Abstract
The colon targeted drug delivery has a number of important implications in the field of pharmacotherapy. Oral colon targeted drug delivery systems have recently gained importance for delivering a variety of therapeutic agents for both local and systemic administration. Targeting of drugs to the colon via oral administration protect the drug from degradation or release in the stomach and small intestine. It also ensures abrupt or controlled release of the drug in the proximal colon. Various drug delivery systems have been designed that deliver the drug quantitatively to the colon and then trigger the release of drug. This review will cover different types of polymers which can be used in formulation of colon targeted drug delivery systems.
Keywords: Biodegradable polymers, colon targeted delivery, controlled delivery, polysaccharides
Conventional orally administered controlled release products normally lack any special property which would facilitate them for targeting of drug to a specific site in gastrointestinal tract. Targeted delivery of drugs to the colon has attracted much interest recently for local treatment of a variety of colonic diseases as well as systemic absorption of protein and peptides[1,2].
The colon is an ideal site for both systemic and local delivery of drugs. Treatment of large intestine disorders such as Crohn's disease, irritable bowel syndrome, ulcerative colitis and colon cancer, where a high concentration of active drug is required, can be improved by colon-targeted drug delivery system. Colon is used for systemic absorption of proteins and peptides also because proteolytic activity of colon mucosa is much less than that observed in small intestine. Drug targeting to specific sites of action offers several advantages over non targeted drugs such as prevention of side effects and reduction of doses. The colon as a site of drug delivery offers various therapeutic advantages because of its near neutral pH and longer transit time.
To reach the colon and release the drug, a dosage form must be formulated taking into account various obstacles introduced by the gastrointestinal tract. Successful delivery of a drug to the colon requires protection of the drug from degradation or release in the stomach and then controlled release of drug in colon[3,4]. The desired properties of colon targeted drug delivery systems can be achieved by using some polymers either alone or in a combination because it is now recognized that polymers can potentially influence the rate of release and absorption of drugs and play an important role in formulating colon targeted drug delivery systems. Hence an attempt to review different polymers used in colon targeted drug delivery system has been made here.
BIODEGRADABLE POLYMERS
Natural polysaccharides are extensively used for the development of solid oral dosage forms for colonic delivery of drugs[5]. Biodegradable polymers are generally hydrophilic in nature and have limited swelling characteristic in acidic pH. Various bacteria present in the colon secretes many enzymes which can cause hydrolytic cleavage of glycosidic bonds e.g. β-D-galactosidase, amylase, pectinase, β-D- glucosidase, dextranase, α-D-xylosidase[1]. These polymers are inexpensive and are available in a variety of structures. Linear polysaccharides remains intact in stomach and small intestine but the bacteria of human colon degrades them and thus make them potentially useful in colon targeted drug delivery systems[6].
Guar gum:
Guar gum is derived from the seeds of the cyomopsis tetragonolobus (Fam. Leguminosae). Chemically, guar gum is a polysaccharide composed of the sugars galactose and mannose. The backbone is a linear chain of β 1,4-linked mannose residues to which galactose residues are 1,6-linked at every second mannose, forming short side-branches (fig. 1)[7]. Guar gum is used in colon targeted drug delivery systems due to its drug release retarding property and susceptibility to microbial degradation in large intestine.
Fig. 1.
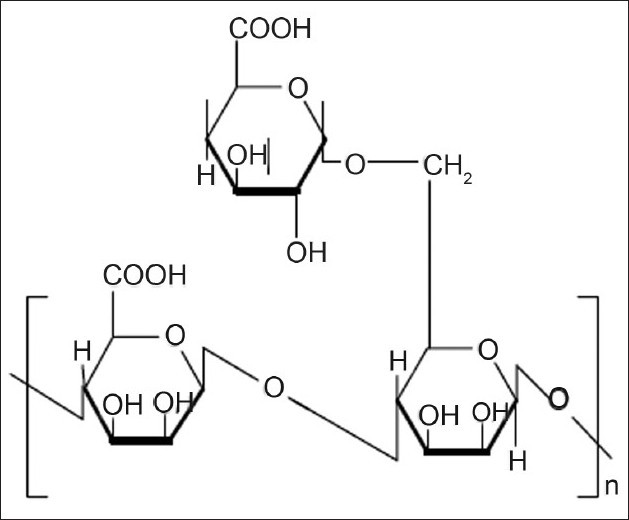
Structure of Guar Gum
Wong et al.[8] studied the dissolution of dexamethasone and budesonide from guar gum-based formulations using reciprocating cylinder dissolution apparatus (USP Dissolution Apparatus III) and observed that the drug release in simulated colonic fluid was markedly increased at galactomannanase concentrations >0.01 mg/ml. Krishnaiah et al.[9] performed a gamma scintigraphic study on guar gum matrix tablet using technetium-99m-DTPA as a tracer, in human volunteers. The scintigraphs showed that some amount of tracer present on the surface of the tablets was released in stomach and small intestine and the bulk of the tracer present in the tablet mass was delivered to the colon. These results indicated that guar gum, in the form of directly compressed matrix tablets, is a potential carrier for colon-specific drug delivery.
