Abstract
AMP-activated protein kinase (AMPK) β1 or β2 subunits are required for assembling of AMPK heterotrimers and are important for regulating enzyme activity and cellular localization. In skeletal muscle, α2β2γ3-containing heterotrimers predominate. However, compensatory up-regulation and redundancy of AMPK subunits in whole-body AMPK α2, β2, and γ3 null mice has made it difficult to determine the physiological importance of AMPK in regulating muscle metabolism, because these models have normal mitochondrial content, contraction-stimulated glucose uptake, and insulin sensitivity. In the current study, we generated mice lacking both AMPK β1 and β2 isoforms in skeletal muscle (β1β2M-KO). β1β2M-KO mice are physically inactive and have a drastically impaired capacity for treadmill running that is associated with reductions in skeletal muscle mitochondrial content but not a fiber-type switch. Interestingly, young β1β2M-KO mice fed a control chow diet are not obese or insulin resistant but do have impaired contraction-stimulated glucose uptake. These data demonstrate an obligatory role for skeletal muscle AMPK in maintaining mitochondrial capacity and contraction-stimulated glucose uptake, findings that were not apparent in mice with single mutations or deletions in muscle α, β, or γ subunits.
Keywords: TBC1D1, PGC1-α, AS160, type 2 diabetes, obesity
AMP-activated protein kinase (AMPK) is an evolutionarily conserved stress-sensing kinase that controls energy metabolism and appetite by responding to nutrients and hormones (1). The regulation of AMPK activity depends on AMP and ADP regulated phosphorylation of the α catalytic subunit at T172 by the upstream kinases LKB1 and Ca2+/CaM-dependent protein kinase kinase (CaMKKβ; refs. 2 and 3). AMPK exists as a heterotrimer, consisting of an α catalytic subunit (α1, α2), a scaffolding β subunit (β1, β2) and a nucleotide-binding γ subunit (γ1, γ2, γ3) (1). The C-terminal of the β subunit contains a highly conserved α and γ subunit-binding sequence (SBS) that is required for the formation of a stable, active AMPK αβγ complex (4). We recently reported on the physiological effects of germ-line deletion of β1 (5) and β2 (6) isoforms in mice. We showed that β1 null mice have reduced AMPK α-subunit expression and activity in liver, adipose tissue and the hypothalamus (5). In contrast, AMPK β2 null mice have reduced AMPK activity in skeletal muscle, are aminoimidazole carboxamide ribonucleotide (AICAR) insensitive and have reduced exercise tolerance despite a greater than 50% increase in muscle β1 protein expression (6). The phenotype of β2 null mice was similar to that of mice lacking α2 (7) or γ3 (8) subunits or muscle-specific overexpression of an α2 kinase dead (KD) mutation (9, 10).
During exercise, AMPK is activated in an intensity-dependent manner (for review, see ref. 11). Mice with reduced AMPK in muscle are exercise intolerant, an effect shown not to be due to cardiac impairments in AMPK (12–14). However, the cause for this reduction in exercise capacity remains largely unknown, because mitochondrial content and glucose uptake are not altered (6, 7, 10, 12, 15–17) or only very modestly reduced (9, 18–20). An important caveat of these studies is that AMPK expression and activity is not abolished and in some cases there is substantial compensatory up-regulation of the remaining isoform(s) (e.g., α1 in α2 null and β1 in β2 null mice), which may be sufficient for maintaining mitochondrial capacity and contraction-stimulated glucose uptake.
Exercise and pharmacological activation of skeletal muscle AMPK improves insulin sensitivity in obese rodents and humans (for review, see ref. 1). Although AMPK plays an important role in regulating a number of metabolic pathways that both interact and are involved with the regulation of insulin-stimulated glucose uptake, current genetic models of AMPK deficiency do not show an essential role for AMPK in maintaining muscle insulin sensitivity. For example, although AMPK α2 null mice have whole-body insulin resistance, this resistance is due to overactivation of the sympathetic nervous system and not due to an intrinsic defect in skeletal muscle insulin-stimulated glucose uptake (21). Similarly, insulin-stimulated glucose uptake is normal in AMPK β2 (6) or γ3 null (8) and α2 KD (9, 22, 23) mice. Although these data suggest that muscle AMPK does not regulate skeletal muscle insulin sensitivity, an alternative explanation may be that the residual AMPK activity is sufficient to maintain skeletal muscle insulin sensitivity.
Because AMPK is central to the regulation of skeletal muscle metabolism and prior whole-body and skeletal muscle-specific genetic alterations in AMPK subunits have produced equivocal results, we generated muscle-specific null mice for AMPK β1 (β1M-KO) and β2 (β2M-KO). We then crossed these mice to generate mice lacking both β1 and β2 (β1β2M-KO) subunits in skeletal muscle to minimize residual AMPK activity and compensatory up-regulation of AMPK α1 and β1 subunits. We found no detectable phenotype in β1M-KO mice, whereas β2M-KO mice had modestly reduced exercise capacity with normal muscle mitochondrial content and contraction-stimulated glucose uptake. In contrast, β1β2M-KO mice had drastically reduced exercise capacity, muscle mitochondrial content, and contraction-stimulated glucose uptake, yet showed normal insulin sensitivity.
