Abstract
Objective:
Coriandrum sativum (Linn.), a glabrous, aromatic, herbaceous annual plant, is well known for its use in jaundice. Essential oil, flavonoids, fatty acids, and sterols have been isolated from different parts of C. sativum. The plant has a very effective antioxidant profile showing 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, lipoxygenase inhibition, phospholipid peroxidation inhibition, iron chelating activity, hydroxyl radical scavenging activity, superoxide dismutation, glutathione reduction and antilipid peroxidation due to its high total phenolic content with the presence of constituents like pyrogallol, caffeic acid, glycitin, etc.
Materials and Methods:
This study was aimed at investigating the hepatoprotective activity of C. sativum against carbon tetrachloride (CCl4), with estimation of serum serum glutamyl oxaloacetic acid transaminase (SGOT), serum glutamyl pyruvate transaminase (SGPT), alkaine phosphatase (ALP) and bilirubin, and with liver histopathology.
Results:
Ethanolic extract was found to be rich in alkaloids, phenolic compounds and flavonoids, and high performance liquid chromatography (HPLC) fingerprinting showed the presence of iso-quercetin and quercetin. C. sativum signifies hepatoprotection by reducing the liver weight, activities of SGOT, SGPT, and ALP, and direct bilirubin of CCl4 intoxicated animals. Administration of C. sativum extract at 300 mg/kg dose resulted in disappearance of fatty deposit, ballooning degeneration and necrosis, indicating antihepatotoxic activity.
Conclusion:
The results of this study have led to the conclusion that ethanolic extract of C. sativum possesses hepatoprotective activity which may be due to the antioxidant potential of phenolic compounds.
Keywords: Antioxidant, carbon tetrachloride, Coriandrum sativum, histopathology, iso-quercetin, quercetin
The liver is an organ of paramount importance and plays an essential role in the metabolism of foreign compounds entering the body. Human beings are being exposed to these compounds through environment exposure, consumption of contaminated food or during exposure to chemical substances in the occupational environment. In addition, human beings consume a lot of synthetic drugs during diseased conditions, which are alien to body organs.[1] Liver diseases remain one of the serious health problems. Conventional drugs used in the treatment of liver diseases are often inadequate. It is therefore necessary to search for alternative drugs for the treatment of liver diseases. Attempts are being made globally to get scientific evidences for these traditionally reported herbal drugs.
Many known and lesser known plants are in folk and tribal medical practices in India. Coriandrum sativum (Linn.) of family Umbelliferae, a glabrous, aromatic, herbaceous annual plant, is well known for its use in jaundice. It is a major component of many hepatoprotective herbal formulations. It has been reported to possess diuretic, carminative, digestive, anthelmintic, antioxidant and antibacterial activities. The name “coriander” in a culinary context may refer to either the seeds of the plant (used as a spice) or its leaves (used as a herb). However, in North American countries, for example, the name cilantro is given to the leaves.[2,3] C. sativum has diuretic, carminative, digestive, anthelmintic, antioxidant and antibacterial activities. A large number of phytoconstituents, viz., essential oil, flavonoids, fatty acids, and sterols have been isolated from different parts of C. sativum.[4]
C. sativum is reported to have a very effective antioxidant activity profile showing 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, lipoxygenase inhibition, phospholipid peroxidation inhibition, iron chelating activity, hydroxyl radical scavenging activity, superoxide dismutation, glutathione reduction and antilipid peroxidation. The ethanolic, methanolic, chloroform, ethyl acetate and water extracts of C. sativum have high total phenolic content with presence of constituents like pyrogallol, caffeic acid, glycitin, etc.[5–8] Scientific studies on the hepatoprotective potential of leaf are lacking. So, this study was planned to investigate the leaf ethanolic extract against carbon tetrachloride (CCl4) induced hepatotoxicity to validate its use as a liver protective agent, correlating with C. sativum antioxidant activity. C. sativum ethanolic extract was evaluated for its hepatoprotective efficacy against CCl4 induced hepatotoxicity in rats.
Materials and Methods
Chemicals
All chemicals used in the study were of analytical grade and obtained from Loba Chemie Pvt. Ltd. (Mumbai, India), Sigma Chemicals Co. (Sigma, USA) and Merck India Ltd. (Mumbai, India).
Plant material
Fresh leaves of C. sativum were collected from the vegetable market of Bhopal, India, in April 2010. The taxonomical identification was carried out by Dr. Tariq Hussain, National Botanical Research Institute, Lucknow, and a voucher specimen (Accession no. 97311) was deposited in the herbarium of the department. The leaves were air dried under shade and powdered in a grinding mill.