Krishnaiah et al.[10] in their study, performed the pharmacokinetic evaluation of guar gum-based colon-targeted tablets of mebendazole against an immediate release tablet in six healthy human volunteers. Colon-targeted tablets showed delayed tmax (9.4±1.7 h) and absorption time, and decreased Cmax (25.7±2.6 μg/ml) and absorption rate constant when compared to the immediate release tablets. The results of the study indicated that the guar gum-based colon-targeted tablets of mebendazole did not release the drug in stomach and small intestine, but delivered the drug to the colon resulting in a slow absorption of the drug and making the drug available for local action in the colon.
The potential of guar gum as a film coating material has been evaluated for colonic delivery of 5-flourouracil[11]. Guar gum based pellet systems were prepared by coating guar gum and pH-sensitive polymer Eudragit FS30D sequentially around drug-loaded non-pareil cores. The study revealed that guar gum coating worked as a time-controlled retardant and offered additional protection to the pellets until it is degraded by microbial enzymes at the proximal colon. In vitro results indicated that guar gum is a feasible coating material to achieve timed and enzyme-triggered fluorouracil release. Pharmacokinetic study in beagle dogs shows delayed absorption of about 5 h.
Core tablets containing 5-aminosalicylic acid (5-ASA) were prepared by wet granulation with starch paste and were compression coated with coating formulations containing different quantities of guar gum. The study confirmed that selective delivery of 5-ASA to the colon can be achieved using guar gum as a carrier in the form of compression coating over the drug core[12].
Pectin:
Pectin is a linear, heterogeneous polysaccharide which is mainly composed of galacturonic acid and its methyl ester. These are predominantly linear polymers of mainly (1→4) linked D-galacturonic acid residue interrupted by 1,2-linked L-rhamnose residue with a few hundred to about one thousand building blocks per molecule (fig. 2)[6]. It is one of the major sources of dietary fiber and is extracted from fruit and vegetable cell walls.
Fig. 2.
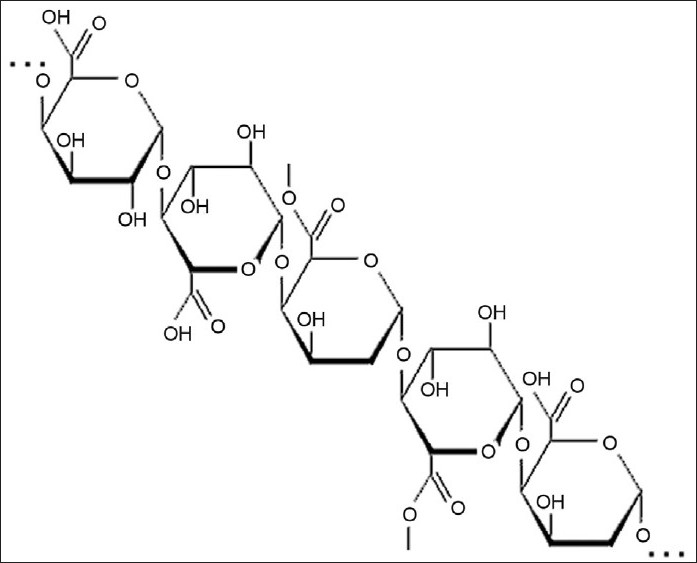
Structure of Pectin
A novel colon targeted tablet formulation using pectin as a carrier and diltiazem hydrochloride and indomethacin as model drugs has been developed[13]. In vitro study showed that prepared dosage forms have limited drug release in stomach and small intestine and released maximum amount of drug in the colon. The study revealed that pectin can be used effectively for colon targeting of both water soluble and insoluble drugs.
Calcium/zinc pectinate is a less water soluble pectin salt used in fabrication of colonic delivery system[4]. Sriamornsak et al.[14] produced cores containing theophylline, calcium acetate and microcrystalline cellulose by extrusion-spheronization and then applied a coating of calcium pectinate by interfacial complexation and reported that theophylline release from the uncoated cores was rapid and linear with the square root of time. The large coated cores released the drug over a period of about 4 h and the small coated cores released the drug over a period of 2 h.
Dupuis et al.[15] used zinc pectinate beads for colonic delivery of ketoprofen and reported similar performance when compared to calcium pectinate in hard capsules, but significant differences when the same pellets were compared encapsulated in enteric hard caspsules. This study revealed that Zinc pectinate beads could protect drug entrapped sufficiently from the upper gastro-intestinal conditions and drug release will be controlled by pectin degradation with colonic microflora. Zinc pectinate beads in enteric hard capsules are promising as a carrier for specific colonic delivery of drugs after oral administration.
Spray drying method has been employed to prepare pectin microspheres for oral colon delivery of indomethacin[16]. The prepared microspheres were crosslinked with calcium chloride. The release of Indomethacin from the cross linked pectin microspheres, was more suppressed than its release from non-cross linked microspheres. Drug release from pectin microspheres was increased by the addition of pectinase. Release of indomethacin from pectin microsphere was less in acidic pH while it was stimulated at neutral pH (pH 7.4). The results of the study clearly demonstrated that pectin microspheres prepared by spray drying and crosslinking methods are potential carriers for colon-specific drug delivery.
