Abstract
A number of pathogenic bacteria target mitochondria to modulate the host's apoptotic machinery. Studies here revealed that infection with the human gastric pathogen Helicobacter pylori disrupts the morphological dynamics of mitochondria as a mechanism to induce host cell death. The vacuolating cytotoxin A (VacA) is both essential and sufficient for inducing mitochondrial network fragmentation through the mitochondrial recruitment and activation of dynamin-related protein 1 (Drp1), which is a critical regulator of mitochondrial fission within cells. Inhibition of Drp1-induced mitochondrial fission within VacA-intoxicated cells inhibited the activation of the proapoptotic Bcl-2–associated X (Bax) protein, permeabilization of the mitochondrial outer membrane, and cell death. Our data reveal a heretofore unrecognized strategy by which a pathogenic microbe engages the host's apoptotic machinery.
Keywords: cytochrome c, mitochondrial dynamics, gastric cancer, gastric ulcer, toxin
Mitochondria preserve cell viability by functioning both as centers for energy production and central regulators of calcium homeostasis, apoptosis, and development. Some pathogenic microbes usurp the mitochondrial apoptotic machinery (1), which, depending on the pathogen and host cell type, can block cell death to preserve a colonization niche or, alternatively, induce cell death to promote escape from an intracellular niche or circumvent immune clearance (2). For the gastric pathogen Helicobacter pylori (Hp), chronic infection is associated with increased apoptosis within the gastric mucosa of humans (3), mice (4), and Mongolian gerbil models (5). Increased apoptosis may alter the gastric environment to promote Hp persistence (6), while at the same time, contribute to gastric disease, including peptic ulcers and gastric adenocarcinoma (7). Earlier studies have indicated that the vacuolating cytotoxin A (VacA), an Hp virulence factor that is important for Hp colonization (8) and disease pathogenesis (9), is essential (10) and sufficient (11) for inducing gastric epithelial cell death.
Several studies investigating the mechanism of VacA-induced cell death indicate that VacA is a mitochondrial-targeting toxin. Subsequent to binding plasma membrane sphingomyelin (12, 13), and potentially additional protein components (14, 15), VacA is internalized and induces mitochondrial dysfunction and mitochondrial outer membrane permeabilization (MOMP) (16). Intracellular VacA localizes to mitochondria (16, 17), and isolated mitochondria rapidly import purified VacA beyond the outer membrane (17, 18), resulting in dissipation of the mitochondrial transmembrane potential (ΔΨm) (19). VacA-dependent MOMP occurs after ΔΨm dissipation (16) and requires activation of the eukaryotic proapoptotic effector Bcl-2–associated X protein (Bax) (19). However, the underlying mechanism by which VacA triggers Bax-dependent MOMP has not been identified.
Here, we report that VacA disrupts the morphological dynamics of mitochondria as a mechanism to induce gastric epithelial cell death. Mitochondria exist in several overall morphologies, which are linked to the health of the cell, and change in a dynamic fashion through frequent and repetitive cycles of fission and fusion that occur in response to cellular energy demands and environmental challenges (20). Deregulation of mitochondrial dynamics has increasingly been linked to the pathologies resulting from inflammatory and neurodegenerative disorders (21) and several cancers (22). However, the extent to which the morphological dynamics of mitochondria may be targeted by pathogenic microbes during host infection, or are associated with the pathophysiology of some infectious diseases, is largely unexplored. Our studies revealed that VacA induces activation of the dynamin-related protein 1 (Drp1), which is a critical regulator of mitochondrial fission within cells. Moreover, inhibition of VacA-mediated Drp1-dependent fission prevented activation of the proapoptotic Bax protein, MOMP, and death of intoxicated cells. Hp disruption of mitochondrial dynamics during infection is a heretofore unrecognized strategy by which a pathogenic microbe engages the host's apoptotic machinery.
Results
Hp Infection and Mitochondrial Fragmentation.