Preparation of extract
Powdered crude drug (500g) was extracted in a soxhlet apparatus with ethanol (60-80°C) for 28 hours. The extract was filtered through muslin cloth and evaporated at 40°C up to one-third of the initial volume; the remaining solvent was completely evaporated using a rotary vacuum evaporator (Superfit, Mumbai, India). The extract was then weighed and the percentage yield (15.7%) calculated. The color and consistency of the extract was noted and the extract was subjected to different tests to detect the presence of various phytoconstituents.[9,10]
Chromatographic study
Thin layer chromatography
The chromatographic profiles were developed for ethanolic extract utilizing different solvent systems. The best resolution of spots for ethanolic extract was found in the solvent system methanol: water (45:55, 65:35, 70:30, 75:25) (v/v) in which four spots were seen (Rf values 0.83, 0.76, 0.35, 0.19; 0.82, 0.76, 0.19, 0.10; 0.77, 0.53, 0.22, 0.11; 0.79, 0.60, 0.26, 0.13). The best resolution of spots for ethanolic extract in the solvent system acetonitrile: phosphate buffer (20: 72; 24: 76; 28: 72; 36: 64) (v/v) showed four spots (Rf values 0.83, 0.66, 0.54, 0.25; 0.80, 0.66, 0.36, 0.19; 0.76, 0.65, 0.46, 0.20; 0.83, 0.71, 0.59, 0.40).
High performance liquid chromatography
Instrumentation and reagents
A Shimadzu HPLC system (SPD-M20A, Kyoto, Japan) equipped with a 680 quaternary pump, ASI-100 autosampler, 200 μl loop injector, a column oven, PDA-100 photodiode array detector and a data system (LC solution) was used. An ultrasonic cleaner was used for extraction. Water purified with reverse osmosis (18 Mμ) was used for all the solutions and dilutions, and the vacuum concentrator system consisted of a rotary evaporator. High performance liquid chromatography (HPLC) grade acetonitrile and methanol were purchased from Molychem (Mumbai, India). Standards of quercetin and iso-quercetin were purchased from Merck (Mumbai, India).
Sample preparation
Powdered leaf (2g) of C. sativum was extracted with 20 ml ethanol: water (6:4, v/v) solution in an ultrasonic water bath for 10 min, and the extraction was repeated thrice. The extracted solution was mixed and filtrated, and evaporated at 45°C to dryness by vacuum. The dry extract was dissolved in 10 ml methanol: water (50:50, v/v) and suspended particles were then filtrated through a 45 μm membrane filter.
High performance liquid chromatography procedures
The chromatographic separation was performed on a reverse-phase Luna 5 μm C-18 100 Å, 250 × 4.6 mm, 5 μm particle size (Phenomenex, Torrance, California, USA) column. The sample was eluted with a mobile phase using linear gradient of acetonitrile (4-27% for 100 mins) and buffer solution (water-KH2PO4-H3PO4, pH 3.0, 96-73% for 100 mins) at a flow rate of 1 ml/min. The detection wavelength and column temperature were set at 300 nm and 28°C, respectively. A loading volume of 10 μl filtered ethanolic extract was injected under these conditions as well as a mixture of authentic sample of quercetin and iso-quercetin. Data analysis was performed using a software named LC solution System provided by Shimadzu, Japan.[11]
In vivo pharmacological study
Experimental animals
Laboratory-based adult Wistar albino rats (120-200g) of either sex were used in the experiment. Animals were housed in polycarbonate cages under standard laboratory conditions at 22 ± 2°C, relative humidity 50 ± 5% and photoperiod (12 h light:12 h dark). The animals were maintained with standard pelleted balanced diet and water ad libitum. Animal study was performed after obtaining due permission from institutional animal ethical committee (IAEC Approval No. IAEC/RCP/2010/11) of our institute and care provided to the animal was as per the WHO “Guidelines for the Care and Use of Animals in Scientific Research”. Animals were divided into six groups of six rats each and all the test drugs were administered intraperitoneally (i.p.) at the calculated dose.
Preparation of dosage form
Dosage form was made in 2% polyvinyl pyrrolidone solution. C. sativum ethanolic (CSE) extract (1g) was taken in mortar and pestle and triturated with 10 ml of 2% polyvinyl pyrrolidone solution continuously to get homogenous suspensions having a concentration of 100 mg/ml of the drug. All the suspensions of test extract and standard drug silymarin were prepared freshly before use.