Pectin is a poor film former and therefore it is often used in combination with other polymers like hydroxypropylmethylcellulose, chitosan, ethylcellulose. Suresh Kumar et al.[3] prepared pectin-hydroxypropyl methylcellulose coated pellets for the colonic delivery of curcumin and reported that pectin-HPMC coated pellets offer a greater degree of protection from premature drug release in the upper GI tract.
A mixed film of pectin:ethylcellulose for colon targeted drug delivery of sennosides and triphala was prepared using non aqueous solvent like acetone and isopropyl alcohol[17]. The results of the study indicated that under simulated colonic conditions, drug release was more pronounced from coated formulations containing higher proportion of pectin.
Wei et al.[18] performed in vivo and in vitro study of pectin/ethylcellulose film-coated pellets of 5-fluorouracil for colonic targeting. The pellet cores were coated to different film thicknesses with three different pectin:ethylcellulose formulations. The 1:2 ratio pectin:ethylcellulose-coated pellets with 30% total weight gain (TWG-30%) produced more satisfactory drug-release profiles in simulated gastric, intestinal and colonic fluids. Most of the coated pellets were eliminated from the stomach in 2 h, moved into the small intestine after 2-4 h, and reached the large intestine after 4 h. The TWG-30% formulation showed delayed Tmax, decreased Cmax and prolonged mean residence time compared with uncoated pellets.
Chondroitin Sulfate:
Chondroitin sulfate is a soluble mucopolysaccharide that is used as a substrate by Bacteroides species in the large intestine mainly by B. thetaiotaomicron and B. ovatus. Chondroitin sulfate consist of β-1,3-D-glucuronic acid linked to N-acetyl-D-galactosamide (fig. 3). Natural chondroitin sulfate is cross linked and readily water soluble but it may not be able to sustain the release of most drugs from the matrix[4,19]. Chondroitin sulfate is degraded by the anaerobic bacteria of the large intestine mainly by Bacteroids thetaiotaoimicron and B. ovatus[20].
Fig. 3.
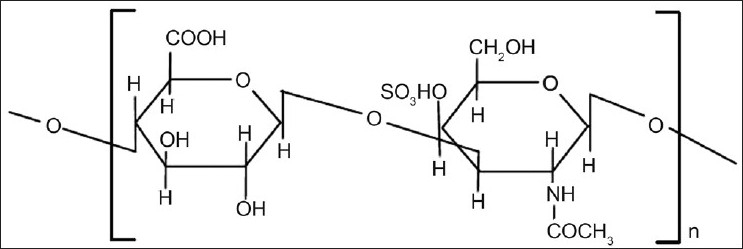
Structure of chondroitin sulfate
Chondroitin sulfate is highly water soluble and this property act as a barrier in the formulation of the colon targeted drug delivery. Rubinstein et al .[21] cross linked chondroitin sulfate with 1,12-diaminododecane. The cross linked chondroitin sulfate was used as a carrier for indomethacin specifically for the large bowel. Cross linking took place between the carboxyl group in chondroitin and the amino group in diaminododecane and formed a dimer of chondroitin sulfate. The degree of cross linking was determined by measuring the amount of methylene blue which was adsorbed as a result of cation exchange. The cross linked polymer was mixed with indomethacin and compressed into tablets. An enhanced release was observed on incubation with rat cecal contents.
Rubistein et al.[22] cross-linked chondroitin sulfate and formulated a matrix form with indomethacin as a drug marker. Cross linking was characterized qualitatively as well as quantitatively and the drug release kinetics was analysed using phosphate buffer saline solution. The amount of drug released was increased linearly with the increase in the degree of cross linking. Results of the study revealed that drug targeting to the colon may be achieved by varying the amount of cross linked chondroitin sulfate in formulations.
Amrutkar et al.[23] have prepared matrix tablet for colon specific delivery of indomethacin using chondroitin sulfate and chitosan as carrier and binder. Chondroitin sulfate was used to form polyelectrolyte complexes (PEC) with chitosan, and its potential as a colon-targeted drug carrier was investigated. The study confirmed that selective delivery of drug to the colon can be achieved using cross-linked chitosan and chondroitin sulfate polysaccharides. Cavalcanti et al. characterized crosslinked chondroitin sulfate for specific drug delivery to colon[24]. Chondroitin sulfate was crosslinked with trisodium trimetaphosphate to reduce its hydrosolubility.
Dextran:
Dextran is a polysaccharide consisting of α-1,6 D-glucose and side chain of α-1,3 D-glucose units (fig. 4)[1,19]. These highly water soluble polymers are available commercially as different molecular weights with a relatively narrow molecular weight distribution. Dextran contains a large number of hydroxyl groups, which can be easily conjugated to drugs and proteins. Dextran gets degraded by the microbial enzyme dextranases, which is found in the colon[5]. Pharmacodynamically, conjugation with dextran has resulted in prolongation of the effect, alteration of toxicity profile, and a reduction in the immunogenicity of drug.
Fig. 4.
