Abstract
The present study investigate the protective effect of aqueous root extract of Desmodium gangeticum in preserving mitochondrial and sarcoplasmic ATPase during ischemia reperfusion injury. The isolated rat hearts in both drug and control group were subjected to warm ischemia (37°), followed by reperfusion with the Langendorff perfusion system. The aqueous root extract of Desmodium gangeticum (L) at a dose of 50 mg/kg body weight was found to be effective in the rat heart for the management of ischemic reperfusion injury. Physiological parameters were significantly (P<0.05) improved in drug treated rat hearts. Creatine phosphokinase in coronary perfusate found to be declined. Moreover, sarcoplasmic ATPase and mitochondrial enzymes were significantly (P<0.05) improved in drug treated rat hearts. In fact, histological analysis of the myocardium also suggested an improved ultra structure in Desmodium gangeticum treated rat heart. These results suggest that Desmodium gangeticum aqueous root extract can preserve the mitochondrial and sarcoplasmic ATPase in the myocardium, resulting in the improvement of cardiac function after ischemia reperfusion injury.
Keywords: Desmodium gangeticum, ischemia, reperfusion, mitochondrial enzymes, sarcoplasmic ATPase
Reperfusion of the ischemic myocardium results in irreversible cell damage and necrosis, leading to decreased cardiac performance[1] and the cell damage is mainly due to oxidative stress mediated by reactive oxygen species (ROS). Reperfusing the ischemic myocardium can cause the release of ROS including hydrogen peroxide (H2O2), O2-, hydroxyl radical (•OH) and peroxynitrite (ONOO-)[2]. In fact cytotoxic ROS can also release from mitochondria during ischemia reperfusion, mainly due to the demand for energy[3]. Perhaps the released ROS from mitochondria can damage the myocyte and thereby reduced the functional recovery of heart[4]. Oxidative stress may cause depressed sarcolemmal Ca2+ -ATPase and Na+ / K+ -ATPase activities leading to altered calcium homeostasis[5].
Many pharmacological interventions were used to render cardio-protection against oxidative stress by ischemic reperfusion injury[6]. The herbal agents like garlic[7], Emblica officinalis[8], Terminalia arjuna[9] were investigated for their effect in the management of ischemic reperfused rat heart. However the searches for pharmacological agents that can ameliorate ischemic injury and delay the development of irreversible cell injury are still continuing.
Desmodium gangeticum (L.) DC (Fabaceae) (DG) is a perennial non-climbing shrub and widely used as medicinal herb in the treatment of ischemic heart diseases[10]. The presence of flavone and isoflavone glycosides was reported in the DG root. Recent study from our laboratory showed that the aqueous extract of Desmodium gangeticum root can reduce the oxidative stress mediated by isoproterenol induced myocardial infarction[11]. However no study was reported on the effect of DG in ischemia reperfusion injury and the present study was designed to investigate the cardio protective effect of DG on ischemic reperfusion injury in an isolated rat heart.
MATERIALS AND METHODS
DL isocitrate and N-Phenyl-p-Phenylenediamine were purchased from Acros organics, New Jersy USA. Cytochrome C and ATP were purchased from sigma chemical Co., St. Louis, MO USA. All other chemicals used were of analytical grade.
Adult male Wistar rats, weighing approximately 250-280 g were obtained from King Institute of Preventive Medicine, Chennai, India. They were acclimatized to animal - house conditions and were fed commercial rat pellet (Hindustan Lever Ltd., Bangalore, India) and had free access to water. The experimental protocol was approved by the institutional animal ethical committee (817/ac/CPCSEA, dated 6/8/04).
Preparation of aqueous extract of the roots of Desmodium gangeticum:
The plant after collection from the herbal garden maintained in the department was washed and cleaned. The plant material was taxonomically identified at the Department of Botany, Saint Berchman's College, Mahatma Gandhi University, Kerala. A voucher specimen A/C no. 3908 was retained in our laboratory for future reference.