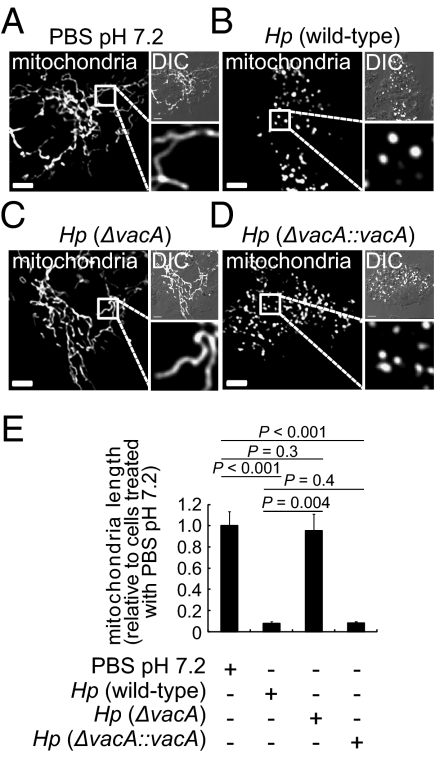
While studying cellular responses to Hp, we observed Hp-dependent alterations in the structure of cellular mitochondria, similar to those previously reported in studies of Hp-infected human stomach adenocarcinoma (AGS) cells (23). In uninfected AZ-521 gastric epithelial cells, mitochondria were predominantly filamentous networks of interconnected strands (∼15–25 μm in length) (Fig. 1 A and E). In contrast, at 8 h postinfection with Hp 60190, the mitochondrial network structure was highly fragmented, and individual mitochondria were visible as punctate organelles that were significantly shorter in length (1–2 μm) (Fig. 1 B and E). The transition from filamentous to punctate structures was also observed with either Hp 26695 or Hp G27, indicating that Hp-dependent fragmentation of mitochondria is not idiosyncratic to a single strain (Fig. S1A). In addition, Hp-dependent fragmentation was recapitulated across cells lines, as observed in studies using AGS (Fig. S1B) and polarized Madin-Darby canine kidney (MDCK) II cells (Fig. S1 C and D). Fragmentation occurred at multiplicity of infection (MOI) 10, but not at MOI 1 (Fig. S1E), and was time dependent, with progressive fragmentation of the filamentous network observed between 2 and 8 h postinfection (Fig. S1F).
Fig. 1.
Hp infection and mitochondrial fragmentation. AZ-521 cells transfected with pDsRed2-Mito were incubated for 8 h with Hp 60190 (B), Hp VM022 (ΔvacA) (C), Hp VM084 (ΔvacA::vacA) (D), all at MOI 100, or mock infected (A). (A–D) (Scale bar, 5 μm.) (E) Data from A–D were rendered as mitochondrial length relative to mock-infected cells. Data were combined from three independent experiments where, in each, 45 randomly chosen mitochondria were analyzed (3 mitochondria in each of 15 cells).
VacA Is Essential for Hp-Induced Mitochondrial Fragmentation.
While characterizing Hp-dependent mitochondrial alterations, we observed fragmentation of the mitochondrial network in AZ-521 cells that had been incubated with culture filtrate prepared from Hp 60190 (Hp culture filtrate, HPCF), but not with HPCF that had been pretreated at 95 °C (Fig. S1G). These results suggested that a proteinaceous factor released by Hp is responsible for mitochondrial fragmentation observed during infection. Consistent with this idea, fragmentation was not detected in cells incubated with heat-killed Hp 60190 (Fig. S1H).
Studies indicating the involvement of an extracellular, heat-labile factor in Hp-mediated mitochondrial network fragmentation prompted us to evaluate a potential role for VacA, which has been previously reported to modulate mitochondrial function (16, 17, 24, 25). In contrast to the extensive fragmentation of mitochondria induced by Hp 60190 (Fig. 1B), an isogenic Hp mutant strain lacking a functional vacA gene [Hp (ΔvacA)] (26) did not induce visible mitochondrial fragmentation within AZ-521 cells (Fig. 1 C and E), whereas the complemented strain [Hp (ΔvacA::vacA)] (27) induced robust mitochondrial fragmentation (Fig. 1 D and E). Differences in the adherence of Hp 60190, Hp (ΔvacA), or Hp (ΔvacA::vacA) to AZ-521 cells were not observed (Fig. S1I), suggesting that the inability of Hp (ΔvacA) to induce mitochondrial fragmentation was not likely due to attenuated cell association. These results support the idea that VacA is essential for Hp-dependent mitochondrial fragmentation.
VacA Is Sufficient to Induce Mitochondrial Fragmentation.