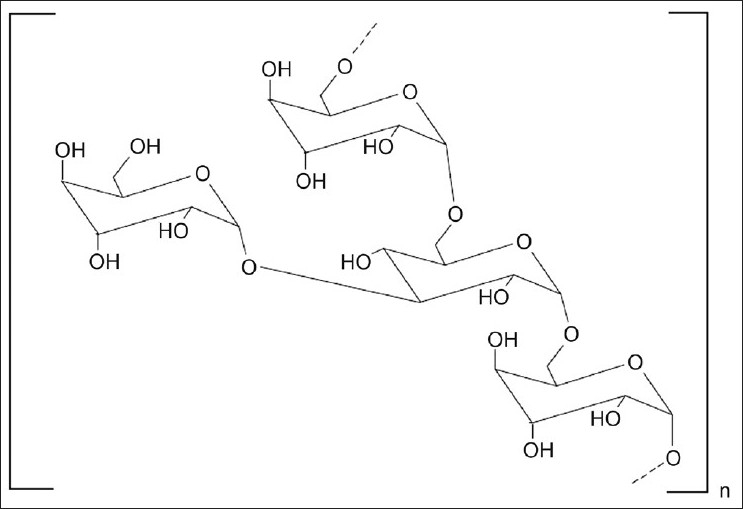
Structure of dextran
Dextran was oxidized using sodium periodate and coupled the aldehyde product with the α-amino group of 5-amino salicylic acid (5-ASA)[25]. It was reported that less oxidized dextran yields the minimum 5-amino salicylic acid conjugation, which were susceptible to dextranase hydrolysis while highly oxidized dextran yields the maximum 5-ASA conjugation, which were resistant to dextranase hydrolysis. Therefore, it was concluded that dextran can potentially be used to treat bowel inflammatory diseases.
The prepared dextran hydrogels were characterized by equilibrium degree of swelling and mechanical strength[26]. Degradation study of the hydrogels was done in vitro using dextranase, in vivo in rats and in a human fermentation model. The study indicated that the equilibrium degree of swelling, mechanical strength and degradability of the hydrogels can be controlled by changing the chemical composition. Dextran hydrogels degraded in vivo in the cecum of rats but not in the stomach suggesting that dextran hydrogels can be used as drug carriers for colon-specific drug delivery.
McLeod et al.[27] synthesized glucocorticoid-dextran conjugates in which dexamethasone and methylprednisolone were attached to dextran using dicarboxylic acid linkers (succinate and glutarate). Dextran conjugates resisted hydrolysis in upper GI tract contents but were rapidly degraded in cecal and colonic contents where the bacterial count is high. The results of this study indicate that dextran conjugates may be useful in selectively delivering glucocorticoids to large intestine for the treatment of colitis.
Chitosan:
Chitosan is functional linear polymer obtained from the alkaline deacetylation of chitin. Chitosan is consisting of the repeated units of (2-amino-2-deoxy-D-gluco-pyranose) which are linked by (1-4) β-bonds (fig. 5)[1,19]. Chitosan is a nontoxic, biodegradable, biocompatible and bioactive polymer. Chitosan is used as excipient and drug carrier in drug delivery systems. Chitosan is used for the colon targeted drug delivery because it has a tendency to dissolve in acidic pH of stomach but get swollen in the intestinal pH.
Fig. 5.
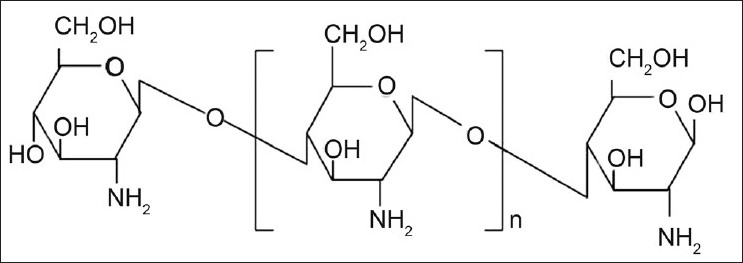
Structure of chitosan
Lorenzo-Lamosa et al .[28] designed a system consist of chitosan microcores entrapped within acrylic microspheres for the colonic delivery of sodium diclofenac. The drug was efficiently entrapped within chitosan microcores using spray-drying and then microencapsulated into Eudragit. The release rate was adjustable by changing the chitosan molecular weight or the type of chitosan salt. Furthermore, by coating the chitosan microcores with Eudragit, perfect pH-dependent release profiles were attained. A combined mechanism of release is proposed, which considers the dissolution of Eudragit coating, the swelling of chitosan microcores and the dissolution of sodium diclofenac and its further diffusion through the chitosan gel cores. This work presented new approaches for the modification of chitosan as well as a new system with a great potential for colonic drug delivery.
Chitosan capsules were used for colonic delivery of an antiulcerative colitis drug. 5-Aminosalicylic acid (5-ASA) was used as model drug. A marked increase in the release of drug from chitosan capsule was observed in the presence of the rat cecal content. From the results of this study it was concluded that chitosan capsules could be an effective carrier for the colon targeted delivery of antiinflammatory drugs[29].
Chitosan film was prepared and cross linked with citrate. Under acidic conditions, the drug was released quickly but in neutral condition, the release of drug was low. To control the release of the drug, chitosan/citrate film was again coated with alginate. The study revealed that chitosan along with citrate can be used for drug targeting to specific site[30]. Hydrogel beads of chitosan were formed with tripolyphosphate and protein release was investigated in vitro under different conditions. It was observed that under colonic environment, protein release was high due to the degradation of the beads[31].