One kilogram of fresh secondary roots of DG were sliced and air-dried at room temperature. The sliced, air-dried roots of the plant were milled into fine powder in a warring commercial blender. The powder was Soxhlet extracted with 2.5 l of distilled water at room temperature for 24 h with shaking. The aqueous extracts were filtered and concentrated to dryness under reduced pressure at 30±1°. The resulting aqueous extract was freeze-dried, finally giving 18.66 g (i.e., 1.866% yield) of a light-brown, powdery crude aqueous root extract of DG. Aliquot portions of the crude root aqueous extract residue were weighed and dissolved in distilled water for use on each day of our experiment.
Acute toxicity studies:
Wistar rats (150-250 g) maintained under standard laboratory condition was used. A total of five animals were used which received a single dose (2000 mg/kg body weight) of DG. Animals were kept overnight fasting prior to drug administration. After the administration of DG, the food was with held for 3-4 h. Animals were observed individually at least once during the first thirty minutes after dosing, periodically during the first 24 h (with special attention during the first 4 h) and daily thereafter for a period of 14 days. Daily cage side observation included changes in skin and fur, eyes and mucous membrane (nasal) and also respiratory rate, circulatory (heart and blood pressure), autonomic (salvation, lacrimation, perspiration, pilorection urinary incontinence and defecation changes[12].
Dosage fixation:
Dose response study of DG with the range of doses of 25, 50, 100 and 150 mg/kg body weight was carried out and the minimal effective dose of DG to cause anti ischemic reperfusion injury effect was found to be 50 mg/kg body weight.
Heart preparation:
Male Wistar rats weighing 250-280 g were anesthetized with 40 mg/kg sodium thiopentenone. After an intravenous injection of 300 U heparin, the heart was rapidly excised via a mid-sternal thoracotomy and arrested in the ice cold Krebs-Henseleit buffer (KH) containing (mM/l) NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 1.8, NaHCO3 25 and glucose 11. The heart was attached to a Langendorff apparatus via the aorta for retrograde perfusion with KH buffer maintained at 37° and pH 7.4 and saturated with a gas mixture of 95% O2 5% CO2. The coronary perfusion pressure was maintained at 80 mm Hg by a fluid column with a overflow pump, and it was measured at the aortic root via a pressure transducer. Left ventricular pressure was measured iso-volumetrically with a transducer (Statham; Gould Electronics, Cleveland, OH) connected to a thin water filled latex balloon inserted into the left ventricle through the mitral valve via a left atrial incision. Left ventricular pressure gave an indication of the mechanical performance of the heart. Heart rate, left ventricular systolic and diastolic pressure, dP/dT were measured by lab chart software (AD instruments, New Zealand). Cardiac function was determined using the product of heart rate and left ventricle developed pressure. Coronary flow was measured simply by collecting the perfusate draining from the heart in a graduated cylinder for a defined time. The heart rate was measured by counting the number of contractions (obtained from the left ventricular pressure record) per minute.
Experimental Protocol:
The rats were divided into three main groups: group I, control; group 2, reperfusion, and group 3, drug. In control group isolated hearts (n=6) were perfused for 90 min with KH buffer and used for the biochemical analysis. Reperfusion group animals were subdivided into three sub groups (n = 6 in each sub-group) namely, group 2.1, 2.2 and 2.3. Hearts in all sub groups were perfused with KH buffer initially for 20 min to equilibrate the heart to get constant hemodynamic. Subsequently the hearts were subjected to 30 min of global ischemia by stopping the perfusion of KH[13] followed by 15 min of reperfusion in subgroup 2.1. In group 2.2 and group 2.3, 30 min global ischemia followed by 30 min reperfusion and 30 min global ischemia followed by 45 min reperfusion respectively. Similarly, rats in drug group were also subdivided into 4 sub-groups namely, group 3.1, 3.2, 3.3 and 3.4. In sub groups 3.1 and 3.2, rat were pretreated with DG orally at a dose of 50 mg/kg body weight for thirty days. After 30 days, isolated rat hearts from group 3.1 were subjected to 30 min of global ischemia and followed by 30 min of reperfusion where in group 3.2, isolated hearts were subjected to 30 min of global ischemia followed by 45 min of reperfusion. In sub group 3.3, hearts (n=6) were infused with standard drug namely verapamil (0.2 mg/kg body weight)[14] for 10 min after 20 min equilibration and subjected to 30 min global ischemia followed by 30 min reperfusion. Isolated hearts (n=6) from sub group 3.4 were initially subjected to 30 min global ischemia after equilibration followed by 10 min administration of verapamil (0.2 mg/kg body weight) and subsequent 45 min reperfusion.
