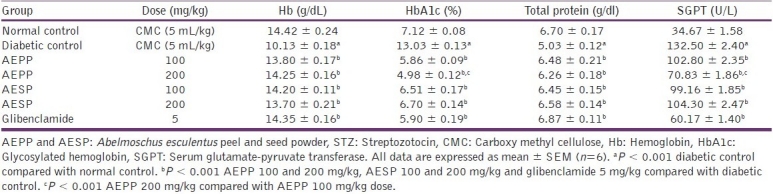
The liver damage produced by administration of STZ leads to leakage of SGPT from liver cytosol into the blood stream which in turn increases its level in serum.[29,33] In the present study, the significant (P < 0.001) increase in serum SGPT was observed in STZ-induced diabetic rats compared to control rats [Table 2]. It represents that liver damage occurred in diabetic rats and oral administration of AEPP and AESP significantly (P < 0.001) reduced the elevated level of SGPT, which supports its protective potential on liver tissue.
Hyperlipidemia was reported as common in adults with diabetes and it is characterized most often by increased triglyceride and reduced HDL cholesterol levels. This is generally observed in both type 1 and type 2 diabetes, representing the defect of insulin action in each, either due to inadequate secretion or resistance. It is well known that hyperlipidemia is accepted as an independent risk factor for cardiovascular disorders (CVD) in diabetic patients.[34] Diabetes induced by STZ in rats significantly (P < 0.001) elevated the TC, TG, LDL, VLDL levels and decreased the HDL levels compared with normal control rats. In the present study, administration of AEPP and AESP at 100 and 200 mg/kg doses to the diabetic rats showed significant (P < 0.001, P < 0.05) reduction in TC, TG, LDL, and VLDL levels than diabetic control rats. The HDL level was increased significantly (P < 0.001) after the treatment of both the dose of AEPP and AESP in diabetic rats compared with diabetic control rats [Table 3]. These actions of AEPP and AESP directly support its ability to reduce hyperlipidemia in diabetes and hence, it may prevent CVD related to diabetes.
Table 3.
Effect of AEPP and AESP on lipid profiles in STZ-induced diabetic rats

Conclusion
The present study, for the first time, confirms that A. esculentus peel and seed possess blood glucose normalization and lipid profiles lowering action in diabetic condition.
Acknowledgments
I thank to Mohammad Akbar, Department of Pharmacology, KMCH College of Pharmacy, Coimbatore for their constant support throughout this research. I thank management, Dr. A. Rajaseakaran, Principal and Mr. K. T. Mani senthil kumar, Head, Department of Pharmacology, KMCH College of Pharmacy, Coimbatore for their given support during the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Xing XH, Zhang ZM, Hu XZ, Wu RQ, Xu C. Antidiabetic effects of Artemisia sphaerocephala Krasch.gum, a novel food additive in China, on streptozotocin-induced type 2 diabetic rats. J Ethnopharmacol. 2009;125:410–6. doi: 10.1016/j.jep.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Shikarwar MS, Patil MB. Antidiabetic activity of Crateva nurvala stem bark extracts in alloxan-induced diabetic rats. J Pharm Bioallied Sci. 2010;2:18–21. doi: 10.4103/0975-7406.62700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Li WL, Wu JL, Ren BR, Zhang HQ. Hypoglycemic effects of a sesquiterpene glycoside isolated from leaves of loquat (Eriobotrya japonica (Thunb.) Lindl.) Phytomedicine. 2008;15:98–102. doi: 10.1016/j.phymed.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Yang K, Jeong SC, Lee HJ, Sohn DH, Song CH. Antidiabetic and hypolipidemic effects of Collybia confluens mycelia produced by submerged culture in streptozotocin diabetic rats. Arch Pharm Res. 2006;29:73–9. doi: 10.1007/BF02977472. [DOI] [PubMed] [Google Scholar]
- 5.Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62:598–605. doi: 10.1016/j.biopha.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Ivorra MD, Payá M, Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol. 1989;27:243–75. doi: 10.1016/0378-8741(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 7.Chandramohan G, Ignacimuthu S, Pugalendi KV. A novel compound from Casearia esculenta (Roxb.) root and its effect on carbohydrate metabolism in streptozotocin-diabetic rats. Eur J Pharmacol. 2008;590:437–43. doi: 10.1016/j.ejphar.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 8.Chopra RN, Nayar SL, Chopra IC. New Delhi: Council of Industrial and Scientific Research; 1956. Glossary of Indian medicinal Plants; pp. 1–133. [Google Scholar]
- 9.Khomsug P, Thongjaroenbuangam W, Pakdeenarong N, Suttajit M, Chantiratikul P. Antioxidative activities and phenolic content of extracts from Okra (Abelmoschus esculentus L.) Res J Biol Sci. 2010;5:310–13. [Google Scholar]
- 10.Lengsfeld C, Titgemeyer F, Faller G, Hensel A. Glycosylated compounds from okra inhibit adhesion of Helicobacter pyroli to human gastric mucosa. J Agric Food Chem. 2004;52:1495–503. doi: 10.1021/jf030666n. [DOI] [PubMed] [Google Scholar]