Acute toxicity study
Acute oral toxicity of CSE was determined according to the Guidelines of Organization for Economic Co-operation and Development (OECD), following the up and down method (OECD guideline No. 425) and fixed dose method (OECD guideline No. 420). Based on these methods, a Limit test was performed to categorize the toxicity class of the compound and then Main test was performed to estimate the exact LD50. The animals (nulliparous and non-pregnant female Wistar albino rats) were fasted overnight with free access to water, weighed, and a single dose of the test substance was administered. Animals were observed individually during the first 30 min for all the reflexes, periodically during 48 hours with special attention given during the first 4 hours (short-term toxicity) and daily thereafter for a total of 14 days (long-term toxicity). The animals were fasted overnight prior to the experiment and maintained under standard conditions. Following the OECD Guidelines, a Limit test was performed at 2000 mg/kg, i.p., which does not show mortality in rats.[12] Dose ranges of 100, 200, and 300 mg/kg were selected for the evaluation of pharmacological activity. For all the studies, overnight fasted animals of either sex were divided randomly into six per group.
Hepatoprotective study
Hepatoprotective study was performed on CCl4 induced hepatotoxic animal models. 10 ml of liquid paraffin was triturated with 10 ml of CCl4 and administered orally in a dose of 1 ml/kg. Group I was kept as vehicle control, Group II was CCl4 control, Group III received silymarin (50 mg/kg, i.p.), and Groups IV, V, and VI were treated with different doses of extract (100, 200 and 300 mg/kg, respectively, i.p.).
The drugs were given once a day for 14 days and CCl4 was administered orally to animals of all the groups on 7th, 9th, 11th and 13th days of the experiment. Body weight of all the animals was recorded on 0 day, 7th day and 14th day of experimentation. On the 14th day, the animals were sacrificed 3 h after the extract treatment, liver and kidney were dissected out, kept in ice-cold saline, wiped and the weights were recorded. Blood samples were collected by cardiac puncture, serum was separated after coagulating at 37°C for 30 mins and centrifuged at 1000 rpm for 10 mins in a cooling centrifuge. In view of multiplicity and complexity of the liver functions, it is obvious that no single test can establish the disturbances in liver function. Thus, a battery of liver function tests was employed for accurate diagnosis, to assess the severity of damage, to judge prognosis and to evaluate therapy. Serum was analyzed for various biochemical parameters. Serum glutamyl oxalacetic acid transaminase (SGOT), serum glutamyl pyruvate transaminase (SGPT), alkaline phosphatase (ALP), total bilirubin, direct bilirubin and indirect bilirubin were estimated using Biosystem A-25 autoanalyser.[13–15]
Histopathological study
Isolated liver slices kept in 10% formalin solution were processed for histopathological assessment of liver damage following the method of Nanji.[16] Briefly, liver slices were dehydrated in ethanol (50-100%), cleared in xylene and embedded in paraffin. Sections of 4-5 μm thickness were cut using a rotary microtome and stained with hematoxylin-eosin dye for photomicroscopic observation.
Statistical analysis
The results were reported as Mean ± SEM of six observations. Experimental data were analyzed using one-way analysis of variance (ANOVA) followed by Student's t-test to compare the difference between the control and treated values. P value less than 0.05 was considered significant. Graph Pad Prism Version 3.02 was used for statistical calculations.
Results
Green-colored extract was obtained with ethanol (yield 15.7%). Ethanolic extract showed the presence of carbohydrates, alkaloids, phenolic compounds and flavonoids. HPLC fingerprint showed the presence of 11 peaks. Peak 5 with retention time 62.82 mins and peak 7 with retention time 70.04 mins were identical with iso-quercetin and quercetin [Figure 1].
Figure 1.
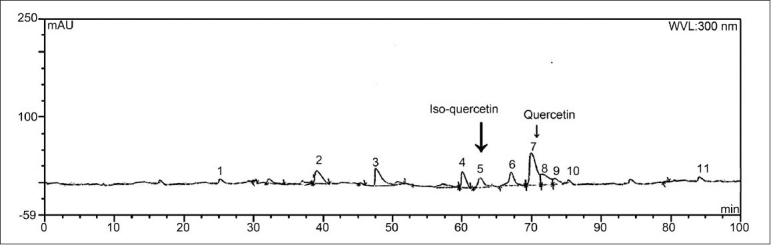
HPLC fingerprint chromatogram of C. sativum ethanolic extract
Acute toxicity
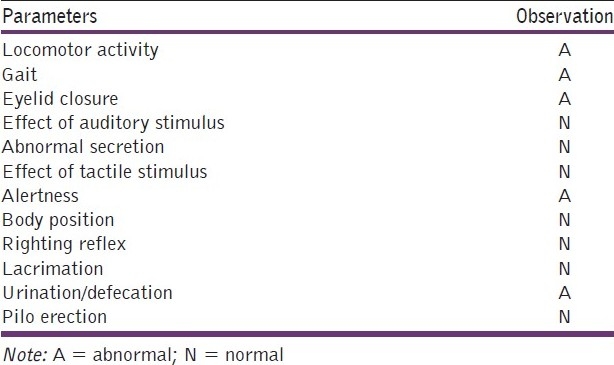
Acute toxicity studies performed on ethanolic extract of C. sativum (according to OECD guidelines) did not show any sign and symptoms of toxicity and mortality up to 2000 mg/kg dose, except centrally induced depression evidenced by alertness, motor activity and increased urination and defecation [Table 1].