Tissue preparation:
The heart was excised, rinsed in ice cold isotonic saline, blotted with filter paper, weighed, homogenized in 0.25 M sucrose at 4° by Polytran homogenizer for 5 s at maximum power. The homogenate was centrifuged for 10 min at 600 g, nuclear and cytoskeleton fractions were discarded. The supernatant was centrifuged for 20 min at 15000×g (Centrifuge 5417 R, Eppendorf instruments, Germany) to pellet mitochondria[15]. The mitochondria were suspended in 0.25 M sucrose containing 10 mM Tris HCl and 1 mM EDTA to a known volume of 3 ml. The post mitochondrial (supernatant) fraction was further centrifuged at 105000 × g (Beckman Coulter ultracentrifuge) for 60 min to isolate the microsomal fraction[16]. The microsomal pellet suspended in 50 mM Tris HCl buffer pH 7.5 containing potassium chloride.
Biochemical assays:
Assay of isocitrate dehydrogenase (ICDH)[17], malate dehydrogenase (MDH)[18], succinate dehydrogenase (SDH)[19], α- ketoglutarate dehydrogenase (α KGDH)[20], NADH dehydrogenase[21] and cytochrome c oxidase[22] were carried out in a UV-1601 Shimadzu spectrophotometer. Protein concentration was measured with Folin phenol reagent, following the procedure described by Lowry[23]. After isolating the sarcoplasmic reticulum (SR), Na+ K+ ATPase[24], Ca2+ ATPase[25], Mg 2+ ATPase[26] and 5’ nucleotidase[27] were assayed.
Gas chromatography mass spectrometry:
All analysis was conducted with a Perkin Elmer Clarus 500 GC equipped with mass spectrometry. The chromatographic conditions were as follows: Column: Elite -1 (100% dimethyl poly siloxane). Helium was used as the carrier gas with a flow rate of 1 ml/min. The 1 μl DG methanol root extract was injected into the GS –MS in split less mode at 250°. The column oven temperature was held at 110° for 2 min, then programmed at 75°/min to 200° for 1 min, 5°/min to 280° and held for 9 min. Helium carrier gas was maintained at a flow rate of 1.0 ml/min.
Light microscopic study:
Myocardial tissue was fixed in 10% formalin, routinely processed and embedded in paraffin. Paraffin sections (3 μm) were cut on glass slides and stained with hematoxylin and eosin (HandE), periodic acid Schiff (PAS) reagent and examined under a light microscope.
Statistical analysis:
All values are reported as mean±SD. Results were statistically analyzed by a one-way analysis of variance (ANOVA) by SPSS software 12.00, followed by Duncan's Multiple range Test (DMRT). P<0.05 was considered to be significant. The groups were also compared with the Mann–Whitney U test and the comparisons within the groups were analyzed with Wilcoxon matched pairs test.
RESULTS AND DISCUSSION
In acute toxicity study, no signs and symptoms of toxicity and mortality were observed. Major compounds of GC/MS analysis comprises of phenol,4-[2-(dimethylamino)ethyl]-(hordenine) retention time (R.T 15.41), tri tetracontane (R.T 32.16), docosanoic acid docosyl ester (R.T 35.58), 5-hydroxy-7-(4-methoxyphenyl)-2,2-dimethyl-10-(3-methyl-2-butenyl)-2H,6H-pyrano32-g] chromen-6-one (R.T 38.57) and 15-isobutyl-(13aH)-isocopalane (R.T 44.96). It represents around 35%. Minor compounds such as 2,2-dimethyl-N-phenethylpropinamide (R.T 20.65), 5 hydroxymethyldihydrofuran-2-one (R.T 8.38) and 3-methyl-2-(2-oxopropyl)furan (R.T 21.73) (fig. 1).
Fig. 1.