A chitosan dispersed system was newly developed for colon-specific drug delivery which was composed of drug reservoir and the outer drug release-regulating layer dispersing chitosan powder in hydrophobic polymer. It was observed that the thickness of the outer layer controls the drug release rate. Since the dispersed chitosan dissolves easily under acidic conditions, an additional outer enteric coating was also provided to prevent the release of drug from chitosan dispersed system in the stomach[32]. Different salts of chitosan were prepared by dissolving chitosan in various acidic solutions and then spray drying these solutions[33]. From the results of the study it was concluded that drug release was reduced in acidic and alkaline pH when drug was mixed with chitosan salts.
Cyclodextrin:
Cyclodextrin is a cyclic oligosaccharide consisting of six to eight glucopyranose units joined by α-(1→4) glucosidic linkage (fig. 6). These are potential high performance carrier molecules that have the ability to alter physical, chemical and biological properties of the drug molecule through the formation of inclusion complexes. Cyclodextrins consist of six, seven or eight glucose monomers arranged in a ring shape and these are denoted as α-cyclodextrin, β-cyclodextrin and γ-cyclodextrin, respectively.
Fig. 6.
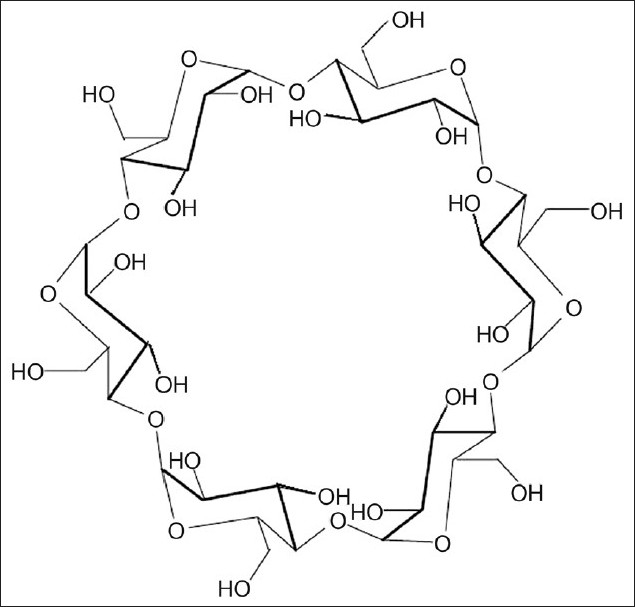
Structure of α-cyclodextrin
Cyclodextrins consist an internal lipophilic cavity, which can make complex with hydrocarbon materials. Cyclodextrins are slowly hydrolysable in upper gastrointestinal tract while it gets fermented to small saccharides by colonic microflora and get absorbed in large intestine. Cyclodextrins are used to improve the drug properties such as solubility, stability, bioavailability[1,19].
Antiinflammatory drug was conjugated with primary hydroxyl groups of alpha, beta, and gamma cyclodextrins through an ester or amide linkage. The in vivo drug release behavior of these drug-cyclodextrin conjugates was investigated in rat. The results reveal that these conjugates were stable in stomach and in small intestine. The study suggested that cyclodextrins can be used for colon specific delivery of drug[34].
Inulin:
Inulin is a naturally occurring glucofructan and consists of β 2-1 linked D-fructose molecule having a glycosul unit at the reducing end (fig. 7). It can resist the hydrolysis and digestion in the upper gastrointestinal tract. Inulin can be fermented by colonic microflora. Vervoort et al[35] developed inulin hydrogels for colonic delivery of drugs and swelling property of these hydrogels was investigated. The influence of various parameters such as the degree of substitution, feed concentration of methacrylated inulin, varying concentrations of the initiators of the polymerisation reaction, the effect of pH, ionic strength on the swelling property of hydrogels were studied. In another study Vervoort and Rombaut[36] investigated the in vitro enzymatic digestibility of the inulin hydrogels using an inulinase preparation derived from Aspergillus niger. It was concluded that the inulinase enzyme can diffuse into the hydrogels resulting in the degradation of the hydrogels.
Fig. 7.
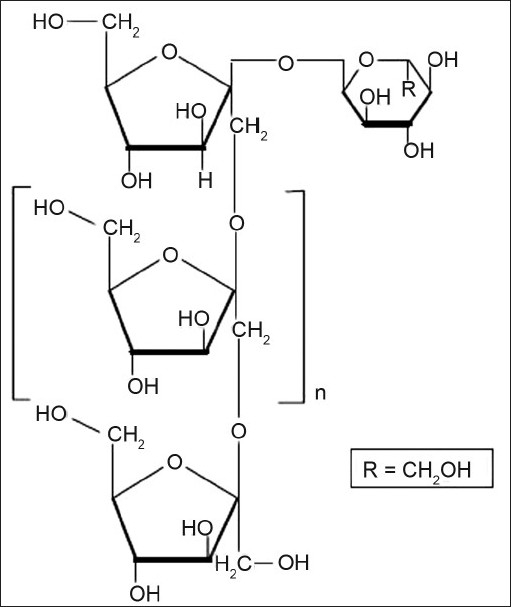
Structure of inulin
Amylose:
Amylose is the polysaccharide which is obtained from the plant extracts and a component of starch. Amylose is unbranched linear polymer of glucopyranose units (α-1,4-D-glucose) linked through α–D-(1-4) linkage (fig. 8). Amylose is resistant to pancreatic amylases in its glassy amorphous form but it gets degraded by the bacteroids, bifidobacterium.
