Abstract
Objective:
to study the probable site of antinociceptive action of SSRI (fluoxetine, escitalopram) and atypical antidepressants (mirtazapine, venlafaxine) and their interaction with morphine and naloxone.
Materials and Methods:
the study was conducted on albino mice (25-35 grams) of either sex. Different doses of morphine (0.5 and 1 mg/kg), fluoxetine (2, 5 and 10 mg/kg), venlafaxine (30, 40 and 50 mg/kg), mirtazapine (3, 5 and 7 mg/kg) and escitalopram (2.5, 5 and 10 mg/kg) were administered subcutaneously to obtain their subanalgesic doses using tail flick analgesiometer. Tail flick latencies were obtained at 15, 30, 60 and 120 min. after drug administration. Naloxone (1 mg/kg) was administered 10 minutes prior to test drug for testing antagonism.
Observations:
fluoxetine (5 and 10 mg/kg), mirtazapine (5 and 7 mg/kg) and venlafaxine (40 and 50 mg/kg) were found to have antinociceptive activity but not at lower doses. Escitalopram failed to show any antinociceptive activity at any of the doses used. The antinociceptive effect of all the drugs was antagonized by naloxone (1 mg/kg). Further, subanalgesic doses of fluoxetine, mirtazapine and venlafaxine showed analgesic activity with suboptimal dose of morphine (0.5 mg/kg).
Result and conclusion:
fluoxetine, mirtazapine and venlafaxine have antinociceptive activity whereas escitalopram doesn’t; their site of action seems to be the same as that of opioid analgesics (‘mue’ receptors). However, other pathways (cholinergic, histaminic, noradrenergic, GABAergic) may be involved in mediation of their analgesic activity, deserving further elucidation. Results apparently show that these drugs may be useful in the management of pain as monotherapy or in combination with other opioids.
Keywords: Analgesia, antidepressants, opioids, selective serotonin reuptake inhibitor
Pain being an unpleasant sensation, is an ill defined, disabling accompaniment of many medical conditions and is often evoked by an external or internal noxious stimulus, with a major affective component. The principal objective of alleviating pain is to remove the cause of pain, but it is not always possible to do so, hence, analgesics are used for the symptomatic treatment of pain.[1]
Although, patients with chronic pain are being treated with various medications such as tricyclic antidepressants (TCA), non-steroidal anti-inflammatory drugs (NSAIDs), anticonvulsants, and opioids, none has shown outstanding efficacy.
Depression and a feeling of hopelessness are common accompaniments of chronic pain.[2] It has also been proved in about 75% of the studies that some antidepressants are superior to placebo in alleviating pain as they have some intrinsic analgesic activity.[3] A wide range of painful conditions are responsive to antidepressants and they have been used as co-analgesics in various clinical conditions, particularly diabetic neuropathy pain, rheumatoid arthritis and migraine.[4] Antidepressants with noradrenergic reuptake inhibition properties (TCA) have been reported to produce varying degrees of pain relief in several persistent or chronic pain syndromes in humans, namely, diabetic neuropathy,[5] post-herpetic neuralgia,[6] fibromyalgia,[7] and chronic rheumatic pain.[8] Unfortunately, adverse effects are not uncommon during antidepressant use, particularly with TCA.[2,8–10] Yet even after forty years of extensive research on the efficacy of antidepressant drugs in the management of chronic pain, their use has not become common.[9]
In recent times, numerous open and controlled studies have shown antidepressant drugs to have an analgesic activity, especially, selective serotonin reuptake inhibitors (SSRIs), which are effective in mixed and chronic pain.[11] On the other hand, there are some studies that have altogather denied the analgesic role of SSRI.[5,12,13]
Another intriguing aspect is the meager understanding regarding their mechanism of action. The underlying mechanisms for the Antinociceptive activity of these agents probably involve a complex interaction between several neurotransmitter systems and neuroreceptors.[14] There is ample evidence to suggest that the pain inhibitory pathway involves monoamines such as noradrenalin (NA) and 5-hydroxy tryptamine (5-HT),[15] and SSRI, by increasing the serotonin level, may inhibit the release of the neurotransmitter carrying the pain sensation from nerve endings.[16]
Despite such an enormity of literature, it is not yet clear whether these can be used as analgesics, and if so, what could be the underlying mechanism. Therefore, the present study was planned with the aim of: (1) Confirming the analgesic/antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazapine), (2) Studying the interaction of these drugs with morphine, and (3) Delineating their probable site of action.