Table 1.
Acute toxicity (LD50) study of C. sativum ethanolic extract on rats (Limit test at 2000 mg/kg, i.p.)
Hepatoprotective activity
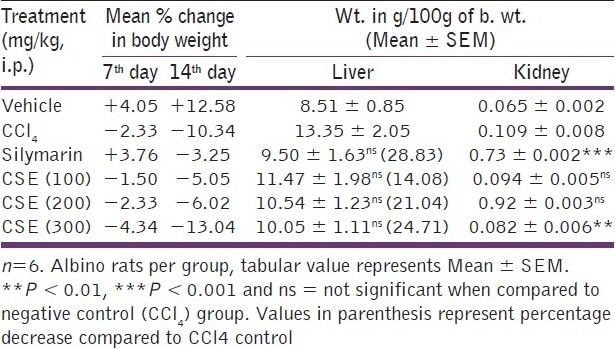
Body weight of all the animals was recorded on the 1st day before commencing the experiment, then on the 7th day after continuous drug treatment and again on the 14th day. Vehicle control group showed 4 and 12.58% increase in body weight on 7th and 14th days, respectively. Negative control group (CCl4 treated) showed 2.33 and 10.34% decrease in body weight on 7th and 14th days, respectively, with reduced food consumption. Standard drug (silymarin) treatment showed 3.76% increase and 3.25% decrease in body weight on 7th and 14th days, respectively, whereas CSE showed dose-dependent body weight loss. CSE at 100, 200 and 300 mg/kg showed 5.05, 6.02 and 13.04% decrease in body weight, respectively, on the 14th day of continuous drug treatment.
Silymarin and ethanolic extract treatment signifies hepatoprotection by reducing the liver weight of CCl4 intoxicated animals. Liver weights (in g/100g b. wt.) in animals treated with vehicle, CCl4 , silymarin and ethanolic extract of C. sativum at 100, 200 and 300 mg/kg b. wt. doses were 8.51 ± 0.85, 13.35 ± 2.05, 9.50 ± 1.63, 11.47 ± 1.98, 10.54 ± 1.23 and 10.05 ± 1.11g, respectively. Silymarin showed 28.83% decrease in liver weight, whereas 300 mg/kg ethanolic extract showed 24.71% decrease, compared to CCl4 control. Vehicle control group showed normal kidney weight/100g of body weight (0.065 ± 0.002g); CCl4 intoxication increased the kidney weight (0.109 ± 0.008g) which was decreased extremely significantly (P < 0.001) by silymarin (0.073 ± 0.002g) and also by 300 mg/kg ethanolic extract (0.082 ± 0.006g; P < 0.01) as represented in Table 2.
Table 2.
Effect of C. sativum ethanolic extract on body weight and relative organ weight of CCL4 treated rats
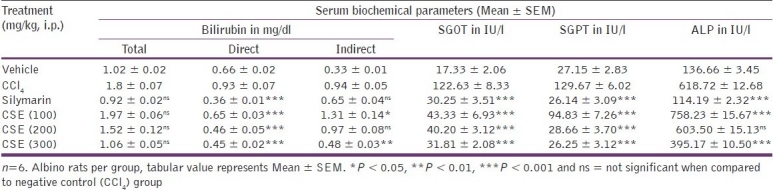
Hepatotoxic CCl4 gets converted into CCl3O-by liver enzymes which attack the unsaturated fatty acids of cell membrane, giving rise to lipid peroxides that alter the functional integrity of liver mitochondria. As a result, the level of marker enzymes in plasma severely increases in CCl4 intoxicated animals. Serum levels of total bilirubin, direct bilirubin, indirect bilirubin, SGOT, SGPT, and ALP were increased to 1.8 mg/dl, 0.93 mg/dl, 0.94 mg/dl, 122.63 IU/l, 129.67 IU/l, and 618.72 IU/l, respectively, in comparison to vehicle control values of 1.02 mg/dl, 0.66 mg/dl, 0.33 mg/dl, 17.33 IU/l, 27.15 IU/l, and 136.66 IU/l, respectively, in CCl4 treated animals.
In contrast, the group treated with silymarin showed extremely significant (P < 0.001) decreases in direct bilirubin, SGOT, SGPT and ALP values toward normalization. Group treated with 200 mg/kg CSE showed nonsignificant decrease in total bilirubin, indirect bilirubin and ALP, whereas it showed extremely significant (P < 0.001) decrease in direct bilirubin, SGOT and SGPT values toward normalization. CSE at 300 mg/kg dose significantly (P < 0.01-0.001) decreased direct bilirubin, indirect bilirubin, SGOT, SGPT and ALP, with nonsignificant effect on total bilirubin [Table 3].