Fig. 8.
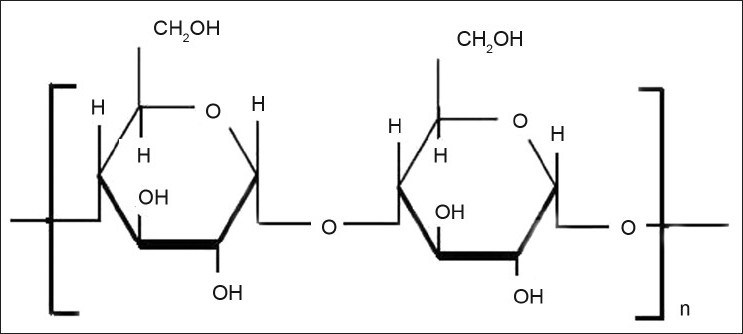
Structure of amylose
Amylose can form film by gelation, which can be used for tablet coating purpose. But coating made up of amylose solely becomes porous and release the drug under simulated gastrointestinal conditions. To avoid this problem, water insoluble polymers are added to the amylose film as these water insoluble polymers control the amylose swelling. Addition of ethylcellulose to amylose gives a suitable polymer mixture for colon targeting. In vitro dissolution of various coated pellets was performed under simulated gastric and simulated intestinal conditions and it was concluded that amylose:ethylcellulose coat (1:4) resist these conditions over a period of 12 h[37].
Pellets were prepared by extrusion and spheronisation using glucose as model drug. In vitro evaluation of these glucose containing pellets coated with an amylose-Ethocel® mixture (ratio 1:4 w/w) was performed. Gastric and small intestine resistance of the formulation was proved in vitro by dissolution release profile. In vitro fermentation study demonstrated the susceptibility towards bacterial enzymatic attack[38]. Lenaerts et al. prepared the cross-linked amylose by epichlorohydrin treatment and used it as a matrix for controlled release of drugs[39].
Cumming et al.[40] used a mixture of amylase and ethocel (1:4) to prepare microspheres of [13C] glucose which was used as a surrogate for drug delivery. The results of the study revealed that combination of amylase and ethylcellulose can be used for coating of pellets which results in controlled release of contents for targeted delivery of drug to the large bowel during a period of 12–24 h.
Locust bean gum:
Locust bean gum contains natural polysaccharides which have a molecular weight of 310000. Locust bean gum is also known as ‘Carob gum’ as it is derived from the endosperm of the seed of the ‘Carob’ (Ceratonia Siliqua Linne, Fam: Leguminosae). It is irregular shaped molecule with branched β-1,4-D-galactomannan units. Locust bean contains about 88% D-galacto-D mannoglycan, 4% of pentane, 6% of protein, 1% of cellulose and 1% of ash.
Studies on the polysaccharides done by Raghavan et al. proved that the combination of locust bean gum and chitosan, as a coating material, is capable of protecting the core tablet containing mesalazine during the condition mimicking mouth to colon transit. The coating was susceptible to the colonic bacterial enzymes which causes the release of drug. It was concluded that the formulation containing locust bean gum and chitosan in the ratio of 4:1 held a better dissolution profile, higher bioavailability and hence a potential carrier for drug targeting to colon[41].
CONCLUSIONS
Interest in the biodegradable polymers is increasing day by day because these are safe, non-toxic, economic and are chemically compatible with the other excipients in the formulation. This article has described the various types of biodegradable polysaccharides that have already been used in the initial approaches for colon specific drug delivery. Polysaccharides exhibit favorable properties for fabrication of colonic delivery system. The colon is rich in harboring excellent microflora, which can be used for targeting of drug release to colon. Formulation containing the microbial degradable polymers passes intact from the upper GIT and release the drug in the colon. Thus polysaccharides appear to be promising agents for obtaining colon-specific drug delivery systems.