Materials and Methods
The study was conducted on healthy albino mice (25 – 35 g) of either sex, maintained at an ambient temperature of 25 – 35°C with food and water ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee and was executed according to the guidelines of the committee for the purpose of control and supervision of the experiments on animals (CPCSEA), India.
The animals were divided into 20 groups of six each. Individual test drug was given to find its subanalgesic dose. Mice in groups 1, 2, and 3 received fluoxetine (2, 5, and 10 mg/kg, respectively), groups 4, 5, and 6 received venlafaxine (30, 40, and 50 mg/kg, respectively), group 7, 8, and 9 received mirtazapine (3, 5, and 7 mg/kg, respectively), group 10, 11, and 12 received escitalopram (2.5, 5, and 10 mg/kg, respectively), and group 13 and 14 received morphine (0.5 and 1 mg/kg). Groups 15, 16, and 17 received combined treatment consisting of subanalgesic doses of test drugs with subanalgesic dose of morphine, that is, fluoxetine (2 mg/kg), venlafaxine (30 mg/kg), and mirtazapine (3 mg/kg), with morphine (0.5 mg/kg), respectively. Groups 18, 19, and 20 received fluoxetine (5 mg/kg), venlafaxine (40 mg/kg), and mirtazapine (5 mg/kg) 10 minutes before naloxone (1 mg/kg). Naloxone was given 10 minutes after the test drug. All the drugs were given subcutaneously. In the groups that received combined treatment, both the drugs were administered simultaneously, at different sites.
Determination of the antinociceptive activity
Effect of test drugs was obtained in terms of tail flick latency period (the time required for flicking of tail, i.e., reaction time), using an analgesiometer at 0, 15, 30, 60, and 120 minutes. Radiant heat was directed to the proximal third of the tail through a hot wire of the analgesiometer and the reaction time was noted, when the mouse tried to pull the tail away. A mean of two pre-drug readings was taken as the basal value (0 minute). Mice with a reaction time of more than six seconds were not used in the test. In order to prevent tissue injury, a cut off time of 10 seconds was maintained. The cut off time was considered as the latency period for the animals not responding up to 10 seconds.
Statistical analysis
The results were expressed as mean ± SD; the paired Student's ‘t’ test was employed for comparison between the two means as a measure of significance. P value of <0.05 was regarded as a statistically significant value.
Results
Effect of test drugs on tail flick latency
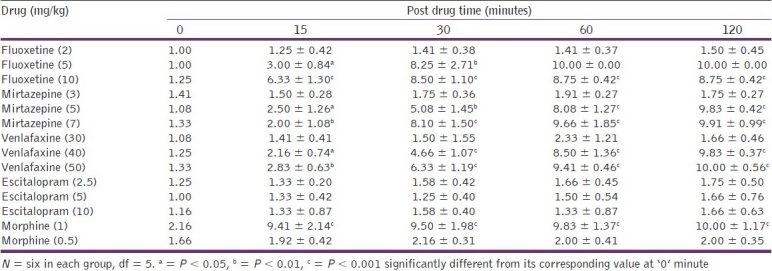
Fluoxetine (2 mg/kg), venlafaxine (30 mg/kg), and mirtazapine (3 mg/kg) showed no significant increase in tail flick latency period as compared to the corresponding 0 minute values in mice, on the tail flick analgesiometer. Fluoxetine (5 and 10 mg/kg), venlafaxine (40 and 50 mg/kg), and mirtazapine (5 and 7 mg/kg) produced a significant increase in tail flick latency at all time intervals (P < 0.05). These drugs produced a dose-dependent antinociception, but escitalopram did not show any antinociceptive effect at 2.5, 5, and 10 mg/kg doses [Table 1].
Table 1.
Effects of different test drugs on the tail.flick latency period (seconds; mean ± SD) at different time intervals
Effect of morphine treatment
Morphine (0.5 mg/kg) showed no significant increase in tail flick latency period at any time interval (P > 0.1). However, at a dose of 1 mg/kg, morphine produced a significant increase in tail flick latency at every time interval tested, from 0 to 120 minutes (P < 0.001) [Table 1].