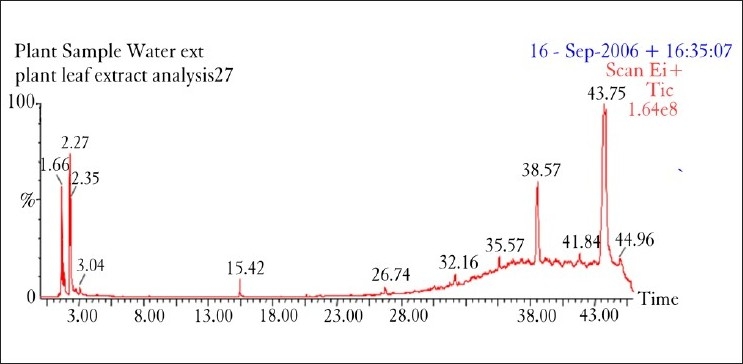
GC mass spectrum for water extract of Desmodium gangeticum root
One of the major compounds in the extract namely, Phenol, 4-(2-(dimethylamino)ethyl) (hordenine) has positive ionotropic effect upon heart and are used in the treatment of irregular heartbeat, angina pectoris, and cardiac neuralgia[28]. Similarly, docosanoic acid, another major compound in the extract, is a substrate for the ω-oxidation system in rat liver microsomes. It exert antiasynchronous effect in rat atrial myocytes by a mechanism which may involve changes in membrane fluidity[29].
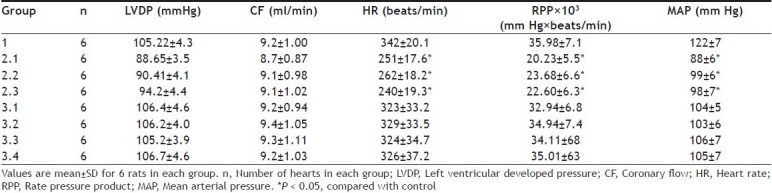
Myocardial dysfunction was observed in IR control rat hearts and was assessed indirectly by observing the significant fall of mean arterial pressure (MAP), heart rate (HR) and left ventricular developed pressure (Table 1). Ischemia reperfusion injury can reduced reflex sympathetic action and thus increased the HR when MAP decreased, indicated in Table 1. However, DG pretreated rat hearts significantly increased both HR and MAP, an indirect measure of cardiac oxygen consumption. The major consequence of the reduced left ventricular end developed pressure (LVEDP) is the increased blood flow through the sub-endocardial region of the ventricular muscle that bears the maximum impact of the ischemia. The disproportionate reduction in blood flow to the subendocardial regions of the heart was observed to be decreased in DG pretreated rat hearts; indicate the functional recovery of myocardium. Thus the favorable modulations of metabolic events by DG during ischemia results in increased myocardial salvage in reperfused myocardium.
TABLE 1.
HEMODYNAMIC CHARACTERISTIC
The functional recovery of myocardium was assessed with the help of cardiac marker enzymes. An increase in cardiac marker enzymes like CK, LDH, AST and ALT in plasma is a well known indicator of necrotic damage. The loss of intracellular constituents to extracellular space and, ultimately, to the vascular space could be divided into three relatively distinct phases: i.e., ions, metabolites, and macromolecules (such as proteins and enzymes), each of which might reflect some different aspects of cellular and metabolic damage. However, in the isolated rat heart, the perfusate from the myocardium represents plasma or serum and thus we measured the LDH level in the perfusate and made the correlation to necrotic damage. Perfusate from ischemia reperfusion control rat heart showed an elevated LDH level (Table 2) and its declined activity in DG pretreated rat heart suggest preserved cardiomyocytes and efficacy of the extract. Moreover the improved ultra structure of myocardium shown by hematoxylin and eosin staining (fig. 2) also confirm the cardio-protection by DG.
TABLE 2.