Table 3.
Effect of C. sativum ethanolic extract on serum biochemical parameters of CCl4 treated rats
Histopathological study
Liver sections of vehicle control animals showed normal hepatocytes. Light microscopic examination of hematoxylin-eosin stained slide of vehicle control animals showed normal architecture without any degeneration, necrosis and fatty depositions. Microscopic examination of liver sections of the animals treated with CCl4 alone exhibited cellular degeneration, hydropic change in central vein, fatty change, widespread cloudy swelling, ballooning degeneration, broad infiltration of lymphocytes, coagulating centro-lobular, lobular, caseative and diffused necrosis, proliferating changes, steatosis and bile duct hyperplasia, reflecting severe liver damage.
Treatment with silymarin alone exhibited reversal of changes induced by CCl4, with slight cellular degeneration, widespread cloudy swelling, coagulating centrolobular, lobular and diffused necrosis. Treatment with ethanolic extract of C. sativum at 100 mg/kg dose showed reversal of changes, with slight cellular degeneration, fatty change, ballooning degeneration, broad infiltration of lymphocytes, coagulating centrolobular, lobular, caseative and diffused necrosis and steatosis also. Treatment with ethanolic extract at 200 mg/kg dose showed significant recovery with little cellular degeneration, broad infiltration of lymphocytes, coagulating lobular and diffused necrosis. C. sativum ethanolic extract at 300 mg/kg dose exhibited tremendous progress with disappearance of fatty deposit with slight cellular degeneration, coagulating lobular and diffused necrosis, indicating antihepatotoxic activity [Table 4 and Figure 2a–f].
Table 4.
Effect of C. sativum ethanolic extract on histopathological parameters of CCl4 treated rat liver
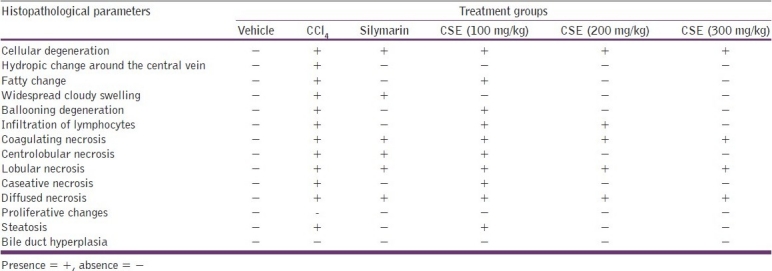
Figure 2.
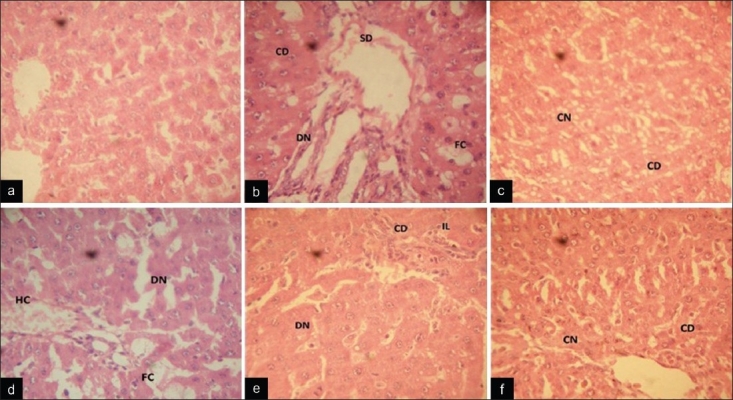
Photomicrograph of paraffin-embedded rat liver sections stained with hematoxylin and eosin. (a) Liver section of a normal control rat liver showing normal architecture with no cellular degeneration and necrosis. (b) Liver sections of CCl4 treated rat showing fatty changes, necrosis, cellular degeneration and sinusoidal dilation. (c) Liver section of silymarin + CCl4 treated rat showing preservation of cellular structure with few coagulating necrosis. (d) CSE 100 mg/kg + CCl4 treated rat liver sections showing hydrophobic and fatty changes with diffused necrosis. (e) CSE 200 mg/kg + CCl4 treated rat liver sections showing cellular degeneration, diffused necrosis with lymphocyte infiltration. (f) CSE 300 mg/kg + CCl4 treated rat liver sections showing well-brought central vein and sinusoid with slight cellular degeneration and coagulative necrosis (CD: cellular degeneration; N: necrosis; CN: coagulating necrosis; DN: diffused necrosis; SD: sinusoidal dilation; HC: hydrophobic changes; FC: fatty changes or steatosis; IL: infiltration of lymphocytes)
Discussion
The most important function of the liver is detoxification of substances like alcohol and different medications such as chemotherapeutic drugs, antibiotics and toxicants. Chemical agents and toxins impose excess stress on the liver filtering function. If accumulation of toxins is faster than the liver metabolizing ability, hepatic damage may occur.[17] CCl4 induced hepatotoxicity has been chosen as the experimental model since the changes associated with the CCl4 induced liver damage are similar to those of viral hepatitis. The liver toxicant CCl4 causes lipid peroxidative degradation of biomembrane, which is one of the principle causes of hepatotoxicity.[18] In liver, CCl4 is biotransformed by cytochrome P450 to produce its active metabolite, trichloromethyl free radical,[19] which binds to the macromolecule and induces peroxidative degradation of membrane lipids of endoplasmic reticulum which are rich in polyunsaturated fatty acids. This leads to the formation of lipid peroxide, which in turn produces a toxic aldehyde that causes damage to liver. When the liver cell plasma membrane is damaged, a variety of enzymes normally located in the cytosol are released into the blood stream. Necrosis or membrane damage releases the enzymes SGOT, SGPT and ALP into circulation; therefore, these can be measured in serum. These enzymes in the serum are useful quantitative markers of the extent and type of hepatocellular damage. High levels of SGOT indicate the loss of functional integrity of liver, as seen in viral hepatitis, as well as cardiac infraction and muscle injury. SGPT catalyzes the conversion of alanine to pyruvate and glutamate and is released in a similar manner. Therefore, SGPT is more specific to liver and thus a better parameter for detecting liver injury.[20]