Effects of combined treatment
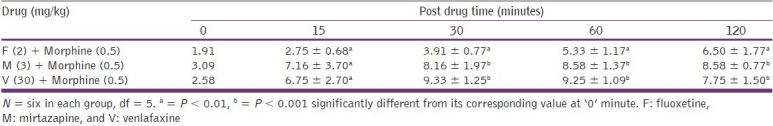
The combination of subanalgesic doses of fluoxetine (2 mg/kg), venlafaxine (30 mg/kg), and mirtazapine (3 mg/kg), with morphine (0.5 mg/kg), respectively, produced a significant (P < 0.01 to P < 0.001) increase in the tail flick latency period, throughout the study [Table 2].
Table 2.
Effects on the tail.flick latency period (seconds; mean ± SD) after combined treatment of test drugs with morphine at different time intervals
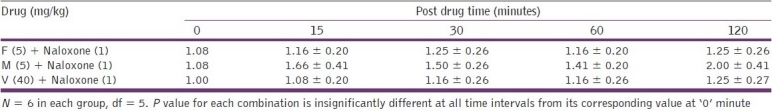
The combination of fluoxetine (5 and 10 mg/kg), venlafaxine (40 and 50 mg/kg), and mirtazapine (5 and 7 mg/kg), with naloxone (1 mg/kg) did not produce significant (P > 0.1) increase in tail flick latency during the entire test period, [Table 3]. This shows that pretreatment with naloxone (1 mg/kg) completely antagonized the antinociceptive effect of fluoxetine, venlafaxine, and mirtazapine, at their analgesic doses.
Table 3.
Effects on the tail. flick latency period (seconds; mean ± SD) after combined treatment of test drugs with naloxone
Discussion
Chronic pain is not only difficult for patients to tolerate, but also for the clinicians to treat effectively. Several pre-clinical and clinical studies have reported the antinociceptive activity of antidepressants.[4–8,11–13]
In the present study, SSRIs (fluoxetine and escitalopram) and atypical antidepressants (mirtazapine and venlafaxine) were used to analyze their analgesic/antinociceptive effect, using the tail-flick analgesiometer. Out of the four above-mentioned drugs, escitalopram did not show any analgesic/antinociceptive effect. Our observations are in accordance with the results of various clinical and animal studies.[12,13]
Fluoxetine showed antinociceptive activity at 5 and 10 mg/kg. These findings are in conjunction with the finding of Max et al.,[5] [Table 4] who in a double blind cross-over study, observed similar results. Goldenberg et al.,[17] compared placebo with fluoxetine in fibromyalgia patients and found a significant pain-reliving activity in fluoxetine. Similarly, Rani et al.,[8] compared fluoxetine with amitriptyline and placebo in patients with chronic rheumatic pain and found significant reduction in pain intensity scores and pain relief scores. They suggested fluoxetine to be an effective analgesic, with fewer side effects.
Table 4.
Various studies showing antinociceptive activity of antidepressants
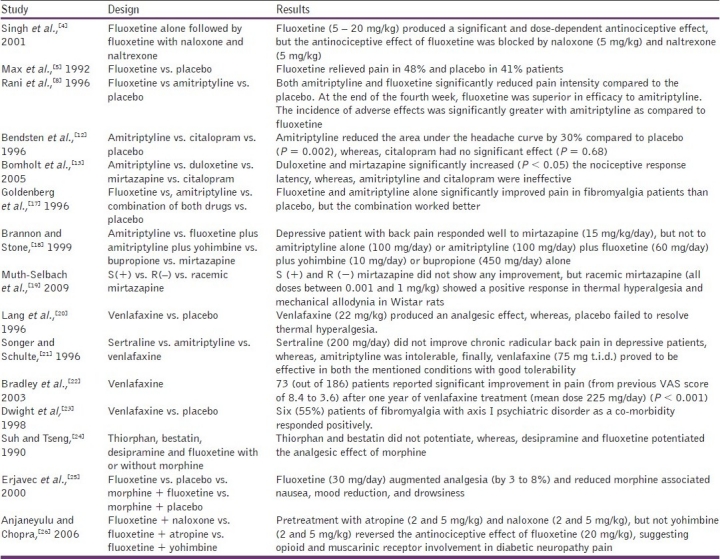
The antinociceptive effect shown by mirtazapine (5 and 7 mg/kg) is supported by the findings of Brannon and Stone[18] for chronic back pain, with major depression. Muth-Selbach et al.,[19] and Bomholt et al.,[13] also showed the same effects in animal models.