LEVEL OF CREATINE KINASE AND LACTATE DEHYDROGENASE IN THE MYOCARDIUM OF ISOLATED RAT HEART AND THE ACTIVITY OF LDH IN THE MYOCARDIAL PERFUSATE
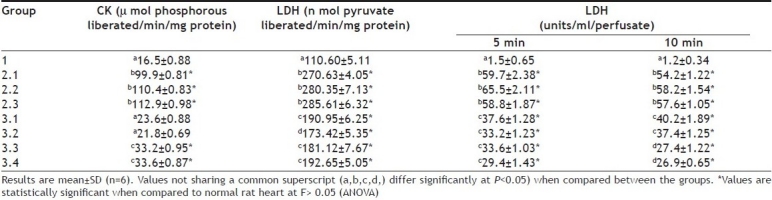
Fig. 2.
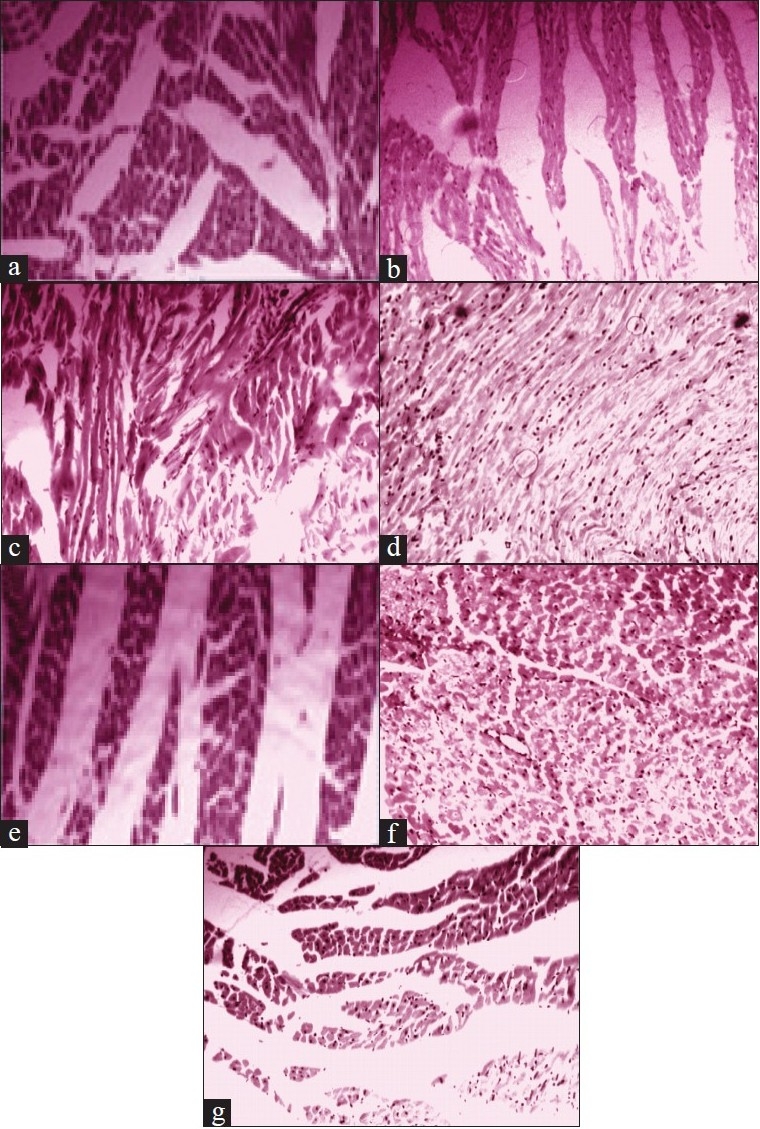
Histological analysis of rat heart with H and E staining and light microscopy. (a) Normal control; most cells are elongated and rod shaped. (b) 30 min ischemia followed by 15 min reperfusion; striated muscles and contracted bands. (c) 30 min ischemia followed by 30 min reperfusion; cell swelling, striated muscles and blood vessels seen. (d) 30 min ischemia followed by 45 min reperfusion; cell swelling and increased congested blood vessels. (e) pre- treatment with Desmodium gangeticum root extract followed by 30 min ischemia and 45 min reperfusion; Elongated cells that suggest the beneficial effect. (f) pre-treatment with Desmodium gangeticum root extract followed by 30 min ischemia and 45 min reperfusion; cells are intact and except few cells all the cells are normal. A layer of blood vessel lined by endothelial cells seen.(g) pre-treated with verapamil followed by 30 min ischemia and 45 min reperfusion; cell swelling and loss of striations.