Results
Generation of AMPKβ1β2M-KO Mice.
We attempted to generate mice with whole-body deletion of both AMPK β1 and β2 isoforms by crossing homozygous AMPK β1 and β2 null mice. The results of this cross generated heterozygous AMPK β1β2 null and WT mice, but no homozygous β1β2 null mice (expected 6.25 out of 144 progeny, P < 0.036) leading us to conclude that at least one of the β subunits is required during embryonic development. To bypass the embryonic lethality of β1β2 null mice, we generated AMPK β1 and β2 floxed mice on a C57Bl6 background and crossed these mice with C57Bl6 mice expressing Cre-recombinase under the control of the muscle creatine kinase (MCK) promoter, which drives transcription in skeletal and heart muscle (24). After two generations, we obtained homozygous AMPK β1 fl/fl (β1M-WT) and AMPK β1 MCK-Cre (β1M-KO) and homozygous AMPK β2 fl/fl (β2M-WT) and AMPK β2 MCK-Cre (β2M-KO) mice. We then crossed these mice for two generations to obtain homozygous AMPK β1β2 fl/fl (β1β2M-WT) and AMPK β1β2 MCK-Cre (β1β2M-KO) mice (Fig. S1). AMPK β1, β2, and β1β2 M-KO mice all had normal litter sizes with typical frequency (Table S1). Body mass, degree of adiposity, and extensor digitorium longus (EDL), soleus, and heart muscle weights were similar between genotypes (Table S1).
AMPK β1β2M-KO Mice Have Dramatic Reductions in AMPK α Expression in Skeletal Muscle.
In the heart, β2- and β1β2 M-KO mice had no change in α1 or β1 expression (Fig. S2A). β2- and β1β2 M-KO mice had reduced β2 expression in the heart that corresponded with lower α2 expression (β2M-KO ∼50%, β1β2M-KO ∼80%) and T172 phosphorylation (β2M-KO ∼18%, β1β2M-KO ∼85%; Fig. S2 A and B).
Skeletal muscle α1 and α2 expression were not altered in the β1M-KO mice (Fig. S2C). In β2M-KO mice, skeletal muscle β1 expression was normal whereas β2 expression was not detected (Fig. 1A). Reductions in β2 corresponded with nondetectable levels of α2 expression but did not alter α1 expression (Fig. 1A). In β1β2M-KO mice, there was no detectable α2 or β2 expression and α1 and β1 expression were reduced by ∼70% and ∼45%, respectively indicating considerable loss of the AMPK heterotrimer (Fig. 1A). We hypothesized that the residual α1β1 heterotrimers in β1β2M-KO muscle may have originated from blood, connective, and vascular cells and/or adipocytes, where expression of β1 heterotrimers is predominant (5). We also hypothesized that muscle contractions would not activate AMPK in these cells. Consistent with these hypotheses, we found that basal AMPK T172 phosphorylation was lower, but still significantly increased in contracting muscles from β2M-KO mice (Fig. S2D), but importantly, was eliminated in muscles from β1β2M-KO mice (Fig. 1B). These data indicate that the remaining α1β1 expression in muscle from β1β2M-KO mice was likely due to contributions from cells other than differentiated muscle fibers.
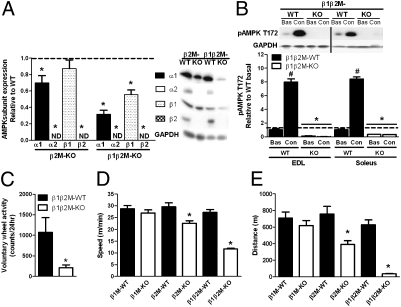
Fig. 1.
Muscle-specific reductions in AMPK phosphorylation and subunit expression in AMPK β2 and β1β2 M-KO mice results in dramatic reductions in exercise tolerance. (A) Expression of AMPK α1, α2, β1, and β2 in extensor digitorium longus (EDL) muscle. ND, not detectable. (B) AMPK T172 phosphorylation in resting (Bas, basal) and electrically stimulated (Con, contraction) EDL and soleus muscles. (C) β1β2M-KO mice have reduced voluntary wheel activity. (D and E) Mean maximal running speed (D) and distance run (E) of β1, β2, and β1β2 M-KO and wild-type (WT) littermates during a progressive treadmill running exercise test. Data are means ± SEM, n = 8−10, *P < 0.05 compared with WT littermate. #P < 0.05 compared with basal. For protein expression, values were corrected for equal protein loading using GAPDH.
AMPK β1β2M-KO Mice Have Reduced Voluntary Wheel Activity and Treadmill-Exercise Tolerance.