- 11.Sengkhamparn N, Verhoef R, Schols HA, Sajjaanantakul T, Voragen AG. Characterisation of cell wall polysaccharides from okra (Abelmoschus esculentus (L.) Moench) Carbohydr Res. 2009;344:1824–32. doi: 10.1016/j.carres.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Facts about okra-Health and nutritional benefits of okra [Internet] [Last accessed on 2009 Aug 19]. Available from: http://www.vegrecipesofindia.com/facts-about-okrahealth-nutritional-benefits-of-okra/
- 13.Tomoda M, Shimizu N, Gonda R, Kanari M, Yamada H, Hikino H. Anticomplementary and hypoglycemic activity of okra and hibiscus mucilage. Carbohydr Res. 1989;190:323–8. doi: 10.1016/0008-6215(89)84136-9. [DOI] [PubMed] [Google Scholar]
- 14.Kahlon TS, Chapman MH, Smith GE. In vitro binding of bile acids by okra, beets, asparagus, eggplant, turnips, green beans, carrot and cauliflower. Food Chem. 2007;103:676–80. [Google Scholar]
- 15.OECD Guidelines for Testing of Chemicals 423. Acute oral toxicity - acute toxic class method. 2001 Dec [Google Scholar]
- 16.Ramachandran S, Asokkumar K, Uma Maheswari M, Ravi TK, Sivashanmugam AT, Saravanan S, et al. Investigation of antidiabetic, antihyperlipidemic, and in vivo antioxidant properties of Sphaeranthus indicus Linn.in type 1 diabetic rats: An identification of possible biomarkers. Evid Based Complement Alternat Med. 2011 doi: 10.1155/2011/571721. 2011 pii: 571721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wintrobe M M. 4th ed. Philadelphia: Lea and Febiger; 1965. Clinical hematology. [Google Scholar]
- 18.Jeppsson JO, Kobold U, Barr J, Finke A, Hoelzel W, Hoshino T, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002;40:78–89. doi: 10.1515/CCLM.2002.016. [DOI] [PubMed] [Google Scholar]
- 19.Lopes-Virella MF, Stone P, Ellis S, Colwell JA. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977;23:882–4. [PubMed] [Google Scholar]
- 20.Deeg R, Ziegenhorn J. Kinetic enzymatic method for automated determination of total cholesterol in serum. Clin Chem. 1983;29:1798–802. [PubMed] [Google Scholar]
- 21.Cole TG, Klotzsch SG, McNamara J. Measurement of triglyceride concentration. In: Rifai N, Warnick GR, Dominiczak MH, editors. Handbook of lipoprotein testing. Washington: AACC Press; 1997. pp. 115–126. [Google Scholar]
- 22.Thomas L. Alanine aminotransferase (ALT), Aspartate aminotransferase (AST) In: Thomas L, editor. Clinical laboratory diagnostics. 1st ed. Frankfurt: TH-Books Verlagsgesellchaft; 1998. pp. 55–65. [Google Scholar]
- 23.Weichselbaum TE. An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. Am J Clin Pathol. 1946;10:40–9. [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of VLDL and LDL-cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Kumar GP, Arulselvan P, Kumar DS, Subramanian SP. Anti-diabetic activity of fruits of Terminalia chebula on streptozotocin induced diabetic rats. J of Health Sci. 2006;52:283–91. [Google Scholar]
- 26.Arumugam S, Kavimani S, Kadalmani B, Ahmed AB, Akbarsha MA, Rao MV. Antidiabetic activity of leaf and callus extracts of Aegle marmelos in rabbit. ScienceAsia. 2008;34:317–21. [Google Scholar]
- 27.Rodríguez T, Alvarez B, Busquets S, Carbó N, López-Soriano FJ, Argilés JM. The increased skeletal muscle protein turnover of the streptozotocin diabetic rats is associated with high concentrations of branched-chain amino acids. Biochem Mol Med. 1997;61:87–94. doi: 10.1006/bmme.1997.2585. [DOI] [PubMed] [Google Scholar]
- 28.Møller N, Nair KS. Diabetes and protein metabolism. Diabetes. 2008;57:3–4. doi: 10.2337/db07-1581. [DOI] [PubMed] [Google Scholar]
- 29.Shokeen P, Anand P, Murali YK, Tandon V. Antidiabetic activity of 50% ethanolic extract of Ricinus communis and its purified fractions. Food Chem Toxicol. 2008;46:3458–66. doi: 10.1016/j.fct.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23:187–91. doi: 10.2337/diacare.23.2.187. [DOI] [PubMed] [Google Scholar]
- 31.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and haemoglobin A1C in diabetes mellitus. N Engl J Med. 1976;295:417–20. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 32.Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European prospective investigation of cancer and nutrition (EPIC-Norfolk) BMJ. 2001;322:15–8. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhu KS, Lobo R, Shirwaikar A. Antidiabetic properties of the alcoholic extract of Sphaeranthus indicus in streptozotocin-nicotinamide induced diabetic rats. J Pharm Pharmacol. 2008;60:909–16. doi: 10.1211/jpp.60.7.0013. [DOI] [PubMed] [Google Scholar]
- 34.Garg A, Grundy SM. Management of dyslipidemia in NIDDM. Diabetes Care. 1990;13:15369. doi: 10.2337/diacare.13.2.153. [DOI] [PubMed] [Google Scholar]