Reperfusion therapy becomes the mainstay of treatment of patients with evolving MI and provides practical approach for salvage of ischemic myocardium. However, the basic patho-physiological reason for ischemia reperfusion injury is mainly the increased outburst of free radicals and calcium overload. Both mitochondrial complexes I and III have been implicated as sources of ROS during reoxygenation. Present study shows that reperfusion of ischemic rat heart exacerbates the decreased activities of mitochondrial enzymes (ICDH, MDH, SDH, NADH dehydrogenase and cytochrome c oxidase) and sarcoplasmic enzymes (Ca2+ ATPase, Mg2+ ATPase and 5’-nucleotidase Tables 3 and 4). This may be due to calcium-mediated inactivation of the enzymes that were complimented by the increased release of free radicals. When the oxygen is re-admitted to the myocardium under oxidative stress (ischemic myocardium) Ca2+ entry into the cells will be triggered, thereby increases the Ca2+ uptake by mitochondria. However, a decrease in SR Ca 2+ ATPase activity observed in the present study may contribute to the intracellular Ca2+ overload[30] as the enzyme is mainly required for the sequestration of cytoplasmic Ca2+ into the sarcoplasmic reticulum. According to Humphries and Szweda[31], α KGDH are more susceptible to free radical mediated inactivation. Presumably, the significant (P<0.05) decline of α KGDH during ischemic reperfusion (Table 3) in the present study emphasis a probable increased outburst of free radicals.
TABLE 3.
EFFECT OF AQUEOUS ROOT EXTRACT OF DG ON MITOCHONDRIAL ENZYMES IN ISOLATED RAT HEART
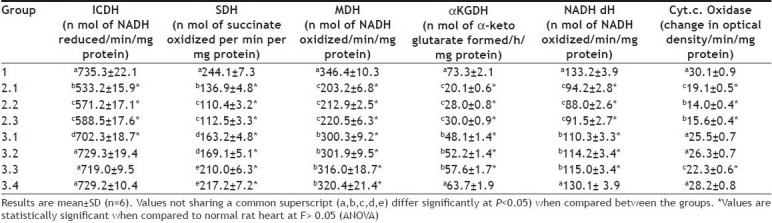
TABLE 4.
EFFECT OF AQUEOUS ROOT EXTRACT DG ON SARCOPLASMIC ATPASE ENZYMES IN ISOLATED RAT HEART
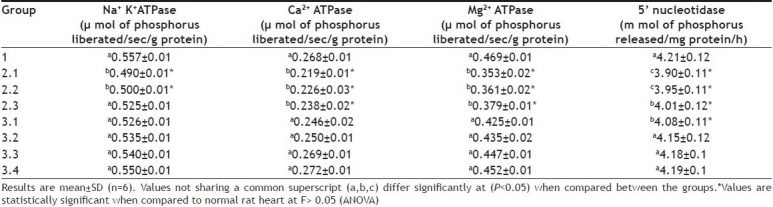
Pre-treatment of ischemic myocardium with DG significantly (P<0.05) brought the activities of the mitochondrial respiratory enzymes and some sarcoplasmic enzymes to a near normal levels (Tables 3 and 4). In fact, cardio protective effect of DG in MI was reported earlier[32]. The improvement of 5’nucleotidase activity in the DG pretreated group indicated an early release of adenosine, which is considered to be cardioprotective[33]. The beneficial effect of adenosine is mediated via the A1 receptor and it stimulates protein kinase c isoform translocation[34]. Since ischemia and reperfusion results in the decline of rate of NADH linked respiration and ATP synthesis in the cardiac mitochondria, the significant improvement in the cardiac mitochondrial enzymes and SR ATPase explained the beneficial effect mediated by DG. These findings led to the conclusion that the pre treatment of DG can reduce the extent of mitochondrial and sarcoplasmic damage during ischemic reperfusion and thereby reduce the oxidative stress. However its mechanism of action is not completely understood.