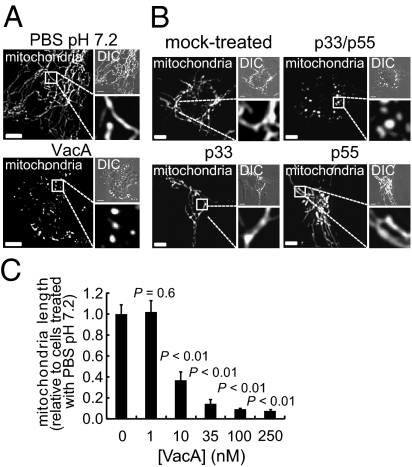
Studies to evaluate the sufficiency of VacA for Hp-dependent mitochondrial fragmentation revealed that VacA, which had been purified from culture filtrates of Hp 60190, induced the transition of mitochondrial networks into significantly shorter punctiform organelles (Fig. 2A), an effect that was abrogated when the toxin was preincubated at 95 °C or with VacA antiserum (Fig. S2A). More convincing, however, were studies conducted with recombinant VacA comprising the toxin's amino- and carboxyl-terminal fragments, called p33 and p55, respectively, that had been expressed separately in Escherichia coli and purified (28). The combined p33–p55 fragments induced robust fragmentation, whereas neither p33 nor p55 alone induced detectable alterations in the mitochondrial network (Fig. 2B). Together, these results indicate that VacA is sufficient to induce mitochondrial fragmentation.
Fig. 2.
VacA and mitochondrial fragmentation. AZ-521 cells transfected with pDsRed2-Mito were incubated for 4 h with VacA (250 nM) (A), or with recombinant p33, p55, or p33 plus p55 (each at 250 nM) (B), or at the indicated concentrations (C). Additionally, the cells were mock intoxicated with PBS pH 7.2 (A and C) or 25 mM Hepes pH 8.8 containing 5% glycerol and 0.1 nM β-ME (B). (A and B) (Scale bar, 5 μm.) (C) The data were combined from three independent experiments.
VacA-mediated mitochondrial fragmentation is not idiosyncratic to AZ-521 cells, as the toxin induced similar morphological changes within AGS cells (Fig. S2B), as previously observed (29), and polarized MDCK cells (Fig. S2C). VacA-dependent mitochondrial fragmentation was also visible within human cervical adenocarcinoma (HeLa) and human larynx carcinoma (HEp-2) cells (Fig. S2B), two nongastric cell lines that have been previously used to study VacA interactions with mitochondria (17, 30). Within AZ-521 cells, mitochondrial fragmentation was detected with VacA at concentrations as low as 10 nM, but not at 1 nM (Fig. 2C). Fragmentation was visible within 60 min after exposure of the cells to VacA (250 nM) and progressed to almost entirely punctiform organelles by 150 min (Fig. S2D).
VacA Induces Mitochondrial Recruitment of Drp1.
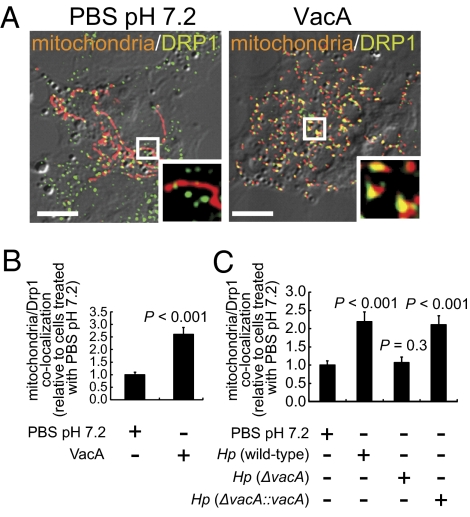
Cellular mitochondria exist in a dynamic state, constantly dividing and fusing by opposing processes (31). An important regulator of mitochondrial fission is Drp1 (31), which upon activation is recruited to focal sites of division on the mitochondrial outer membrane (32). Studies to evaluate whether VacA-dependent mitochondrial fragmentation might involve the cellular fission machinery revealed that, compared with mock-intoxicated cells, incubation for 4 h with purified VacA (from Hp 60190) resulted in a significant increase in Drp1 localization to mitochondria (Fig. 3 A and B). VacA-induced Drp1 localization to mitochondria was dose dependent, with significant increases observed at toxin concentrations as low as 10 nM (Fig. S3). A significant increase in mitochondrial localization of Drp1 was also detected in cells infected with Hp 60190 and Hp (ΔvacA::vacA), but not with Hp (ΔvacA), indicating that increased Drp1 recruitment to mitochondria within infected cells is VacA dependent (Fig. 3C). These results suggested a possible involvement of the cellular fission machinery in VacA-dependent mitochondrial network fragmentation.
Fig. 3.
Drp1 localization to mitochondria. AZ-521 cells transfected with pDsRed2-Mito were incubated with VacA (250 nM) or PBS pH 7.2 (A and B) for 4 h. Additionally, pDsRed2-Mito transfected AZ-521 cells were infected with Hp 60190, Hp VM022 (ΔvacA), Hp VM084 (ΔvacA::vacA), or mock infected (C) for 8 h. (Scale bar, 5 μm.) (B and C) The data were combined from three independent experiments.
Drp1 GTPase Activity Is Important for VacA-Dependent Mitochondrial Fragmentation.