In the present study, CCl4 treatment causes severe hepatic damage through a substantial increment in the serum level of biochemical markers like SGPT, SGPT, ALP and bilirubin. Pretreatment with C. sativum ethanolic extract before introduction of CCl4 has significantly reduced the elevated serum levels of SGOT, SGPT, ALP and bilirubin. The biochemical mechanism involved in the development of CCl4 induced toxicity is oxidative damage; so, antioxidant activity or inhibition of free radical generation is generally correlated with protection against CCl4 induced liver lesion.[21,22] The highly reactive free radical (trichloromethyl, CCl3°-) in the presence of oxygen leads to auto-oxidation of fatty acids and causes functional and morphological changes in the cell membrane.[23] Hepatic microsomal antilipid peroxidation, glutathione reduction, and catalase and superoxide dismutase activities are effective indicators of inhibition of oxidative damage of hepatocytes.[24]
Hashim reported significantly increased superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase activities by methanol, chloroform and ethyl acetate extracts of C. sativum, in comparison to quercetin.[7] Iron chelating, DPPH radical scavenging and antilipid peroxidation activities of water and methanol extracts of C. sativum have also been reported by Wong and Kitts.[5] Silymarin, catachin and phenolic acids have an inhibitory action against lipid peroxidation in human platelets, as reported by Koch and Loffler.[25] A major component of the cellular antioxidant system in mammalian cells consists of three enzymes, namely, superoxide dismutase, catalase and glutathione peroxidase. The enzymes work in concert to detoxify O2* and H2O2 in cells. Polyphenols of C. sativum act as antiperoxidative agents, representing it as a promising drug for the prevention of oxidative damage in living systems.[5] Ethanol and water extracts of culinary herbs are reported to be found with higher concentration of phenolic compounds. Phenolic compounds in plants constitute a major class of secondary metabolite are attributed to anti-oxidant and anti-bacterial activities. The phenolic compounds present in (C. sativum) are relatively more polar. Based on these findings in our present study ethanol was selected as extraction solvent to get more fraction of phenolics extracted. C. sativum leaf and stem were found to be contained 63.2-189.0 mg caffeic acid/100g of fresh weight as per Wong and Kitts, Wangensteen reported 1.9-5.5g gallic acid equivalent/100g extract and Hashim reported presence of 49.8-397.5 mg quercetin as major anti-oxidant phenolic acid per 100g of dry extract.[5,7,8]
CCl4 is an experimental protocol drug used extensively to investigate hepatoprotective effect of various under trial drug on experimental animals. Drugs used for liver diseases usually have to be given for several days to get effective therapeutic response. Two weeks schedule (one week pre-treatment) was followed here to stimulate a clinically effective course of treatment. C. sativum at 300 mg/kg dose effectively restores the functional integrity of liver by preventing penetration of the toxin into the interior of the cell. The pre-treatment has prevented oxygen free radicals and thereby the formation of peroxy radicals. A major defense mechanism involves the anti-oxidant enzymes, like SOD, catalase, glutathione peroxidase which converts reactive oxygen molecules into non-toxic compounds. Lipid peroxidation is accelerated when free radicals are formed as a result of losing a hydrogen atom from the double bond in the structure of unsaturated fatty acids. Scavenging of free radicals is one of the major anti-oxidation mechanisms to inhibit the chain reaction of lipid peroxidation.