In our study, venlafaxine showed an antinociceptive effect at 40 and 50 mg/kg. Various other studies also suggested the antinociceptive effect of venlafaxine, namely, Lang et al.,[20] in mitigating thermal hyperalgesia in animals, Songer and Schulte[21] in radicular back pain associated with depression, Bradley et al.,[22] in migraine, chronic back pain, and chronic regional pain syndrome (CRPS), and Dwight et al.,[23] in fibromyalgia with axis I psychiatric disorders.
Further, morphine at a subanalgesic dose (0.5 mg/kg) potentiated all the three above-mentioned antidepressants (supported by the work of Suh and Tseng and Erjavec et al.,)[24,25]
Naloxone (1 mg/kg) abolished the analgesic effect of fluoxetine, mirtazapine, and venlafaxine. These results suggest that the antinociceptive activity of these three antidepressant drugs could involve opioid mechanisms. These observations are in agreement with the findings of Singh et al., and Anjaneyulu and Chopra [Table 4].[4,26]
As the analgesic activity of morphine is mediated through mue (μ) receptors, it is likely that fluoxetine, mirtazapine, and venlafaxine act through opioid pathways involving the μ opioid receptors. However, these drugs interact with other receptor systems also, such as, cholinergic, muscarinic, histaminergic, noradrenergic, and even the GABAergic system.[26–28] Hence, it would not be unreasonable to suggest that antidepressant drugs would involve at least some of these systems for their analgesic effect.
In the present study, a combination of subanalgesic doses of morphine and fluoxetine, mirtazapine, and venlafaxine produced a significant additive antinociceptive/analgesic effect. Therefore, the above combinations of antidepressants with morphine would theoretically minimize the dose requirements, and thus, the potential adverse effects of morphine. Above all, further clinical studies are needed to prove the same.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kurlekar PN, Bhatt JD. Study of the antinociceptive activity of Fluoxetine and its interaction with morphine and Naloxone in mice. Indian J Pharmacol. 2004;36:369–72. [Google Scholar]
- 2.McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22:139–56. doi: 10.2165/00023210-200822020-00005. [DOI] [PubMed] [Google Scholar]
- 3.Rafieian-Kopaei M, Sewell RD. Newer antidepressants: Analgesic and relative monoamine reuptake inhibitory potency. J Pharm Pharmacol. 1994;46:1088. [Google Scholar]
- 4.Singh VP, Jain NK, Kulkarni SK. On the antinociceptive effect of Fluoxetine, a selective Serotonin reuptake inhibitor. Brain Res. 2001;915:218–26. doi: 10.1016/s0006-8993(01)02854-2. [DOI] [PubMed] [Google Scholar]
- 5.Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326:1250–6. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- 6.Kishore-Kumar R, Max MB, Schafer SC, Gaughan AM, Smoller B, Gracely RH, et al. Desipramine relieves postherpetic neuralgia. Clin Pharmacol Ther. 1990;47:305–12. doi: 10.1038/clpt.1990.33. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg DL, Felson DT, Dinerman H. A randomized controlled trial of amitryptaline and naproxen in the treatment of patients with Fibromyalgia. Arthritis Rheum. 1986;29:1371–7. doi: 10.1002/art.1780291110. [DOI] [PubMed] [Google Scholar]
- 8.Rani PU, Naidu MU, Prasad VB, Rao TR, Shobha JC. An evaluation of antidepressants in rheumatic pain conditions. Anesth Analg. 1996;83:371–5. doi: 10.1097/00000539-199608000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Barkin RL, Fawcett J. The management challenges of chronic pain: The role of antidepressants. Am J Ther. 2000;7:31–47. doi: 10.1097/00045391-200007010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Egbunike IG, Chaffee BJ. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10:262–70. [PubMed] [Google Scholar]
