Abstract
Plants are an important source of neutraceuticals that have proved to be effective against important microbial infections of humans. Lower plants are gaining importance in this regard. The present study is aimed at investigating the antimicrobial properties of three selected ferns, Psilotum nudum, Nephrolepis biserrata and Nephrolepis cordifolia. The aerial parts of the selected ferns, P. nudum, N. biserrata and N. cordifolia, were fractionated in different solvents. These fractions were concentrated to obtain a powder and were tested against nine bacterial and three fungal strains according to disc diffusion method. The water and ethanol fractions were active against most of the tested bacterial and fungal strains, some of these were more effective than the controls tested. Present study suggests that the pteridophytes, P. nudum, N. biserrata and N. cordifolia could be good source of antimicrobials. These natural compounds might be more effective as the microbes may have lesser chance of developing resistant mutants.
Keywords: Antibacterial, antifungal, dermatophytes, disc diffusion assay, Nephrolepis biserrata, Nephrolepis cordifolia, Psilotum nudum, pathogenic bacteria
Use of antibiotics is often associated with adverse effects such as hypersensitivity, depletion of beneficial gut and mucosal microorganisms, immune-suppression and allergic reactions in the user. Besides, the indiscriminate use of antimicrobial drugs has created immense clinical problems in the treatment of infectious diseases[1], as the microorganisms develop resistance to often used antibiotics. This necessitates development of alternative antimicrobial drugs, for the treatment of infectious diseases, from the medicinal herbs which are a rich source of novel antibacterial and antifungal chemotherapeutics[2]. Angiosperms are an immense source of therapeutics. However, lower plants are attracting more attention in recent times for the search of new and effective molecules. The medicinal value of the pteridophytes has been known for several years. Antimicrobial properties of ferns are remarkable as compared to the higher plants may be because of the presence of a large number of defensive biochemical compounds[3,4].
The present study has been undertaken to assess the antimicrobial properties and therapeutic value of three pteridophytes P. nudum, N. biserrata and N. cordifolia. This is the first report of the antibacterial and antifungal properties of the non-aqueous frond extracts of these three pteridophytes. P. nudum is an epiphyte that grows as a terrestrial plant in rocky crevices or in sandy soils and being a spore-producing vascular plant is considered to be a fern ally[4]. Ethnomedicinal uses of P. nudum are as general pain reliever especially dental pains and for easing bowls[5]. This pteridophyte is reported to contain an alkaloid psilotin, an insect feeding deterrent and growth reducer[6] and 3’-hydroxypsilotin, a glycoside[7]. N. biserrata and N. cordifolia belong to the family Davalliaceae and have been reported to be of immense ethnobotanical importance. N. biserrata is widely distributed and having escaped cultivation has become naturalized in many countries. Its ethnomedicinal importance in boils, abscesses and blisters is well known[5]. Incidentally, boils[8] and abscess[9] are due to bacterial infections and blisters are caused by fungal infection[10]. Thus, N. biserrata can be a potential fern to fight against pathogenic microbes. N. cordifolia is a pantropical species growing from tropical to temperate areas[11] and ethnomedically used in general disorders of renal and liver systems, skin diseases and as a contraceptive[5]. Aqueous extracts of N. cordifolia have been earlier reported to have antimicrobial activity[12].
The extracts were prepared from the fronds of P. nudum, N. biserrata and N. cordifolia growing in the fern house of National Botanical Research Institute, Lucknow. The fronds were dried in shade, powdered and extracted with 50% ethanol in a percolator. The extracts were concentrated in a rotavapour (Eyela, Japan) at 45°. The concentrate was lyophilized to a dry yellowish to brownish powder and weighed. The lyophilized powder was then fractionated three times separately in hexane, chloroform, ethanol and water in that order. Each of these fractions was used as a test extract[3,13–16].
