Abstract
The primary aim of this study is to identify and analyze the importance of adverse drug reaction due to drug-drug interaction as a contributing factor towards drug safety. Patients more than 18 years of age admitted in multidisciplinary intensive care unit of a tertiary care hospital were included in this study. Patients who stayed less than 48 h and patients in whom all treatment modalities have been withdrawn and were on comfort measures only (no drugs were prescribed), were excluded. All the drugs that were given during intensive care unit stay were checked for presence of potential interactions which led to adverse drug reaction. Drug-drug interactions that were detected clinically or through investigations were recorded and also any therapeutic actions taken for drug-drug interactions were noted. From June 2006 to April 2007, 400 patients-prescriptions were analyzed. Adverse drug reactions due to drug-drug interactions were identified in 64% patients. Among those patients 38.67% had a single drug-drug interaction. Potential drug-drug interactions were 602. Clinically significant drug-drug interactions among the potential were 208 (34.55%). Clinically relevant drug-drug interactions were 103 (49.52% of 208 episodes). The adverse drug reactions due to drug-drug interactions in our sample were managed either by substituting another drug (50.48% of 103 episodes) or by adjusting the dose (1% of 103 episodes) or by omitting the drug (48.54% of 103 episodes). Among the 208 observed drug-drug interactions induced adverse drug reactions 21.63% was severe drug-drug interactions induced adverse drug reactions, 23.08% was moderate drug-drug interactions induced adverse drug reactions and 55.29% was minor drug-drug interactions induced adverse drug reactions. The interactions which were life threatening and/ or require medical intervention to minimize or prevent serious adverse effects were considered as severe drug-drug interactions and those interaction which resulted in an exacerbation of the patient's condition and/ or require an alteration in therapy were considered as moderate drug-drug interactions. The interactions which were limited clinical effects and manifestations may include an increase in the frequency or severity of side effects but generally would not require a major alteration in therapy were classified as minor drug-drug interactions. The correlation coefficient was 0.86 between the number of drugs given to the patient & number of average potential adverse drug reactions found among the patients. Increase in number of prescribed drug significantly (one way) increases number of potential adverse drug reaction due to drug-drug interaction (p<0.0001). Critically ill patients are more susceptible to drug-drug interactions due to the administration of multiple drugs and complex drug combinations. Several drug-drug interactions were clinically irrelevant.
Keywords: Adverse drug event, adverse drug reaction, critically ill patients, drug-drug interaction, intensive care unit, polypharmacy
An adverse drug reactions has been defined by the World Health Organization as ‘any response to a drug which is noxious and unintended, and occurs at doses normally used in man for prophylaxis, diagnosis or therapy of disease, or for modification of physiological function’[1]. Modern intensive care has become much more complex over year than providing support for failure of a single organ system. Intensive care unit patients are more susceptible to adverse drug reaction due to polypharmacy. One of the consequences of multiple drug use is the risk of one drug influencing the activity, the availability or the effect of a second drug. This so-called drug interaction can be desired or can result in adverse effects like reduced effectiveness or increased toxicity of the involved drugs[2,3]. The rapidly changing physiology of critically ill patients causes variations in the absorption, distribution, metabolism, excretion, and pharmacodynamic effect of drugs used to treat these patients. Alterations in fluid status, cardiac, renal and hepatic function, and circulating serum proteins necessitate increased attention to drug selection and dosage modification. Cardiac failure results in decreased absorption, metabolism, and excretion of drugs while renal failure results in parent drug and metabolite accumulation, increases in unbound drug, and changes in distribution volume. The changes in hepatic blood flow and protein binding, and decreases in hepatocellular mass and enzyme function that occur in hepatic failure may alter the clearance of several drugs. Serum drug concentrations are sometimes helpful in defining the pharmacokinetics and ultimately the pharmacodynamic effect of the drugs used in critically ill patients. The serum drug concentrations must be interpreted in association with their pharmacodynamic effect and the clinical situation[4–7].