Footnotes
Rajpurohit, et al.: Polymers for Colon Targeted Drug Delivery
REFERENCES
- 1.Wilson CG, Mukherji G, Sha HK. Modified-release Drug Delivery Technology. In: Rathbone MJ, Had graft J, Roberts MS, Lane ME, editors. Biopolymers and Colonic Delivery. 2nd ed. Vol. 1. New York: Informa Healthcare; 2008. pp. 295–309. [Google Scholar]
- 2.Jose S, Dhanya K, Cinu TA, Litty J, Chacko AJ. Colon Targeted drug delivery: Different approaches. J Young Pharm. 2009;1:13–9. [Google Scholar]
- 3.Kumar RS, Kumar M, Ganesh GN, Jawahar N, Nagasamyvenkatesh D, Senthil V, et al. Formulation and evaluation of pectin-hydroxypropyl methylcellulose coated curcumin pellets for colon delivery. Asian J Pharm. 2009;3:138–42. [Google Scholar]
- 4.Vyas SP, Khar RK. Systems for colon specific delivery. In: Vyas Sp, Khar RK., editors. Controlled drug delivery: Concept and advantages. 1st ed. Delhi: Vallabh Prakashan; 2002. pp. 218–56. [Google Scholar]
- 5.Jain S, Jain NK. Polymers in Pharmaceutical Sciences. In: Jain NK, editor. Pharmaceutical Product Development. 1st ed. New Delhi: CBS Publisher and Distributor; 2006. pp. 218–26. [Google Scholar]
- 6.Shirwaikar A, Shirwaikar AN, Prabu SL, Kumar GA. Herbal Excipients in Novel Drug Delivery Systems. Indian J Pharm Sci. 2008;70:415–22. doi: 10.4103/0250-474X.44587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Available from: http://en.wikipedia.org/wiki/Guar_gum .
- 8.Wong D, Larrabee S, Clifford K, Tremblay J, Friend DR. USP Dissolution Apparatus III (reciprocating cylinder) for screening of guar-based colonic delivery formulations. J Control Release. 1997;47:173–9. [Google Scholar]
- 9.Krishnaiah YS, Satyanarayana S, Prasad YV, Rao SN. Gamma Scientigraphic studies on guar-gum matrix tablet for colonic drug delivery in healthy human volunteers. J Control Release. 1998;55:245–52. doi: 10.1016/s0168-3659(98)00057-1. [DOI] [PubMed] [Google Scholar]
- 10.Krishnaiah YS, Rajua PV, Kumarb BD, Satyanarayanaa V, Karthikeyana RS, Bhaskara P. Pharmacokinetic evaluation of guar gum-based colon-targeted drug delivery systems of mebendazole in healthy volunteers. J Control Release. 2003;88:95–103. doi: 10.1016/s0168-3659(02)00483-2. [DOI] [PubMed] [Google Scholar]
- 11.Ji CM, Xu HN, Wu W. Guar Gum as Potential Film Coating Material for Colon-specific Delivery of Fluorouracil. J Biomat Appl. 2009;23:311–29. doi: 10.1177/0885328208089617. [DOI] [PubMed] [Google Scholar]
- 12.Krishnaiah YS, Satyanarayana S, Prasad YV. Studies of guar-gum compression coated 5-aminosalicylic acid tablet for colonic specific drug delivery. Drug Develop Ind Pharm. 1999;25:651–7. doi: 10.1081/ddc-100102221. [DOI] [PubMed] [Google Scholar]
- 13.Ravi V, Kumar TM, Siddaramaiah Novel colon targeted drug delivery system using natural polymers. Indian J Pharm Sci. 2008;70:111–3. doi: 10.4103/0250-474X.40346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sriamornsak P, Prakongpan S, Puttipipatkhachoran S, Kennedy RA. Development of sustained release theophylline pallets coated with calcium pectanate. J Control Release. 1997;47:221–32. [Google Scholar]
- 15.Dupuis G, Chambin O, Genelot C, Champion D, Pourcelot Y. Colonic drug delivery: Influence of cross linking agent on pectin beads properties And role of capsule shell type. Drug Dev Ind Pharm. 2006;32:847–55. doi: 10.1080/03639040500536718. [DOI] [PubMed] [Google Scholar]
- 16.Lee CM, Kim DW, Lee HC, Lee KY. Pectin microspheres for oral colon delivery: Preparation using spray drying method and in vitro release of indomethacin. Biotech Bioproc Eng. 2004;9:191–5. [Google Scholar]
- 17.Momin M, Pundarikakshudu K, Nagori SA. Design and development of mixed film of pectin: ethyl cellulose for colon specific drug delivery of sennosides and triphala. Indian J Pharm Sci. 2008;70:338–43. doi: 10.4103/0250-474X.42998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei H, Qing D, De-Ying C, Bai X, Li-Fang F. In vitro and in vivo studies of pectin/ethylcellulose film-coated pellets of 5-fluorouracil for colonic targeting. J Pharm Pharmacol. 2008;60:35–44. doi: 10.1211/jpp.60.1.0005. [DOI] [PubMed] [Google Scholar]
- 19.Jain A, Gupta Y, Jain SK. Perspectives of biodegradable natural polysaccharides for site specific delivery to the colon. J Pharm Pharm Sci. 2007;10:86–128. [PubMed] [Google Scholar]
- 20.Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci. 2003;6:33–66. [PubMed] [Google Scholar]
- 21.Rubinstein A, Nakar D, Sintov A. Colonic drug delivery: Enhanced release of indomethacin from cross linked chondroitin matrix in rat cecal content. Pharm Res. 1992;9:276–8. doi: 10.1023/a:1018910128452. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein A, Nakar D, Sintov A. Chondroitin sulfate: A potential biodegradable carrier for colon-specific drug delivery. Int J Pharm. 1992;84:141–50. [Google Scholar]