Preliminary phytochemical screening of the extracts obtained from the three plants was performed for, flavonoids using Shinoda's test[17], tannins through Ferric chloride test[18], alkaloids using Dragendroff's test[19], reducing sugars with Fehling's test[20], triterpenoids with Liebermann-Burchard's test[21] and steroids as described by Hardman and Sofowora[22]. The antibacterial effect of the fern extracts was studied on nine bacterial strains, namely, Proteus mirabilis (MTCC-1429), Pseudomonas aeruginosa (MTCC-424), Salmonella typhimurium (MTCC-98), Bacillus subtilis (MTCC-121), Streptococcus faecalis (MTCC 1927), Staphylococcus aureus (MTCC-96), Klebsiella pneumoniae (MTCC-109), Bacillus cereus (MTCC-430), Shigella flexneri (MTCC 1456) and Escherichia coli (MTCC-443), chosen because of their pathogenecity. The pure cultures of these bacterial strains were obtained from the Institute of Microbial Technology (IMTECH), Chandigarh, India. Antifungal activity of the extracts was tested on three fungal species, namely, Microsporum gypseum (ATCC-24102), Trichophyton mentagrophytes (ATCC-9533) and Trichophyton rubrum (ATCC-28188) that are important dermatophytes. The fungal strains were procured from Himedia, India.
The bacteria were maintained and tested on Mueller Hinton Agar/Broth (MHA/MHB, Oxoid). The fungi were maintained and tested on Saubouraud Dextrose Agar/broth (SDA/SDB, Oxoid). Prior to pouring into petriplates all media were sterilized at 121° and 15 lbs for 20 min.
Agar disc diffusion method[23] was used to evaluate the antimicrobial activity. A final inoculum of 100 μl suspension containing 108 CFU/ml of each bacterium and fungus was used. The bacterium was spread on Mueller Hinton Agar (MHA) medium and each fungus was spread on Saubouraud Dextrose Agar. The disc (6 mm diameter) was impregnated with 10 μl of 50 mg/ml extracts and placed on the seeded agar. Gentamycin (30 μg/disc) and erythromycin (30 μg/disc) were used as positive controls for bacterial pathogens and ketoconazole (30 μg/disc) and amphoterecin-B (30 μg/disc) were used as positive controls for the fungal pathogens. The test plates were incubated at 37° for 24 h for bacterial pathogens and 27° for 72 h for fungal pathogens or for a period required for a visible growth[24]. Each set of experiment was performed in triplicates[12].
The phytochemical tests indicated the presence of all the metabolites such as flavonoids, tannins, alkaloids, reducing sugars, triterpenoids and steroids in the three pteridophytes, P. nudum, N. biserrata and N. cordifolia. The yield on fractionation of the crude extract varied depending on the solvent used. The water fraction gave the maximum yield and it was as follows: P. nudum - 662 0μg/ml, N. biserrata - 679 μg/ml and N. cordifolia - 788 μg/ml. In the chloroform fraction the yield was maximum for P. nudum (59 μg/ml) and quiet less for N. biserrata (9 μg/ml) and N. cordifolia (3 μg/ml). The yield of hexane fraction was maximum for P. nudum (62 μg/ml) followed by N. biserrata (33 μg/ml) while N. cordifolia yielded only 6 μg/ml. The ethanol fraction yielded more or less the same amount for P. nudum (34 μg/ml) and N. cordifolia (37 μg/ml) and was only marginally less for N. biserrata (24 μg/ml).
The results of the effect of various extracts and controls are presented in Table 1 for the bacterial strains and in Table 2 for the fungal strains. Water extract of all the three species was the most effective in inhibiting growth of all the bacterial strains (Table 1). The water extracts of P. nudum was least effective in inhibiting the growth of P. aerugenosa and most effective against Salmonella typhimurium, that of N. biserrata was less inhibitory to the growth of Enterobacter aerogenes and most inhibitory against Escherichia coli and N. cordifolia extract was least inhibitory on the growth of Proteus mirabilis and Streptococcus feacalis and most effective against Salmonella typhimurium and Bacillus cereus. None of the water extracts were as effective as the gentamycin or erythromycin controls in inhibiting the growth of the bacteria (Table 1).
TABLE 1.
ANTIBACTERIAL SCREENING OF P. NUDUM, N. BISERRATA AND N. CORDIFOLIA

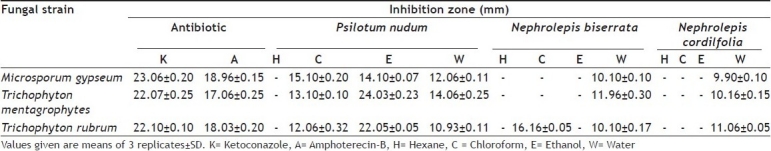
TABLE 2.