In β2- and β1β2 M-KO mice, spontaneous activity, VO2, and VCO2 were not altered between genotypes (Table S2). However, when given the option to run on an exercise wheel that was placed in their cages, β1β2M-KO mice were extremely inactive (Fig. 1C). Therefore, we analyzed exercise capacity by conducting a progressive treadmill running test starting at 10 m/min (a fast walk for mice) and found that maximal running speed and distance in β1M-KO mice was not different compared with WT littermates (Fig. 1 D and E). β2M-KO had an ∼25% reduction in running capacity, in line with previous models of AMPK deficiency (12, 15, 23). However, β1β2M-KO mice were extremely exercise intolerant and had dramatic reductions in both maximal running speed (∼57%) (Fig. 1D) and distance covered (∼94%) compared with WT littermates (Fig. 1E).
AMPK β1β2M-KO Have Reductions in Mitochondrial Content.
Oxidative metabolism is dependent on mitochondrial function. There was a trend for modest reductions in citrate synthase (Fig. 2A) and cytochrome c oxidase (Fig. 2B) activities in β2M-KO mice (∼17%, P > 0.05) whereas in β1β2M-KO mice these reductions were significant (∼30%, P < 0.05). Succinate dehydrogenase (SDH, mitochondrial complex II) activity also tended to be lower in β1β2M-KO mice (∼47%, P = 0.06, Fig. S3A). OXPHOS protein expression was also reduced in β1β2M-KO (Fig. 2C) but not β1 or β2 M-KO mice (Fig. S3 B and C). Mitochondrial content in skeletal muscle is proportionate to mitochondrial DNA copy number (25), and we found that this was reduced by ∼56% in β1β2M-KO compared with WT (Fig. 2D). Consistent with reduced muscle mitochondrial enzyme activity and DNA copy number, β1β2M-KO mice had reduced mRNA expression of citrate synthase (Cs; ∼35%); beta-hydroxy acyl-CoA dehydrogenase (βhad; ∼41%); NADH:ubiquinone oxidoreductase (Nd1; mitochondrial subunit of complex I; ∼59%), and SDH subunit D (Sdhd; ∼58%; Fig. 2E). Interestingly, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc1a) mRNA expression was increased by ∼62% in β1β2M-KO mice (Fig. 2E).
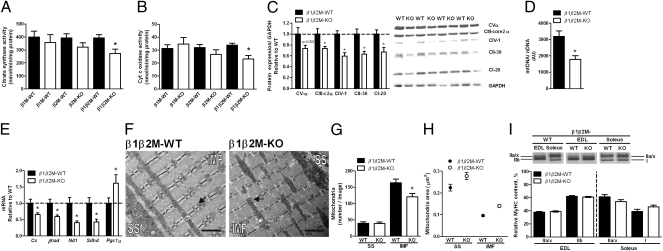
Fig. 2.
AMPK β1β2M-KO mice have reduced mitochondrial biogenesis independent of fiber type switch. (A and B) Mitochondrial (mt.) citrate synthase (A) and cytochrome c oxidase (Complex IV; B) activities in quadriceps from β1, β2, and β1β2 M-KO mice. (C) β1β2 M-KO OXPHOS protein expression in extensor digitorium longus (EDL) muscle. (D) mtDNA relative to nuclear DNA and (E) mRNA expression of nuclear and mt. encoded genes in quadriceps muscle. (F) Representative transmission electron microscopy (EM) image of tibialis anterior muscle sections analyzed for mt. content (G) and size (H) in subsarcollemal (SS) and intermyofibrillar (IMF) regions; arrows indicate representative mt. (Scale bar, 2 μm.) (I) Resolution of myosin heavy chain (MyHC) isoforms in EDL and soleus muscles. Data are means ± SEM, n = 8−10, n = 2 for EM analyses. CI-20, Complex I subunit NDUFB8; CII-30, Complex II subunit of 30 kDa; CIII-core2, Complex III subunit Core 2; CIV-I, Complex IV subunit I; CV-α, ATP synthase subunit alpha. *P < 0.05 compared with WT littermate. For protein expression, values were corrected for equal protein loading using GAPDH.
To assess whether there were morphological differences in skeletal muscle mitochondria from β1β2M-KO mice, sections from tibialis anterior (TA) muscle were visualized by transmission electron microscopy (EM) and analyzed for mitochondrial distribution [intermyofibrillar (IMF), ∼80% of total skeletal muscle mitochondria, or subsarcolemmal (SS)] and size (Fig. 2F). β1β2M-KO mice had lower content of IMF (∼26%, Fig. 2G) but not SS mitochondria. Interestingly, mitochondria found in both SS and IMF regions were larger in β1β2M-KO mice compared with WT (Fig. 2H). Importantly, these changes in mitochondrial content and size were not due to a fiber type switch (Fig. 2I). Together, these data indicate an indispensable role for AMPK in regulating mitochondrial number and size but not fiber type and suggest that reductions in exercise capacity of β1β2M-KO mice may be related to reductions in mitochondrial content.