- 23.Amrutkar JR, Gattani SG. Chitosan–Chondroitin Sulfate Based Matrix Tablets for Colon Specific Delivery of Indomethacin. AAPS PharmSciTech. 2009;10:670–7. doi: 10.1208/s12249-009-9253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavalcanti OA, Silva CC, Pineda EG, Hechenleitner AW. Synthesis and characterization of phosphated crosslinked chondroitin sulfate: Potencial ingredient for specific drug delivery. Acta Farmaceutica Bonaerense. 2005;24:234–8. [Google Scholar]
- 25.Ahmad S, Tester RF, Corbett A, Karkalas J. Dextran and 5-aminosalicylic acid conjugates: Synthesis, characterisation and enzymic hydrolysis. Carbohydr Res. 2006;341:2694–701. doi: 10.1016/j.carres.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Hovgaard L, Brondsted H. Dextran hydrogels for colon-specific drug delivery. J Control Release. 1995;36:159–66. [Google Scholar]
- 27.McLeod AD, Friend DR, Tozer TN. Glucocorticoid-dextran conjugates as potential prodrugs for colon-specific delivery: Hydrolysis in rat gastrointestinal tract contents. J Pharm Sci. 2006;83:1284–8. doi: 10.1002/jps.2600830919. [DOI] [PubMed] [Google Scholar]
- 28.Lorenzo-Lamosa ML, Lopez CR, Vila-Jato JL, Alonso MJ. Design of microencapsulated chitosan microspheres for colonic drug delivery. J Control Release. 1998;52:109–18. doi: 10.1016/s0168-3659(97)00203-4. [DOI] [PubMed] [Google Scholar]
- 29.Tozakia H, Odoribaa T, Okadaa N, Fujitaa T, Terabeb A, Suzuki T, et al. Chitosan capsules for colon-specific drug delivery: Enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J Control Release. 2002;82:51–61. doi: 10.1016/s0168-3659(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 30.Shu XZ, Zhu KJ, Song W. Novel pH-sensitive citrate cross-linked chitosan film for drug controlled release. Int J Pharm. 2001;212:19–28. doi: 10.1016/s0378-5173(00)00582-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Alsarra IA, Neau SH. An in vitro evaluation of a chitosan-containing multiparticulate system for macromolecule delivery to the colon. Int J Pharm. 2002;239:197–205. doi: 10.1016/s0378-5173(02)00112-6. [DOI] [PubMed] [Google Scholar]
- 32.Shimono N, Takatori T, Ueda M, Mori M, Higashi Y, Nakamura Y. Chitosan dispersed system for colon-specific drug delivery. Int J Pharm. 2002;245:45–54. doi: 10.1016/s0378-5173(02)00344-7. [DOI] [PubMed] [Google Scholar]
- 33.Orienti I, Cerchiara T, Luppi B, Bigucci F, Zuccari G, Zecchi V. Influence of different chitosan salts on the release of sodium diclofenac in colon-specific delivery. Int J Pharm. 2002;238:51–9. doi: 10.1016/s0378-5173(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 34.Minami K, Hirayama F, Uekama K. Colon-specific drug delivery based on a cyclodextrin prodrug: Release behavior of biphenylylacetic acid from its cyclodextrin conjugates in rat intestinal tracts after oral administration. J Pharm Sci. 1998;87:715–20. doi: 10.1021/js9704339. [DOI] [PubMed] [Google Scholar]
- 35.Vervoort L, Mooter GV, Augustijns P, Kinget R. Inulin hydrogels. I. Dynamic and equilibrium swelling properties. Int J Pharm. 1998;172:127–35. [Google Scholar]
- 36.Vervoort L, Rombaut P, Mooter GV, Augustijns P, Kinget R. Inulin hydrogels. II. In vitro degradation study. Int J Pharm. 1998;172:137–45. [Google Scholar]
- 37.Milojevic S, Newton JM, Cummings JH, Gibson GR, Botham RL, Ring SC, et al. Amylose as a coating for drug delivery to the colon: Preparation and in vitro evaluation using 5-aminosalicylic acid pellets. J Control Release. 1996;38:75–84. [Google Scholar]
- 38.Milojevic S, Newton JM, Cummings JH, Gibson GR, Botham RL, Ring SC, et al. Amylose as a coating for drug delivery to the colon: Preparation and in vitro evaluation using glucose pellets. J Control Release. 1996;38:85–94. [Google Scholar]
- 39.Lenaerts V, Dumoulin Y, Mateescu MA. Controlled release of theophylline from cross-linked amylose tablets. J Control Release. 1991;15:39–46. [Google Scholar]
- 40.Cummings JH, Milojevic S, Harding M, Coward WA, Gibson GR, Botham RL, et al. In vivo studies of amylose and ethylcellulose coated [13C]glucose microspheres as a model for drug delivery to the colon. J Control Release. 1996;40:123–31. [Google Scholar]
- 41.Raghavan CV, Muthulingam C, Amaladoss J, Jenita JL, Ravi TK. An in vitro and in vivo Investigation into the Suitability of Bacterially Triggered Delivery System for Colon Targeting. Chem Pharm Bull. 2002;50:892–5. doi: 10.1248/cpb.50.892. [DOI] [PubMed] [Google Scholar]