ANTIFUNGAL SCREENING OF P. NUDUM, N. BISERRATA AND N. CORDIFOLIA
The hexane extracts of the three plants did not have antimicrobial activity against any of the nine bacterial strains tested. The chloroform extract of the two species of Nephrolepis and ethanol extract of N. biserrata also did not display any antibacterial activity. The ethanol extract of N. cordifolia was only marginally effective against Proteus mirabilis, Enterobacter aerogenes, E. coli and Klebsiella pneumonia. The ethanol extract of P. nudum was marginally effective in inhibiting the growth of all the bacterial strains except B. cereus which was not inhibited at all, while the chloroform extract was not effective against Bacillus cereus and Streptococcus feacalis.
The water extracts of all the three pteridophytes exhibited some degree of inhibition against the fungi tested. None of the hexane extracts of any of the plants had inhibitory effect. Ethanol extract of the two species of Nephrolepis did not have any inhibitory effect on the fungi tested, while chloroform extract of only N. biserrata exhibited inhibitory effect against only one fungal strain, Trichophyton rubrum. P. nudum was the only pteridophyte amongst the three studied whose chloroform, ethanol and water extracts exhibited antifungal properties against all the fungi tested. Of these the ethanol extract was the most effective and its inhibition of the two species of Trichophyton was marked. The chloroform extract though less effective than the ethanol extract was marginally better than the water extract. However, the water extract of P. nudum was marginally better than that of the two species of Nephrolepis. None of the extracts could equal the inhibition by ketoconazole or amphoterecin-B controls (Table 2).
The three pteridophytes, P. nudum, N. biserrata and N. cordifolia possess both antibacterial and antifungal properties. Most of the extracts in the present study were effective against both gram positive and gram negative bacteria unlike Lantana extract which was more effective against gram positive bacteria[25]. The water extract of the three pteridophytes had the most inhibitory effect against the bacterial and fungal strains tested. The findings of the present study validate the earlier investigation that aqueous extract of pteridophytes is more effective in inhibiting microbial growth[12]. The two species of Nephrolepis did not show any appreciable antifungal properties. All the three extracts of P. nudum were effective against the fungal pathogens tested. In an earlier exhaustive study covering 114 species of pteridophytes 73 displayed antimicrobial properties, but Psilotum nudum and Nephrolepis biserrata were not part of it and only the water extract of N. cordifolia was shown to exhibit antimicrobial property[12]. Adiantum species has been reported to contain significant antimicrobial properties[15]. The present study confirms the earlier findings that pteridophytes and other lower plants should be further screened for generating biologically active compounds. P. nudum, a primitive pteridophyte, possesses better antibacterial and antifungal properties than the ferns, N. biserrata and N. cordifolia. The above findings advocate further investigations of the extracts from various parts of P. nudum and the two species of Nephrolepis to identify the active constituents.
ACKNOWLEDGEMENTS
One of the authors Dolly Rani would like to thank the Director, National Botanical Research Institute, Lucknow, for providing facility to carry out the present work.
Footnotes
Rani, et al.: Antimicrobials from Pteridophytes
REFERENCES
- 1.Davis MA, Hancock DD, Besser TE, Rice DH, Gay JM, Gay C, et al. Changes in antimicrobial resistance among Salmonella enterica. Serovar Typhimurium Isolates from humans and Cattles in the North Western United States. Emerg Infect Dis. 1995;5:802–6. doi: 10.3201/eid0506.990610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones CG, Firn RD. The role of phytoecdysteroids in bracken fern, Pteridium aquilinum (L.) Kuhn as a defense against phytophagous insect attack. J Chem Eco. 1977;4:117–38. [Google Scholar]
- 3.Hansel CG, Lagare VB. Antimicrobial Screening of Maranao Medicinal Plants. Terminal Report. [Last accessed on 2009 Apr 25]. Available from: http://www.msumain.edu.ph/mindanaojournal/pdf/mj4-2005.pdf .
- 4.Bir SS. Pteridophytic flora of India: Rare and Endangered elements and their conservation. Indian Fern J. 1987;4:95–101. [Google Scholar]
- 5.Baltrushes N. Medical ethnobotany, phytochemistry and bioactivity of the ferns of Moorea, French Polynesia. Moorea Digital Flora Project. [Last accessed on 2009 Apr 25]. Available from: http://ucjeps.berkeley.edu/moorea/Baltrushes2006pdf .