AMPK β1β2M-KO Mice Have Normal Whole-Body Insulin Sensitivity and Insulin-Stimulated Glucose Uptake.
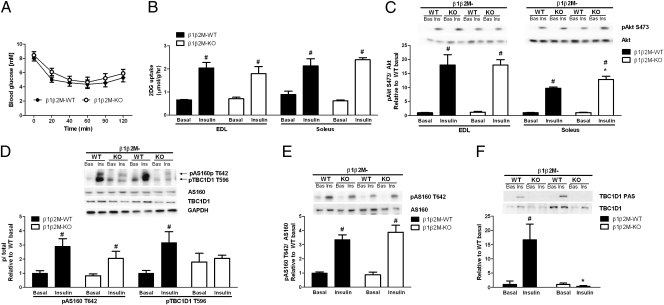
Because reductions in mitochondrial density and exercise capacity have been associated with the development of insulin resistance (for review, see ref. 26), we examined insulin sensitivity in a cohort of young male mice (∼12 wk old). Fasting blood glucose as well as serum insulin and adipokines were not different between WT and β1β2M-KO mice (Table S3). An i.p. insulin tolerance test (ITT) revealed similar whole-body insulin sensitivity between WT and β1β2M-KO mice (Fig. 3A). Skeletal muscle insulin-stimulated glucose uptake (Fig. 3B) and Akt S473 phosphorylation (Fig. 3C) were also comparable. The RabGAP GTPases, Akt substrate of 160 kDa (AS160) and tre-2/USP6, BUB2, cdc16 domain family member 1 (TBC1D1) sequester glucose transporter type 4 (GLUT4) within intracellular vesicles, which limits glucose uptake (27, 28). In response to insulin, Akt phosphorylates AS160 and TBC1D1, which promotes binding to 14-3-3 proteins and subsequent inhibition of their activity; allowing GLUT4 translocation to the plasma membrane and glucose uptake (27, 28). We found that AS160 expression in β1β2M-KO muscle was comparable to WT (Fig. S4A), but TBC1D1 expression was reduced by ∼50% in EDL (Fig. S4B). We then measured AS160 T642 phosphorylation, which is the predominant Akt site, and found that in EDL muscle, with a 4.5% PAGE-electrophoresis, this antibody also detected a second lower band of ∼150 kDa (Fig. 3D), which we confirmed was the equivalent T596 phosphorylation site on TBC1D1 (Fig. S4C). In β1β2M-KO EDL and soleus muscles, increases in AS160 T642 phosphorylation in response to insulin were similar to WT (Fig. 3 D and E). In contrast, insulin-stimulated TBC1D1 T596 phosphorylation was eliminated in β1β2M-KO EDL (Fig. 3D). To verify these findings, we also examined phosphorylated Akt substrate (PAS) motifs from TBC1D1 immunoprecipitates and found that PAS phosphorylation was eliminated in EDL muscles from β1β2M-KO mice (Fig. 3F). These data demonstrate that skeletal muscle AMPK is not required for the maintenance of whole-body insulin sensitivity or skeletal muscle insulin-stimulated glucose uptake.
Fig. 3.
AMPK β1β2M-KO mice have normal insulin sensitivity and insulin-stimulated glucose uptake. (A) Whole-body insulin sensitivity in β1β2M-KO mice was assessed by performing an insulin tolerance test (ITT) (0.6 U/kg). (B and C) Insulin-stimulated 2-deoxyglucose (2DG) uptake (B) and Akt S473 phosphorylation (C) in isolated extensor digitorum longus (EDL) and soleus muscles. (D) Insulin-stimulated AS160 T642 and TBC1D1 T596 and phosphorylation in EDL muscle lysates. (E) AS160 T642 phosphorylation in soleus muscle lysates (note TBC1D1 is not detectable in soleus muscle). (F) Insulin-stimulated TBC1D1 PAS phosphorylation in EDL following TBC1D1 immunoprecipitation. Data are means ± SEM, n = 8 ITT and n = 4 ex vivo experiments. *P < 0.05 compared with wild type (WT), same condition. #P < 0.05 compared with basal, same genotype. For protein expression and phosphorylation, values were corrected for equal protein loading using GAPDH or corresponding total antibody.
AMPK β1β2M-KO Mice Have Reduced Skeletal Muscle Glucose Uptake During Exercise and Muscle Contractions.