In a study conducted by Smith et al[8], the frequency of drug ‘reaction’ in hospitalized patients had been studied, and they had shown that a linear increase in the number of drug led to an exponential increase in the number of reactions. The author(s) attributed the excess of morbidity to drug interaction and concluded that there was a 24% chance of an ‘adverse interaction’ when ten or more drugs are administered concurrently. However, in day-to-day practice, drug interactions are not so commonly seen. The possible explanation may be, the interactions usually do not pose a problem if they are rapidly recognized and treated; drugs for specific organ system are more being used by trained persons; qualitative nature of interactions is predictable even though the magnitude of response might not be known with certainty or any ‘drug reaction’ is not being recognized as ‘drug interaction’. Furthermore, the pharmacologic effects of many drugs are altered in critically ill patients. For these reasons, critically ill patients may be more susceptible to adverse drug events related to drug interactions. Studies on opioids[9] and hypnotics[10] showed that different patients may have a three to five fold difference in the therapeutic and toxic effect of a drug, given alone, which may be due to genetic and environmental factors. Hence introduction of more drugs also can alter the variability to a greater extent. Clinically significant interaction involves drugs with small therapeutic window. Sometimes when a drug fails to produce an effect, it is considered that the patient is tolerant or resistant and almost never considered as drug interaction. The usefulness of the data evolved from such study is immense. Data generated from this study can be used to develop protocols for the clinical management of adverse drug reaction, dose modification for additional drugs or combination medications and help us to consider methodological issues in adverse drug reactions studies. This study will also help us to evaluate interactions with newer drugs used currently. Lastly, knowledge of the epidemiology of drug interactions may help critical care practitioners reduce the risk for adverse drug events in this patient population.
The objectives of this study were to identify incidences of adverse drug reactions (ADRs) due to drug-drug interactions (DDIs), to study implications of adverse drug reaction due to drug-drug interactions and how they changed management in individual critically ill patient.
It was a prospective and observational study. A survey was carried out in the Department of Intensive Care, AMRI Hospital, Kolkata, from June 2006 to April 2007. Four hundred patients of either gender admitted in the medical-surgical unit of the ICU of the hospital were included in the study.
The study was approved by the Ethics Committee of the involved institutions. Confidentiality was maintained, patients and physicians were not identified for collection, informed consent was not considered necessary by the Ethics Committee of the involved institutions because it was a survey involving the collection or study of existing data, documents, records of pathological and diagnostic and the information is recorded by the investigators in such manner that the subjects can’t be identified, directly or through identifier linked to the subjects.
Patients less than 18 years of age and patients staying less than 48 h in intensive care unit (ICU) were excluded from this study. Patients in whom all treatment modalities had been withdrawn and were on comfort measures only were also excluded. All medical and post surgical patients admitted to the above-mentioned ICU were included in the study.
The drug list of every patient was taken from the nursing record sheet, used during ICU stay. The study was limited to drugs that were ordered on ICU admission and during ICU stay. Every patient had a data sheet enumerating all the drugs given to the patient. Any new events, clinical or laboratory or the action taken for that event was recorded, drug events/ interactions were documented as clinical symptoms and signs, laboratory changes, ECG changes, any increased or decreased requirement of the same or other drugs or any other therapeutic intervention required like introduction of other therapy. It was followed by extensive literature search, books, online database to find out whether this could be attributed to drug-drug interaction. The patients’ medication records were reviewed daily along with patients’ daily progress record for evidence of any new adverse drug events. Known adverse drug reactions for each drug were followed in every patient and those that were manifested were recorded. Daily prescriptions, dosage, consumption and route of administration of all medical and pharmaceutical agents were recorded manually.