Footnotes
Kurian and Paddikkala: Effect of DG Extract on Ischemia Reperfusion Injury
REFERENCES
- 1.Park JL, Lucchesi BR. Mechanisms of myocardial reperfusion injury. Ann Thorac Surg. 1999;68:1905–12. doi: 10.1016/s0003-4975(99)01073-5. [DOI] [PubMed] [Google Scholar]
- 2.Garlickm PB, Davies MJ, Hearse DJ, Slater TF. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Cardiovas Res. 1987;61:757–60. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- 3.Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovas Res. 1995;47:446–56. doi: 10.1016/s0008-6363(00)00078-x. [DOI] [PubMed] [Google Scholar]
- 4.Becker LB, Vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–6. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 5.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bandyopadhyay D, Chattopadhyay A, Ghosh G, Datta AG. Oxidative stress-induced ischemic heart disease: Protection by antioxidants. Curr Med Chem. 2004;11:369–87. doi: 10.2174/0929867043456016. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee SK, Dinda AK, Manchanda SC, Maulik SK. Chronic garlic administration protects rat heart against oxidative stress induced by ischemic reperfusion injury. BMC Pharmacol. 2002;2:16. doi: 10.1186/1471-2210-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajak S, Banerjee SK, Sood S, Dinda AK, Gupta YK, Gupta SK, et al. Emblica officinalis causes myocardial adaptation and protects against oxidative stress in ischemic-reperfusion injury in rats. Phytother Res. 2004;18:54–60. doi: 10.1002/ptr.1367. [DOI] [PubMed] [Google Scholar]
- 9.Gauthaman K, Banerjee SK, Dinda AK, Ghosh CC, Maulik SK. Terminalia arjuna protects rabbit heart against ischemic-reperfusion injury: Role of antioxidant enzymes and heat shock protein. J Ethnopharmacol. 2005;96:403–9. doi: 10.1016/j.jep.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 10.Kirthikar KR, Basu BD. Vol. 1. Allahabad: Lalith Mohan Basu; 1975. Indian Medicinal Plants; pp. 758–60. [Google Scholar]
- 11.Kurian GA, Philip S, Varghese T. Effect of aqueous extract of the Desmodium gangeticum DC root in the severity of myocardial infarction. J Ethnopharmacol. 2005;97:457–61. doi: 10.1016/j.jep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Guideline 423, adopted 23.03.1996. In: Eleventh Addendum to the OCED guidelines for the testing of chemicals organization for economic cooperation and development. Paris: 2000. OCED. Acute oral toxicity- Acute oral toxicity class method. [Google Scholar]
- 13.Chevion M, Jiang R, Har El R, Berenshtein E, Uretzky G, Kitrossky N. Copper and iron are mobilized following myocardial ischemia: Possible predictive criteria for tissue injury. Proc Natl Acad Sci USA. 1993;90:1102–6. doi: 10.1073/pnas.90.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maulik SK, Seth SD, Manchanda SC, Reddy KS, Gupta YK, Maulik MG. Effect of verapmil post-treatment in myocardial reperfusion injury. Indian J Exp Biol. 1993;31:120–4. [PubMed] [Google Scholar]
- 15.Johnson D, Lardy H. Isolation of liver or kidney mitochondria. In: Estrabrook RW, editor. Methods in Enzymology. Vol. 10. London: Academic Press; 1967. pp. 94–6. [Google Scholar]
- 16.Lee CY, McKinney JD. Identity of microsomal glutathione S-transferases. Mol Cell Biochem. 1982;48:91–6. doi: 10.1007/BF00227609. [DOI] [PubMed] [Google Scholar]
- 17.Bell JL, Baron DN. Subcellular distribution of the isoenzymes of NADP isocitrate dehydrogenase in rat liver and heart. Enzymol Biol Clin (Basel) 1968;9:393–9. doi: 10.1159/000458268. [DOI] [PubMed] [Google Scholar]
- 18.Mehler AH, Kornberg A, Grisolia S, Ochoa S. The enzymatic mechanism of oxidation reductions between malate or isocitrate and pyruvate. J Biol Chem. 1948;174:961–77. [PubMed] [Google Scholar]