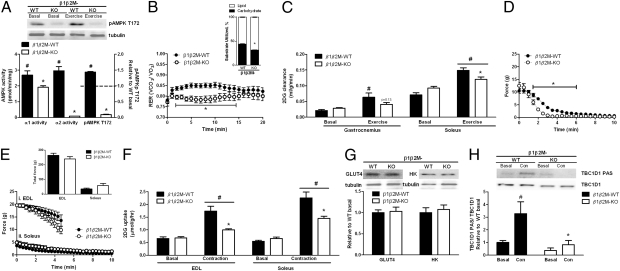
We next examined substrate utilization in WT and β1β2M-KO mice during 20 min of treadmill running at 50% of each mouse's maximal running speed (∼13 m/min and ∼6 m/min, respectively). At the completion of exercise AMPK α2 and α1 activities and AMPK T172 phosphorylation were substantially higher in WT compared with β1β2M-KO mice (Fig. 4A). Serum lactate and nonesterified free fatty acids (NEFA) were similar between WT and β1β2M-KO mice but at the completion of exercise serum glucose levels were ∼25% higher in β1β2M-KO mice (Table S4). We also measured muscle lactate and nucleotides [ATP, ADP, AMP, phosphocreatine (PCr), and creatine (Cr)] and found that there were no differences between genotypes (Table S4) indicating that relative-workloads were comparable. Despite accurate matching of the workloads, which would be expected to result in a similar percentage of substrate oxidation, β1β2M-KO mice had a lower RER (Fig. 4B), indicating a reduced percentage of energy was coming from carbohydrate oxidation (Fig. 4B, Inset). Reduced carbohydrate oxidation in β1β2M-KO mice was not due to lower glycogen utilization, which was comparable between genotypes (Table S4). We then measured 2-deoxyglucose (2DG) clearance in WT and β1β2M-KO mice at rest and during treadmill running at the same relative intensity as above, and found that although treadmill exercise increased 2DG clearance by ∼threefold in gastrocnemius and ∼twofold in soleus of WT mice, this effect was blunted in β1β2M-KO mice (Fig. 4C).
Fig. 4.
AMPK β1β2M-KO mice have reduced carbohydrate utilization and skeletal muscle glucose uptake during treadmill running and contractions. (A–D) Exercise (50% maximal running speed, 20 min). (A) AMPK α1 and α2 activities and T172 phosphorylation in quadriceps muscle. (B) RER kinetics and average percent carbohydrate and lipid used (Inset). (C) 2-deoxyglucose (2DG) clearance in soleus and gastrocnemius muscles. (D–F) Muscle contractions ex vivo (D) Increased fatigue in β1β2M-KO extensor digitorum longus (EDL) muscles (6 tetani/min, 50 Hz, 350 ms pulse duration, 20 min). (E) New matched force fatigue and total force produced (inset) contraction protocol (i: EDL 3 tetani/min, 100 Hz, 350 ms pulse duration, 5 min; ii: soleus 12 tetani/min, 30 Hz, 600-ms pulse duration, 10 min). (F) 2DG uptake in β1β2M-KO and wildtype (WT) muscles. (G) GLUT4 and hexokinase (HK) protein expression in quadriceps. (H) TBC1D1 PAS phosphorylation in resting (Bas) and contracting (Con) EDL muscles from β1β2M-KO mice. Data are means ± SEM, n = 6–8. *P < 0.05 compared with WT, same condition. #P < 0.05 compared with basal, same genotype. For protein expression and phosphorylation, values were corrected using tubulin or corresponding total antibody.
We also measured 2DG glucose uptake ex vivo in isolated soleus and EDL muscles. In agreement with studies from whole-body β2 KO mice (6), we found that contraction-stimulated glucose uptake and force production were maintained in β2M-KO mice (Fig. S5 A and B). However, using the same contraction protocol we found β1β2M-KO EDL muscles fatigued more rapidly than WT muscles (Fig. 4D). Therefore, we devised a modified contraction protocol with a reduced duty cycle (EDL 3 vs. 6 tetani/min) over a shorter duration (EDL 5 vs. 20 min and soleus 10 vs. 20 min). As such, EDL and soleus force curves and total force generated were similar for WT and β1β2M-KO mice (Fig. 4E). Importantly, despite accurate matching of force generation, contraction-stimulated glucose uptake was impaired by ∼70% in EDL and ∼54% in soleus muscles from β1β2M-KO mice (Fig. 4F). These findings demonstrate a major role for AMPK β subunits in regulating glucose uptake during treadmill exercise in vivo and muscle contractions ex vivo.
Skeletal muscle glucose uptake during muscle contractions is dependent on hexokinase II and GLUT4 (29, 30), but the expression of these proteins was not altered in β1β2M-KO mice (Fig. 4G). In contrast to the effects of insulin, recent studies have indicated that TBC1D1 PAS phosphorylation (which also detects proposed AMPK phosphorylation sites) is important for regulating contraction-stimulated glucose uptake (27, 28). We subsequently immunoprecipitated TBC1D1 from resting and contracted EDL muscles and immunoblotted with the PAS antibody, and found that although contraction increased TBC1D1 PAS phosphorylation in WT EDL, this increase was eliminated in β1β2M-KO mice (Fig. 4H). These data suggest that reductions in contraction-stimulated glucose uptake in β1β2M-KO mice may be due to reduced phosphorylation of TBC1D1.