- 6.Arnason JT, Philogene BJ, Donskov N, Muir A, Towers GHN. Psilotin, an insect feeding deterrent and growth reducer from Psilotum nudum. Biochem Syst Eco. 1986;14:287–9. [Google Scholar]
- 7.Balza F, Muir AD, Towers GHN. 3’-Hydroxypsilotin, a minor glycoside from Psilotum nudum. Phytochemistry. 1985;24:529–31. [Google Scholar]
- 8.Lindenmayer JM, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med. 1998;158:895–9. doi: 10.1001/archinte.158.8.895. [DOI] [PubMed] [Google Scholar]
- 9.Smit LH, Leemans R, Overbeek BP. Nocardia farcinica as the causative agent in a primary psoas abscess in a previously healthy cattle inspector. Clin Microbiol Infect. 2003;9:445–8. doi: 10.1046/j.1469-0691.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- 10.The MERCK Manuals. Online Medical Library. [Last accessed on 2010 Aug 26]. Available from: http://www.merck.com/mmhe/sec18/ch203/ch203c.html .
- 11.Dixit RD. Howrah, India: Botanical Survey of India; 1984. A Census of the Indian Pteridophytes. [Google Scholar]
- 12.Banerjee RD, Sen SP. Antibiotic activity of pteridophytes. Eco Bot. 1980;34:284–98. [Google Scholar]
- 13.Ramesh N, Vishwanathan MB, Saraswathy A, Balakrishna K, Brindha P, Lakshmanaperumalsamy P. Phytochemical and antimicrobial studies on Drynaria quercifolia. Fitoterapia. 2001;72:934–6. doi: 10.1016/s0367-326x(01)00342-2. [DOI] [PubMed] [Google Scholar]
- 14.Singh M, Rawat AKS, Govindarajan R. Antimicrobial activity of some Indian mosses. Fitoterapia. 2007;78:156–8. doi: 10.1016/j.fitote.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Singh M, Srivastava S, Rawat AKS. Antimicrobial activities of Indian Berberis species. Fitoterapia. 2007;78:574–6. doi: 10.1016/j.fitote.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Singh M, Singh N, Khare PB, Rawat AKS. Antimicrobial activity of some important Adiantum species used traditionally in indigenous systems of medicine. J Ethanopharmacol. 2008;115:327–9. doi: 10.1016/j.jep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Krishnaswamy NR. Learning Organic Chemistry through Natural Products. A Practical Approach. Resonance. 1996;1:12–8. [Google Scholar]
- 18.Reeve RM. Histochemical tests for polyphenols in plant tissues. Stain Technol. 1951;26:91–6. doi: 10.3109/10520295109113187. [DOI] [PubMed] [Google Scholar]
- 19.Svendsen AB, Verpoorte R. New York: Elsevier Scientific Publishing Company; 1983. Chromatography of Alkaloids. [Google Scholar]
- 20.Purvis MJ, Collier DC, Walls D. London: Butterworths; 1964. Laboratory Techniques in Botany. [Google Scholar]
- 21.Aryantha NP, Adinda A, Kusmaningat S. Occurrence of triterpenoids and polysaccharides on Ganoderma tropicum with Ganoderma lucidum as reference. Aust Mycol. 2002;20:123–9. [Google Scholar]
- 22.Hardman R, Sofowora EA. Antimonium trichloride as a test reagent for steroids especially diogenin and yamogenin in plant tissues. Stain Technol. 1972;47:205–08. doi: 10.3109/10520297209116486. [DOI] [PubMed] [Google Scholar]
- 23.Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH. 6th ed. Wahington DC: American Society for Microbiology; 1995. Manual of Clinical Microbiology. [Google Scholar]
- 24.Beneke ES. Minneapolis, Minn: Burgess Publishing Company; 1918. Medical Mycology. [Google Scholar]
- 25.Venkataswamy R, Doss A, Sukumar M, Mubarack HM. Preliminary phytochemical screening and antimicrobial studies of Lantana indica Roxb. Indian J Pharm Sci. 2010;72:229–31. doi: 10.4103/0250-474X.65020. [DOI] [PMC free article] [PubMed] [Google Scholar]