- 19.Slater EC, Bonner WD. Effect of fluoride on the succinate oxidase system. Biol Chem. 1952;52:185–96. doi: 10.1042/bj0520185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed DJ, Savage MK. Influence of metabolic inhibitors on mitochondrial permeability transition and glutathione status. Biochim Biophys Acta. 1999;1271:43–50. doi: 10.1016/0925-4439(95)00008-r. [DOI] [PubMed] [Google Scholar]
- 21.Minakami S, Ringle RL, Singer TP. Studies on the respiratory chain-linked dihydrodiphosphopyridine nucleotide dehydrogenase. I. Assay of the enzyme in particulate and in soluble preparations. J Biol Chem. 1962;237:569–76. [PubMed] [Google Scholar]
- 22.Pearl W, Cancercao J, Zweifach BW. Micro-determination of cytochrome oxidase in rat tissue by the oxidation of N-Phenyl p-phenylenediamine or ascorbic acid. J Histochem Cytochem. 1963;11:102–7. [Google Scholar]
- 23.Lowry OH, Rosenbrough NT, Farr AL. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 24.Fujita M, Nagano K, Mizuno N, Tashima Y, Nakao T. Ouabain-sensitive Mg++- ATPase, K+- ATPase and Na+- ATPase activities accompanying a highly specific Na+- K+- ATPase preparation. J Biochem. 1967;61:473–7. doi: 10.1093/oxfordjournals.jbchem.a128570. [DOI] [PubMed] [Google Scholar]
- 25.Narayanan N. Comparison of ATP-dependent calcium transport and calcium-activated ATPase activities of cardiac sarcoplasmic reticulum and sarcolemma from rats of various ages. Mech Ageing Dev. 1987;38:127–43. doi: 10.1016/0047-6374(87)90073-x. [DOI] [PubMed] [Google Scholar]
- 26.Hidalgo C, Gonzalez ME, Lagos R. Characterization of the Ca2+- or Mg2+- ATPase of transverse tubule membranes isolated from rabbit skeletal muscle. J Biol Chem. 1983;258:13937–45. [PubMed] [Google Scholar]
- 27.Smith K, Varon HH, Race GJ, Paulson DL, Urschel HC, Mallams JT. Serum 5’-nucleotidase in patients with tumor in the liver. Cancer. 1996;19:1281–5. doi: 10.1002/1097-0142(196609)19:9<1281::aid-cncr2820190914>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Hapke HJ, Strathmann W. Pharmacological effects of hordenine. Dtsch Tierarztl Wochenschr. 1995;102:228–32. [PubMed] [Google Scholar]
- 29.Jahangiri A, Leifert WR, Patten GS, McMurchie SA. Termination of asynchronous contractile activity in rat atrial myocytes by n-3 polyunsaturated fatty acids. Mol Cell Biochem. 2000;206:33–41. doi: 10.1023/a:1007025007403. [DOI] [PubMed] [Google Scholar]
- 30.Piper HM, Abdallah Y, Schafer C. The first minutes of reperfusion: A window of opportunity for cardioprotection. Cardiovasc Res. 2004;61:365–71. doi: 10.1016/j.cardiores.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Humphries KM, Szweda LI. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: Reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15835–41. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- 32.Kurian GA, Sachu P, Thomas V. Effect of aqueous extract of Desmodium Gangeticum root in the severity of isoproterenol induced myocardial infarcted rats. J Ethnopharmacol. 2005;97:457–61. doi: 10.1016/j.jep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 33.Headrick JP, Hack B, Kevin JA. Acute adenosinergic cardioprotection in ischemic-reperfused hearts. Am J Physiol Heart Circ Physiol. 2003;285:H1797–818. doi: 10.1152/ajpheart.00407.2003. [DOI] [PubMed] [Google Scholar]
- 34.Zhao TC, Kukreja RC. Protein kinase C-delta mediates adenosine A3 receptor-induced delayed cardioprotection in mouse. Am J Physiol Heart Circ Physiol. 2003;285:H434–41. doi: 10.1152/ajpheart.00095.2003. [DOI] [PubMed] [Google Scholar]