Discussion
Over the past decade, numerous studies have reported that skeletal muscle AMPK is an important regulator of energy metabolism in response to physiological stimuli such as nutrients and hormones. Given the undetectable levels of skeletal muscle AMPK activity, we were surprised to find that β1β2M-KO mice had normal muscle weights and were not obese. We also demonstrated that young β1β2-MKO mice had normal whole-body and skeletal muscle insulin sensitivity. Consistent with recent reports (27, 28, 31), our data suggest a dispensable role for TBC1D1 but not AS160 phosphorylation in regulating insulin-stimulated glucose uptake. Therefore, in young healthy mice housed under sedentary conditions skeletal muscle AMPK is not required to maintain metabolic homeostasis. In future studies, it will be interesting to examine whether up-regulation/compensation by alternative “energy sensing” pathways may be important for the maintenance of resting skeletal muscle metabolism in β1β2M-KO mice.
β1β2M-KO mice had considerable reductions in voluntary wheel activity suggestive of impaired exercise capacity. To investigate this further, we performed a treadmill exercise tolerance test and found that β1β2M-KO were extremely exercise intolerant and became fatigued almost immediately when the treadmill speed was increased beyond a fast walk (∼10 m/min). This exercise intolerance is much greater than the ∼25–30% reduction in exercise capacity reported for other models of partial AMPK deficiency (12, 15, 23). These data demonstrate that skeletal muscle AMPK is essential for maintaining the ability to buffer against acute increases in metabolic demand.
Reductions in mitochondrial capacity are associated with impaired exercise capacity. Despite the well documented role that both pharmacological (32, 33) and genetic (16, 17) activation of AMPK increases skeletal muscle mitochondrial density, previous studies in genetic models of partial AMPK deficiency suggested that muscle AMPK played a relatively minor to negligible role in the maintenance of muscle mitochondrial content (16, 17, 23, 32, 34). In contrast, we found that β1β2M-KO mice had large reductions in muscle mitochondrial DNA, mRNA, enzyme activities, and OXPHOS protein expression. Electron microscopy imaging demonstrated that reductions in the number of mitochondria were specific to the IMF region of the muscle, because SS mitochondrial density was unchanged in β1β2M-KO mice. Interestingly, despite β1β2M-KO mice having fewer mitochondria, we found that the mitochondria that were present were larger than those found in the muscles of WT littermates. What causes the formation of these larger mitochondria is not known, but we speculate it may be related to the recently described role of AMPK activating mitophagy (35) or alternatively may reflect an adaptive response designed to compensate for reductions in mitochondrial number. These data demonstrate an obligatory role for muscle AMPK in maintaining mitochondrial content and size; however, future studies determining the mechanisms mediating these effects are required.
A critical regulator of mitochondrial biogenesis in muscle is PGC-1 (36). Muscle-specific PGC-1α null mice have reductions in mitochondrial biogenesis and undergo a fiber-type switch whereby muscles take on a more glycolytic profile (37). Interestingly, reductions in mitochondrial content in β1β2M-KO mice occurred despite an ∼60% increase in PGC-1α mRNA, suggesting that AMPK-mediated posttranslational modifications, such as phosphorylation (38) and deacetylation (39) are essential for the control of PGC-1α activity. We found that despite reductions in mitochondrial content, there was no shift in the skeletal muscle fiber-type of β1β2M-KO mice. These data suggest that control of muscle fiber-type is independent of AMPK activity. In future studies, it will be interesting to examine PGC-1α activity and mitochondrial/fiber type adaptations in β1β2M-KO mice following exercise training or treatment with AMPK activators that induce mitochondrial biogenesis such as adiponectin (40) and resveratrol (41).
Despite running at a comparable relative workload β1β2M-KO mice used a lower percentage of carbohydrate for energy compared with WT littermates. Surprisingly, this was not due to alterations in glycogen utilization, but lower muscle glucose clearance. It is possible that the lower glucose clearance and impaired exercise capacity of β1β2M-KO mice may have been due to reduced blood flow to working muscles as a result of reductions in heart AMPK α2 and β2 expression. Although we did not directly assess cardiac function, in contrast to LKB1 MCK-Cre mice (42, 43), we did not detect any change in heart size suggesting that heart function was not adversely affected. Reductions in blood flow may also be related to reduced skeletal muscle capillarization, which has been observed in AMPK α2 KD mice (44–46). In addition to regulating angiogenesis, skeletal muscle AMPK is also important for regulating nitric oxide synthase (NOS), which is important for controlling blood flow and glucose delivery during exercise (44–46). Future studies examining heart function, muscle capillary density, and glucose delivery/blood flow in β1β2M-KO mice are warranted.