The toxic metabolite CCl3°- radical produced by CCl4 further reacts with oxygen to give trichloromethyl peroxy radical. Cytochrome P450 P2E1 is the enzyme responsible for this conversion. This radical binds covalently to macromolecule and causes peroxidative degradation of lipid membrane of the adipose tissue. In this view, the reduction in levels of AST and ALT by the extracts is an indication of stabilization of plasma membrane as well as repair of hepatic tissue damage by CCl4. This effect is agreement with the commonly accepted view that serum levels of transaminases return to normal with the healing of hepatic parenchyma and regeneration of hepatocytes.[26] Alkaline phosphate is the prototype of these enzymes that reflects the pathological alteration in biliary flow.[27] CCl4 induced elevation of this enzymatic activity in the serum is in line with high level of serum bilirubin content. The ethanolic extract induced suppression of the increased ALP activity with the concurrent depletion of raised bilirubin suggesting the possibility of the extract having ability to reestablish biliary dysfunction in rat liver during hepatic injury with CCl4. Thus administration of ethanolic extracts of revealed hepatoprotective activity of C. sativum against the toxic effect of CCl4, which was also supported by histological studies. Hepatotoxin induced fatty liver is mainly due to accumulation of triglycerides.[28] C. sativum significantly reverse CCl4 induced steatosis indicating protective and curative effect against fatty liver. Histopathological studies under light microscope confirm the curative efficacy of C. sativum against CCl4 induced liver damage as shown by decrease in severity of toxic effects in 300 mg/kg treated dose comparable to silymarin.
Conclusion
Ethanolic extract of C. sativum possess hepatoprotective activity on CCl4 induced liver injury. The hepatoprotective effect may be due to its antioxidant potential. It can be concluded that possible mechanism of hepatoprotective activity of C. sativum herb may be due to the presence of phenolic compounds. Biochemical, pharmacological and histopathological parameters indicates the structural and functional integrity of hepatocytes supporting further need of mechanistic studies to be done on C. sativum for it anti-hepatotoxic potential. C. sativum can be a safe and effective future alternative therapy for the treatment of various liver diseases. However further studies are required to confirmed involvement of cytochrome P450 (P2E1) enzyme inhibition.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mohan H. New Delhi: Jaypee Brothers Medical Publishers (p) LTD; 2006. A Text book of Pathology; pp. 608–69. [Google Scholar]
- 2.Abderahim A, Jaouad EH, Zafar HI, Badiâa L. Acute diuretic effect of continuous intravenous infusion of an aqueous extract of Coriandrum sativum L.in anesthetized rats. J Ethnopharmacol. 2008;115:89–95. doi: 10.1016/j.jep.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Eguale T, Tilahun G, Debella A, Feleke A, Makonnen E. in vitro and in vivo anthelmintic activity of crude extracts of Coriandrum sativum against Haemonchus contortus. J Ethnopharmacol. 2007;110:428–33. doi: 10.1016/j.jep.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Kiritikar KR, Basu BD. India (Allahabad): Lalit Mohan Basu; 2006. A textbook of Indian medicinal plants; pp. 1225–7. [Google Scholar]
- 5.Wong PY, Kitts DD. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006;97:505–15. [Google Scholar]
- 6.Melo EA, Filho JM, Guerra NB. Charecterization of antioxidant compounds in aqueous Coriander extract (Coriandrum sativum L.) Lebenson Wiss Technol. 2005;38:15–9. [Google Scholar]
- 7.Hashim MS, Lincy S, Remya V, Teena M, Anila L. Effect of polyphenolic compounds from Coriandrum sativum on H2O2-induced oxidative stress in human lymphocytes. Food Chem. 2005;92:653–60. [Google Scholar]
- 8.Wangensteen H, Samuelsen AB, Malterud KE. Antioxidant activity in extracts from coriander. Food Chem. 2004;88:293–97. [Google Scholar]