During a 6 month period, a total of 400 prescriptions were taken. Drug-drug interactions were identified using the Epocrates online 2006 and the Medclik online 2006 systems. ADR of the prescribed drugs were identified theoretically by Epocrates online system. The Epocrates system contains DDI, mechanism of DDI, ADR. Medclik online system contains DDI, mechanism of DDI, the systems also provided information about clinical consequences that could result from a DDI. Those theoretical ADR and DDI were documented as potential ADR and potential DDI respectively. The laboratory records, ECG records, daily patients’ clinical signs and symptoms monitoring reports etc were checked. It was tried to find out those clinical reports were in support of the documented ADR were DDI. Affirmative results were considered as significant DDI and ADR. If any DDI of two drugs found the ADR of either one or both drugs or if any DDI increased the chances of adverse drug reaction of any one drug by known pharmacology of those drugs then those adverse drug reactions were considered and documented as an ADR precipitated due to DDI. The incidence of drug interaction was analyzed from recorded data. Drug interactions are ADRs that caused by drug-drug or drug-food interactions[11]. In our study only ADRs that caused by drug-drug interactions were observed. The interactions observed were classified according to severity and undesirable effect as per Anatomical Therapeutic Chemical Criteria[12,13].
Severe, where the interaction was life threatening and/or required medical treatment or intervention to minimize or prevent severe adverse effects[12,13]. Moderate, where the interaction resulted in exacerbation of the disease of the patient and/ or change in therapy[12,13]. Mild is when the interaction limited the clinical effects and the manifestations included an increase in frequency or severity of adverse effects, but did not require change in therapy[12,13].
All raw data was recorded in Excel (Microsoft office XP Version) sheet. Results were expressed as proportions and as means±standard deviations (SD) or medians with the corresponding range and correlation coefficient. Statistical significance was defined as P-value <0.05. Statistical analysis was performed using Medcalc software.
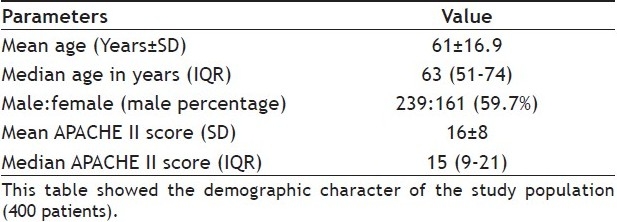
The study encompassed data on 400 patients admitted in ICUs of AMRI hospital, Kolkata for various medical and surgical illnesses. The study was carried out for a period of ten months extending from June 2006 to April 2007. The mean age of the study population was 61±16.9. The median age of the study population was 63 (51-74) years. Male:female 239:161 (59.7%). Mean Acute Physiological Chronic Health Evaluation (APACHE) II Score 16±8. APACHE II Score was taken into consideration to find the severity of illness of the patient cohort (Table 1).
TABLE 1.
DEMOGRAPHIC CHARACTER OF STUDY POPULATION
Average drugs used per patients were 9 (SD±4). Each prescription had a median of 8 drugs (IQR 6-11) (fig. 1). Average number of interaction found per patient was 2 (SD±2). Median interaction found was one (IQR 0-2). From the 400 patients’-prescriptions analyzed, ADR due to DDIs was identified in 256 (64%) of 400 patients. Only 99 (38.67% of 256) had a single DDI, while 157 (61.33% of 256) prescription presented more than one. Total number of potential ADR due to DDIs found in our sample was 602 episodes. Total number of observed ADR due to DDI was 208 (34.55% of 602). Total number of undocumented ADR due to DDI was 124 (20.59% of 602).
Fig. 1.