To overcome the potential limitations of the in vivo model where matching of workloads and substrate delivery to muscle may be compromised, we measured glucose uptake in isolated EDL and soleus muscles during muscle contractions ex vivo under conditions in which the total force generated by WT and β1β2M-KO mice was comparable. However, under these conditions β1β2M-KO mice had substantial increases in muscle fatigue compared with our previous studies in AMPKα2 KD (47, 48), β2 KO (6), or β2M-KO (Fig. S5A) mice, suggesting that factors independent of blood flow contribute to the exercise intolerance of β1β2M-KO mice. Importantly, even with accurate matching of muscle force, we detected an ∼54 and 70% reduction (soleus and EDL, respectively) in contraction-stimulated glucose uptake in β1β2M-KO mice. This reduction in contraction-stimulated glucose uptake in β1βM-KO mice is much greater than previously reported in other models of AMPK deficiency where force fatigue curves were matched (10, 19, 20); suggesting that remaining AMPK activity may have been sufficient for the maintenance of contraction-stimulated glucose uptake in these models.
What might the mechanism be for the reduced contraction-stimulated glucose uptake in β1β2M-KO mice? Recent studies have indicated that TBC1D1 phosphorylation on four sites detected by the phospho-Akt substrate (PAS) antibody are essential for controlling glucose uptake in response to muscle contractions (28). Consistent with this concept, we show that TBC1D1 PAS phosphorylation does not increase in response to muscle contractions in β1β2M-KO mice. Importantly, these findings are different from AMPK α2KO (49) and α2iTg (50) mice, where modest increases in TBC1D1 PAS phosphorylation are accompanied by normal contraction-stimulated glucose uptake (7, 10). These data suggest that reductions in contraction-stimulated glucose uptake in β1β2M-KO mice may be the result of reduced TBC1D1 phosphorylation, which impairs 14-3-3 binding and GLUT4 translocation to the plasma membrane. As phosphorylation-site specific antibodies become commercially available, it will be important to investigate the contribution of the individual phosphorylation sites in regulating glucose uptake in response to contraction in β1β2M-KO mice.
In conclusion, by generating β1β2M-KO mice, we have found that skeletal muscle AMPK is not required for the maintenance of body mass or insulin sensitivity. These data suggest that alternative pathways are able to compensate for a lack of skeletal muscle AMPK in young healthy mice under sedentary conditions. In contrast, there appears to be no substitute for AMPK in regulating muscle metabolism during exercise as β1β2M-KO mice exhibit reduced voluntary wheel activity, extreme exercise intolerance and increased muscle fatigue. Exercise intolerance in β1β2M-KO mice was likely due to dual reductions in skeletal muscle mitochondrial content and contraction-stimulated glucose uptake.
Materials and Methods
β1 and β2 floxed mice were generated on a pure C57BL/6 background by OZGENE (Perth, Australia) and were crossed with MCK-Cre mice that were backcrossed for at least 10 generations onto a C57BL/6 background (24). In vivo and ex vivo 2-DG uptake in muscle was determined as described (6, 15). Protein expression, phosphorylation, and enzymatic activities as well as RT-qPCR of mRNA transcripts were completed as described (5, 6, 51). For a detailed description of all methods and statistics, see SI Materials and Methods.
Supplementary Material
Acknowledgments
These studies were supported by grants and fellowships from the National Health and Medical Research Council (to B.K. and G.R.S.), Australian Research Council and the Victorian Government Operational Infrastructure Support Scheme (to B.K.), Natural Sciences and Engineering Research Council and Canadian Institutes of Health Research (to G.R.S.), Melbourne University Postgraduate Award (H.M.O.), St. Vincent’s Institute of Medical Research (H.M.O.) Canadian Diabetes Association (to G.S. and J.D.S.), McMaster University Degroote Academic Fellowship (to J.D.S.), the Danish Medical Research Council (to S.B.J. and E.A.R.), and the Lundbeck Research Foundation (to E.A.R.). G.R.S. is a Canada Research Chair in Metabolism and Obesity.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105062108/-/DCSupplemental.