- 11.Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12:384–9. doi: 10.1046/j.1525-1497.1997.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendtsen L, Jensen R, Olesen J. A non-selective (amitriptyline), but not a selective (citalopram), serotonin reuptake inhibitor is effective in the prophylactic treatment of chronic tension-type headache. J Neurol Neurosurg Psychiatry. 1996;61:285–90. doi: 10.1136/jnnp.61.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bomholt SF, Mikkelsen JD, Blackburn-Munro G. Antinociceptive effects of the antidepressants amitriptyline, duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology. 2005;48:252–63. doi: 10.1016/j.neuropharm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Gray AM, Pache DM, Sewell RD. Do alpha2-adrenoceptors play an integral role in the antinociceptive mechanism of action of antidepressant compounds? Eur J Pharmacol. 1999;378:161–8. doi: 10.1016/s0014-2999(99)00464-1. [DOI] [PubMed] [Google Scholar]
- 15.Rang HP, Dale MM, Ritter JM, Flower RJ. 6th ed. Edinburgh: Churchill Livingstone; 2007. Rang and Dale's Pharmacology. [Google Scholar]
- 16.Guyton AC, Hall JE. 9th ed. Philadelphia: WB Saunders Co; 1996. Medical physiology. [Google Scholar]
- 17.Goldenberg D, Mayskiy M, Mossey C, Ruthazer R, Schmid C. A randomized, double-blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis Rheum. 1996;39:1852–9. doi: 10.1002/art.1780391111. [DOI] [PubMed] [Google Scholar]
- 18.Brannon GE, Stone KD. The use of mirtazapine in a patient with chronic pain. J Pain Symptom Manage. 1999;18:382–5. doi: 10.1016/s0885-3924(99)00098-6. [DOI] [PubMed] [Google Scholar]
- 19.Muth-Selbach U, Hermanns H, Driehsen C, Lipfert P, Freynhagen R. Racemic intrathecal mirtazapine but not its enantiomers acts anti-neuropathic after chronic constriction injury in rats. Brain Res Bull. 2009;79:63–8. doi: 10.1016/j.brainresbull.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Lang E, Hord AH, Denson D. Venlafaxine hydrochloride relieves thermal hyperalgesia in rats with an experimental mononeuropathy. Pain. 1996;68:151–5. doi: 10.1016/S0304-3959(96)03223-X. [DOI] [PubMed] [Google Scholar]
- 21.Songer DA, Schulte H. Venlafaxine for the treatment of chronic pain (letter) Am J Psychiatry. 1996;153:737. doi: 10.1176/ajp.153.5.737a. [DOI] [PubMed] [Google Scholar]
- 22.Bradley RH, Barkin RL, Jerome J, DeYoung K, Dodge CW. Efficacy of venlafaxine for the long term treatment of chronic pain with associated major depressive disorder. Am J Ther. 2003;10:318–23. doi: 10.1097/00045391-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Dwight MM, Arnold LM, O’Brien H, Metzger R, Morris-Park E, Keck PE., Jr An open clinical trial of venlafaxine treatment of fibromyalgia. Psychosomatics. 1998;39:14–7. doi: 10.1016/S0033-3182(98)71375-1. [DOI] [PubMed] [Google Scholar]
- 24.Suh HH, Tseng LL. Intrathecal administration of thiorphan, bestatin, desipramine and fluoxetine differentially potentiate the antinociceptive effects induced by beta-endorphin and morphine, administered intracerebroventricularly. Neuropharmacology. 1990;29:207–14. doi: 10.1016/0028-3908(90)90003-a. [DOI] [PubMed] [Google Scholar]
- 25.Erjavec MK, Coda BA, Nguyen Q, Donaldson G, Risler L, Shen DD. Morphine-fluoxetine interactions in healthy volunteers: Analgesia and side effects. J Clin Pharmacol. 2000;40:1286–95. [PubMed] [Google Scholar]
- 26.Anjaneyulu M, Chopra K. Possible involvement of cholinergic and opioid receptor mechanisms in fluoxetine mediated antinociception response in streptozotocin-induced diabetic mice. Eur J Pharmacol. 2006;538:80–4. doi: 10.1016/j.ejphar.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 27.Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs) Int Clin Psychopharmacol. 1994;9:19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- 28.Maitre L, Riezen HV. Amine re-uptake block by antidepressant: Virtue or vice. In: Leonard B, Spencer P, editors. Antidepressants: Thirty years on. London: CNS (Clinical Neuroscience) Publishers; 1990. [Google Scholar]