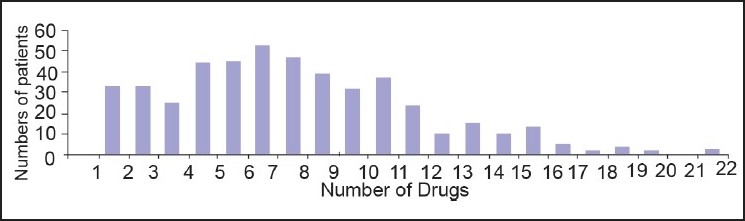
Number of drugs given to different patients. Maximum numbers of patients were prescribed more than 7 drugs simultaneously. Poly-Pharmacy is a one of the reason of adverse drug reaction due to drug-drug interaction in ICU
Clinically relevant ADR means where ADR altered the course of treatment and interventions of any form were required. Total number of clinically relevant ADR was 103 of 208 episodes (49.52% of 208). The ADR in our sample were managed either by substituting another drug (50.48%) or by adjusting the dose (1%) or by omitting the drug (48.54%).
Based on the ATC classification[12,13] we found ADR precipitated due to DDI, 45 episodes (21.63%) were severe ADR, 48 episodes (23.08%) were moderate ADR and 115 episodes (55.29%) were minor DDI among the 208 observed ADR in the study cohort.
Correlation coefficient between the number of drugs given to the patients and number of average potential adverse drug reactions found among the patients was 0.86. The Relationship between the number of drugs given and number of adverse drug reactions is given in fig. 2. Increase in number of prescribed drugs significantly (one way) increase potential adverse drug reaction due to drug-drug interaction (P<0.0001). From this value, it was predicted that number of average potential interaction was positively correlated with the number of drugs given to the patients.
Fig. 2.
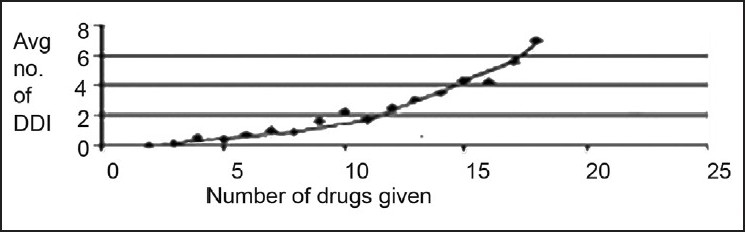
Relationship between the number of drugs given and number of adverse drug reactions
Number of adverse drug reactions due to drug-drug interactions increases with the increase of number of prescribed drugs.
Our study demonstrated that overall, approximately 64% of all patients’ population (400 patients) experienced potential ADRs due to DDIs. This fig. was higher than the study in ambulatory patients, where the overall incidences of drug related complications were approximately 20% among the total patients population[14]. In another out patient population >65 years of age, 10% ADR were found, of which 10% (0.7% of the studied population) were sent to hospital[15]. In our study the mean age of the study population was 61±16.9 median age 63 (51-74) in years.
In our study total number of clinically relevant ADR was 103 of 208 episodes (49.52% of 208). The ADR due to DDIs in our sample were managed either by substituting another drug (50.48% of 103 episodes) or by adjusting the dose (1% of 103 episodes) or by omitting one of the interacting drugs (48.54% of 103 episodes). This result was also comparable with the previous studies where 30–60% of ADRs were considered to be potentially preventable[16–21]. Reasons for the variation of the rates may be in part the methods of detection (ADR – definition, computerized or manual surveillance) and case ascertainment (all hospitalized patients focused hospital unit surveillance[21]. In some previous studies fatality were reported to be 14-5% in patients, who were hospitalized due to an ADR or who experienced an ADR during hospitalization[22].
We found ADR, 45 (21.63%) being major ADR, 48 (23.08%) being moderate ADR and 115 (55.29%) being minor ADR among 208 observed ADR. A recent study with out patients found a rate of 50 ADR in 1000 patient years, of which 27.6% were preventable[23]. Reasons for low preventability were inadequate patient monitoring by health care providers (36.6%), prescription error (wrong medication/ wrong indication 27.1%), wrong dose (24.0%), and patient non compliance (21.1%) and in 13.3% established DDIs. The majorities of ADEs were iatrogenic (88.8%) and were caused by overdose, wrong indication or interaction. As a large amount of ADRs and ADEs are preventable, more attention should be addressed to prevention management. Among patients visiting in an Emergency Department in Italy 4% experienced an ADE, of which another 4% were due to a DDI[24]. The ADE led to hospitalization in 19% of all patients who had suffered an ADE of which 11% were caused by a DDI. The incidence of DDI–associated hospital admission per 100 admissions was 0.27%.