- 9.Evans WC. 1st ed. New York: WB Saunders company (A Division of Harcourt Brace and Company); 2007. Trease and Evans Pharmacognosy; p. 414. [Google Scholar]
- 10.Harborne JB, Boardley M, Linder HP. Variations in flavonoid patterns within the genus Chondropetalum (Restionaceae) Phytochemistry. 1985;24:273–8. [Google Scholar]
- 11.Irena M, Maria S. Flavonoid compounds in the flowers of Abutilon indicum (L.) Sweet (Malvaceae) Acta Poloniae Pharmaceutica Drug Res. 2002;59:227–9. [PubMed] [Google Scholar]
- 12.Diener W, Mischke U, Kayser D, Schlede E. The evaluation of the OECD modified version of acute toxicity class method (oral) Arch Toxicol. 1995;69:729–34. doi: 10.1007/BF03035438. [DOI] [PubMed] [Google Scholar]
- 13.Reitman S, Frankel S. A colorimetric method for determination of serum glutamic-oxaloacetic and glutamic-pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Kind PRN, King EJ. Method of Practical Clinical Biochemistry. In: Varley H, Gowenlock AH, Bell M, editors. London: Heinman; 1980. pp. 899–900. [Google Scholar]
- 15.Jendrassik L, Gorf P. Verainfachte photometrische Methoden zur Bestimmung des Blutbilirrubins. Biochem Zeitsch. 1938;297:81–9. [Google Scholar]
- 16.Nanji AA, Jakelainen K, Fotouhinia M, Rahemtulla A, Thomas P, Tipoe LG, et al. Increased severity of alcoholic liver injury in female rats: Role of oxidative stress, endotoxin and chemotoxin. Am J Physiol Gastrointestinal Liver Physiol. 2002;281:G1348–56. doi: 10.1152/ajpgi.2001.281.6.G1348. [DOI] [PubMed] [Google Scholar]
- 17.Bigoniya P, Shukla A, Singh CS. Evaluation of Hepatic Microsomal Enzyme Functional Integrity on Picroliv Pretreatment Against CCl4 Induced Hepatotoxicity. Int J Pharmacol. 2010;6:200–7. [Google Scholar]
- 18.Contran RS, Kumar V, Robbins SL. 5th ed. USA (Philadelphia): WB Saunder Company; 1994. Pathologic Basis of Diseases; pp. 178–89. [Google Scholar]
- 19.Kaplowitz N, Aw TY, Simon FR, Stolz A. Drug induced hepatotoxicity. Annal Int Med. 1986;104:826–39. doi: 10.7326/0003-4819-104-6-826. [DOI] [PubMed] [Google Scholar]
- 20.Willianson EM, Okpako DT, Evans FJ. England (Chichester): John Wiley and Sons Ltd; 1996. Selection, Preparation and Pharmacological Evaluation of Plant Material; pp. 131–54. [Google Scholar]
- 21.Castro JA, Ferrya GC, Casro CR, Sasame H, Fenos OM, Gillette JR. Prevention of carbon tetrachloride-induced necrosis by inhibitors of drug metabolism.Further studies on the metabolism of their action. Biochem Pharmacol. 1974;23:295–302. doi: 10.1016/0006-2952(74)90420-1. [DOI] [PubMed] [Google Scholar]
- 22.Maling HM, Eichelbaum FM, Saul W, Sipes IG, Brown GA, Gillette JR. Nature of the protection against carbon tetrachlorideinduced hepatotoxicity produced by pretreatment with dibenamine N-chloroethyldipenzylamine. Biochem Pharmacol. 1974;23:1479–91. doi: 10.1016/0006-2952(74)90385-2. [DOI] [PubMed] [Google Scholar]
- 23.Hruszkewycz AM, Glende EA, Recknagel RO. Destruction of microsomal cytochrome P450 and glucose 6-phosphatase by lipids extracted from peroxidized microsomes. Toxicol Applied Pharmacol. 1978;46:695–702. doi: 10.1016/0041-008x(78)90314-9. [DOI] [PubMed] [Google Scholar]
- 24.Jain A, Soni M, Deb L, Lain A, Rout SP, Gupta VB, et al. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb, Leaves. J Ethanopharmacol. 2008;115:61–6. doi: 10.1016/j.jep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Koch HP, Loffler E. Influence of silymarin and some flavonoids on lipid peroxidation in human platelets. Methods Find Exp Clin Pharmacol. 1985;7:13–8. [PubMed] [Google Scholar]
- 26.Thabrew MI, Joice PD, Rajatissa WA. Comparative study of efficacy of Paetta indica and Osbeckia octandra in the treatment of liver dysfunction. Planta Med. 1987;53:239–41. doi: 10.1055/s-2006-962691. [DOI] [PubMed] [Google Scholar]
- 27.Ploa GL, Hewitt WR. Principle and Methods of Toxicology. In: Wallace HA, editor. 2nd ed. New York: Raven Press; 1989. p. 399. [Google Scholar]
- 28.Seakins A, Robinson DS. The effect of administration of carbon tetrachloride on the formation of plasma lipoproteins in the rat. Biochem J. 1963;86:401–7. doi: 10.1042/bj0860401. [DOI] [PMC free article] [PubMed] [Google Scholar]