References
- 1.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 2.Xiao B, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 3.Oakhill JS, et al. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 4.Iseli TJ, et al. AMP-activated protein kinase beta subunit tethers alpha and gamma subunits via its C-terminal sequence (186-270) J Biol Chem. 2005;280:13395–13400. doi: 10.1074/jbc.M412993200. [DOI] [PubMed] [Google Scholar]
- 5.Dzamko N, et al. AMPK beta1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem. 2010;285:115–122. doi: 10.1074/jbc.M109.056762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg GR, et al. Whole body deletion of AMP-activated protein kinase beta2 reduces muscle AMPK activity and exercise capacity. J Biol Chem. 2010;285:37198–37209. doi: 10.1074/jbc.M110.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jørgensen SB, et al. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 8.Barnes BR, et al. The 5′-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J Biol Chem. 2004;279:38441–38447. doi: 10.1074/jbc.M405533200. [DOI] [PubMed] [Google Scholar]
- 9.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 10.Fujii N, et al. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem. 2005;280:39033–39041. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- 11.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418:261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee-Young RS, et al. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem. 2009;284:23925–23934. doi: 10.1074/jbc.M109.021048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell RR, 3rd, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing Y, et al. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 15.Maarbjerg SJ, et al. Genetic impairment of {alpha}2-AMPK signaling does not reduce muscle glucose uptake during treadmill exercise in mice. Am J Physiol Endocrinol Metab. 2009;297:E924–E934. doi: 10.1152/ajpendo.90653.2008. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Roves PM, Osler ME, Holmström MH, Zierath JR. Gain-of-function R225Q mutation in AMP-activated protein kinase gamma3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J Biol Chem. 2008;283:35724–35734. doi: 10.1074/jbc.M805078200. [DOI] [PubMed] [Google Scholar]
- 17.Röckl KS, et al. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. 2007;56:2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- 18.Jørgensen SB, Jensen TE, Richter EA. Role of AMPK in skeletal muscle gene adaptation in relation to exercise. Appl Physiol Nutr Metab. 2007;32:904–911. doi: 10.1139/H07-079. [DOI] [PubMed] [Google Scholar]
- 19.Lefort N, St-Amand E, Morasse S, Côté CH, Marette A. The alpha-subunit of AMPK is essential for submaximal contraction-mediated glucose transport in skeletal muscle in vitro. Am J Physiol Endocrinol Metab. 2008;295:E1447–E1454. doi: 10.1152/ajpendo.90362.2008. [DOI] [PubMed] [Google Scholar]
- 20.Jensen TE, Schjerling P, Viollet B, Wojtaszewski JF, Richter EA. AMPK alpha1 activation is required for stimulation of glucose uptake by twitch contraction, but not by H2O2, in mouse skeletal muscle. PLoS ONE. 2008;3:e2102. doi: 10.1371/journal.pone.0002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viollet B, et al. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujii N, et al. Ablation of AMP-activated protein kinase alpha2 activity exacerbates insulin resistance induced by high-fat feeding of mice. Diabetes. 2008;57:2958–2966. doi: 10.2337/db07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck Jorgensen S, et al. Reduced AMP-activated protein kinase activity in mouse skeletal muscle does not exacerbate the development of insulin resistance with obesity. Diabetologia. 2009;52:2395–2404. doi: 10.1007/s00125-009-1483-8. [DOI] [PubMed] [Google Scholar]
- 24.Brüning JC, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 25.Williams RS. Mitochondrial gene expression in mammalian striated muscle. Evidence that variation in gene dosage is the major regulatory event. J Biol Chem. 1986;261:12390–12394. [PubMed] [Google Scholar]
- 26.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S, Wasserman DH, MacKintosh C, Sakamoto K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab. 2011;13:68–79. doi: 10.1016/j.cmet.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An D, et al. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes. 2010;59:1358–1365. doi: 10.2337/db09-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fueger PT, et al. Hexokinase II protein content is a determinant of exercise endurance capacity in the mouse. J Physiol. 2005;566:533–541. doi: 10.1113/jphysiol.2005.085043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fueger PT, et al. Glucose kinetics and exercise tolerance in mice lacking the GLUT4 glucose transporter. J Physiol. 2007;582:801–812. doi: 10.1113/jphysiol.2007.132902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pehmøller C, et al. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E665–E675. doi: 10.1152/ajpendo.00115.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zong H, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winder WW, et al. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 34.Jørgensen SB, et al. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- 35.Egan DF, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zechner C, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 2010;12:633–642. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handschin C, et al. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 38.Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantó C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Civitarese AE, et al. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab. 2006;4:75–87. doi: 10.1016/j.cmet.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakamoto K, et al. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am J Physiol Endocrinol Metab. 2006;290:E780–E788. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomson DM, et al. Skeletal muscle and heart LKB1 deficiency causes decreased voluntary running and reduced muscle mitochondrial marker enzyme expression in mice. Am J Physiol Endocrinol Metab. 2007;292:E196–E202. doi: 10.1152/ajpendo.00366.2006. [DOI] [PubMed] [Google Scholar]
- 44.Stahmann N, et al. Activation of AMP-activated protein kinase by vascular endothelial growth factor mediates endothelial angiogenesis independently of nitric-oxide synthase. J Biol Chem. 2010;285:10638–10652. doi: 10.1074/jbc.M110.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen ZP, et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 46.Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP. AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. J Physiol. 2008;586:6021–6035. doi: 10.1113/jphysiol.2008.159871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dzamko N, et al. AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol. 2008;586:5819–5831. doi: 10.1113/jphysiol.2008.159814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merry TL, Steinberg GR, Lynch GS, McConell GK. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am J Physiol Endocrinol Metab. 2010;298:E577–E585. doi: 10.1152/ajpendo.00239.2009. [DOI] [PubMed] [Google Scholar]
- 49.Frøsig C, Pehmøller C, Birk JB, Richter EA, Wojtaszewski JF. Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle. J Physiol. 2010;588:4539–4548. doi: 10.1113/jphysiol.2010.194811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vichaiwong K, et al. Contraction regulates site-specific phosphorylation of TBC1D1 in skeletal muscle. Biochem J. 2010;431:311–320. doi: 10.1042/BJ20101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watt MJ, et al. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med. 2006;12:541–548. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.