In our study average drugs used per patients were 9 (SD±4). Each prescription had a median of 8 drugs (IQR 6-11). These frequencies were similar to those reported by other groups[25,26]. Correlation coefficient between the number of drugs given to the patient and number of average potential adverse drug reactions found among the patients was 0.86. Increase in number of prescribed drugs significantly increase number of potential adverse drug reaction due to drug-drug interaction (P<0.0001).
The probability of adverse drug reactions due to drug-drug interactions increases with number of drugs administered. Further more the pharmacologic effects of many drugs altered in critically ill patient population. Polypharmacy is an important problem in critically ill patient population. For these reasons, critically ill patients may be more susceptible to adverse drug events related to drug-drug interactions.
ACKNOWLEDGEMENTS
We would like to thank to Prof (Dr.) Biswanth Sa of Pharmaceutical Technology, Jadavpur University, Kolkata-700 032 for rendering us valuable help and necessary facilities to carry out this work smoothly. We would like to thank Dr. T. K. Chatterjee, Reader, Pharmaceutical Technology, Jadavpur University, Kolkata-700 032. We would also like to express our thanks to all our respected teachers, librarian for their immense help and support.
Footnotes
Ray, et al.: Drug-Drug Interactions Induced Adverse Drug Reactions
REFERENCES
- 1.WHO Collaborating Centre for International Drug Monitoring. Definition Section: Uppsala monitoring centre. 2004. [Last Retrieved on 2005 Aug 01]. Available from: http://www.who-umc.org/umc.html/
- 2.Schaefer TJ, Wolford RW. Disorders of potassium. (8-9).Emerg Med Clin North Am. 2005;23:723–47. doi: 10.1016/j.emc.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Perazella MA. Drug-induced hyperkalaemia: Old culprits and new offenders. Am J Med. 2000;109:307–14. doi: 10.1016/s0002-9343(00)00496-4. [DOI] [PubMed] [Google Scholar]
- 4.Crone CC, Gabriel GM. Treatment of anxiety and depression in transplant patients: pharmacokinetic considerations. Clin Pharmacokinet. 2004;43:361–94. doi: 10.2165/00003088-200443060-00002. [DOI] [PubMed] [Google Scholar]
- 5.DeBellis RJ. Drug Dosing in Critically Ill Patients with Renal Failure: A Pharmacokinetic Approach. Intensive Care Med. 2000;15:273–313. [Google Scholar]
- 6.Perea JR, Díaz De Rada BS, Quetglas EG, Juarez MJ. Oral versus intravenous therapy in the treatment of systemic mycosis. Clin Microbiol Infect. 2004;10:96–106. doi: 10.1111/j.1470-9465.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- 7.Grüneberg RN, Wilson AP. Anti-infective treatment in intensive care: The role of glycopeptides. Intensive Care Med. 1994;20(Suppl 4):S17–22. doi: 10.1007/BF01713978. [DOI] [PubMed] [Google Scholar]
- 8.Smith JW, Seidl LG, Cluff LE. Studies on the epidemiology of adverse drug reactions: V clinical factors influencing susceptibility. Ann Intern Med. 1966;65:629–40. doi: 10.7326/0003-4819-65-4-629. [DOI] [PubMed] [Google Scholar]
- 9.Ausems ME, Hugg CC, Jr, Stanski DR, Burm AG. Plasma concentrations of alfentanyl required to supplement nitrous oxide anesthesia for general surgery. Anesthesiology. 1986;65:362. doi: 10.1097/00000542-198610000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Dundee JW, Robinson FP, McCullum JS, Patterson CC. Sensitivity to propofol in the elderly. Anasthesia. 1986;41:482–5. doi: 10.1111/j.1365-2044.1986.tb13271.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalanchnik JE. Measuring Side Effects of psychopharmacologic medication in individuals with mental retardation and developmental disabilities. Ment Retard Dev Disabil Res Rev. 1999;5:348–59. [Google Scholar]
- 12.Cerulli J. The role of Community pharmacist in identifying, preventing and resolving drug-related problems [text on the internet] [Last cited on 2007 Mar 21]. Available from: http://www. medscape.com/viewarticle/421293_print .
- 13.Anatomical Therapeutic Chemical Classification System [text on the internet] [Last cited on 2007 Jan 25]. Available from: http://en.wikipedia.org/wiki/Anatomical_Therapeutic_Chemical_Classification_System .
- 14.Gandhi TK, Burstin HR, Cook EF, Puopolo AL, Haas JS, Brennan TA, et al. Drug complications in out patients. J Gen Intern Med. 2000;15:149–54. doi: 10.1046/j.1525-1497.2000.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrischilles EA, Segar ET, Wallace RB. Self reported adverse drug reactions and related resource use: A study of community – dwelling persons 65 years of age and older. Ann Intern Med. 1992;117:634–40. doi: 10.7326/0003-4819-117-8-634. [DOI] [PubMed] [Google Scholar]
- 16.Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events Implications for prevention: ADE prevention Study Group. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 17.Leape LL, Cullen DJ, Clapp MD, Burdick E, Demonaco HJ, Erickson JI, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267–70. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]
- 18.Dartnell JG, Anderson RP, Chohan V, Galbraith KJ, Lyon ME, Nestor PJ, et al. Hospitalization for adverse events related to drug therapy, incidence avoidability and costs. Med J Aust. 1996;164:659–62. doi: 10.5694/j.1326-5377.1996.tb122235.x. [DOI] [PubMed] [Google Scholar]
- 19.Evans RS, Pestotnik SL, Classen DC, Horn SD, Bass SB, Burke JP. Preventing adverse drug events in hospitalized patients. Ann Pharmacother. 1994;28:523–7. doi: 10.1177/106002809402800417. [DOI] [PubMed] [Google Scholar]
- 20.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A metaanalysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 21.Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients: Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301–6. [PubMed] [Google Scholar]
- 22.Einarson TR. Drug – related hospital admissions. Ann Pharmacother. 1993;27:832–40. doi: 10.1177/106002809302700702. [DOI] [PubMed] [Google Scholar]
- 23.Gurwitz JH, Avon J. The ambiguous relation between aging and adverse drug reactions. Ann Intern Med. 1991;114:956–66. doi: 10.7326/0003-4819-114-11-956. [DOI] [PubMed] [Google Scholar]
- 24.Raschetti R, Morgutti M, Menniti – Ippolito F, Belisari A, Rossignoli A, Longhini P, et al. Suspected adverse drug events requiring emergency department visits or hospital admissions. Eur J Clin Pharmacol. 1999;54:959–63. doi: 10.1007/s002280050582. [DOI] [PubMed] [Google Scholar]
- 25.Herr RD, Caravati EM, Tyler LS, Iorg E, Linscott MS. Prospective evaluation of adverse drug interactions in the emergency department. Ann Emerg Med. 1992;21:1331–6. doi: 10.1016/s0196-0644(05)81897-9. [DOI] [PubMed] [Google Scholar]
- 26.Gaddis GM, Holt TR, Woods M. Drug interactions in at-risk emergency department patients. Acad Emerg Med. 2002;9:1162–7. doi: 10.1111/j.1553-2712.2002.tb01571.x. [DOI] [PubMed] [Google Scholar]