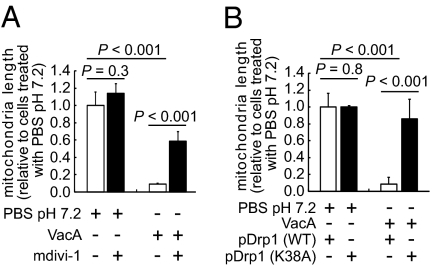
A critical step during mitochondrial fission is the assembly of Drp1 into spiral chains at the mitochondrial scission sites (33), which is driven by the intrinsic GTPase activity of Drp1 (34). To evaluate the importance of Drp1 function for VacA-dependent mitochondrial fission, AZ-521 cells were intoxicated with VacA in the absence or presence of the mitochondrial division inhibitor mdivi-1, which specifically blocks Drp1 self-assembly and GTPase activity (35). These studies revealed that mdivi-1 significantly inhibits VacA-dependent mitochondrial fragmentation in AZ-521 cells (Fig. 4A and Fig. S4A) and in AGS cells (Fig. S4B). Mitochondrial fragmentation was also significantly inhibited by mdivi-1 in AZ-521 cells infected with Hp 60190 (Fig. S4C). Together, these data indicate that Hp- and VacA-dependent mitochondrial fragmentation requires Drp1 GTPase activity, thereby implicating the involvement of the cellular mitochondrial fission machinery.
Fig. 4.
The importance of Drp1-mitochondrial localization and GTPase activity for VacA induced mitochondrial fragmentation. AZ-521 cells transfected with pDsRed2-Mito (A) or cotransfected with pDsRed2-Mito and either pEGFP-Drp1 or pEGFP-DN-Drp1 (K38A) (B) were intoxicated for 4 h with VacA (250 nM) (A and B), in the presence or absence of Drp1 inhibitor mdivi-1 (50 μM) (A). The data were combined from three independent experiments.
Drp1-Mitochondrial Localization Is Important for VacA-Dependent Mitochondrial Fragmentation.
Further validation of Drp1 involvement came from studies using AZ-521 cells overexpressing either Drp1 fused to enhanced green fluorescence protein, EGFP-Drp1 or a dominant-negative form of Drp1, EGFP-DN-Drp1 (K38A), which inhibits Drp1 association with the mitochondrial outer membrane (36). VacA-intoxicated cells overexpressing EGFP-DN-Drp1 (K38A) demonstrated significantly reduced mitochondrial fragmentation than those overexpressing EGFP-Drp1 (Fig. 4B and Fig. S4D). These results further support the functional importance of the cellular fission machinery for VacA-mediated mitochondrial fragmentation.
Drp1-Dependent Mitochondrial Fission Precedes and Is Important for VacA-Induced Bax Activation.
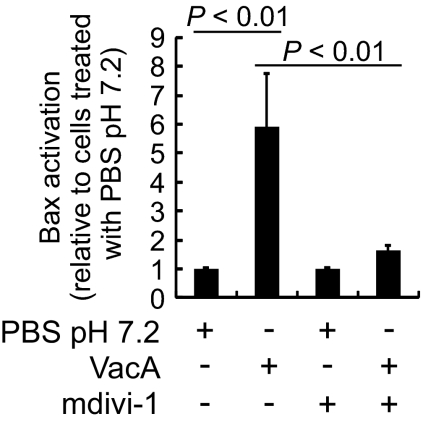
To evaluate whether VacA-mediated mitochondrial fragmentation may be linked to cell death, we first investigated whether Drp1-dependent mitochondrial fission is required for activation of the proapoptotic Bcl-2 effector, Bax, as reported within VacA-intoxicated cells (19). Notably, several studies have reported significant cross-talk between Drp1- and Bax-mediated cellular processes (37, 38). Within this study, we observed a significant decrease in Bax activation in AZ-521 cells incubated with VacA in the presence of the Drp1 inhibitor mdivi-1 (Fig. 5). The overall cellular levels of Bax were unaltered in cells that had been incubated with mdivi-1 and/or VacA (Fig. S5A). Consistent with the results obtained using mdivi-1, VacA-dependent Bax activation was also inhibited in AZ-521 cells overexpressing EGFP-DN-Drp1 (K38A) (Fig. S5B). Although mitochondrial fragmentation was clearly visible by 60 min in AZ-521 cells incubated with VacA (Fig. S2E), a significant increase in the fraction of cells with activated Bax was not detected until 2 h, and the fraction of cells with activated Bax continued to increase through 8 h (Fig. S5C). Together, these results suggest that within VacA-intoxicated cells, Drp1-mediated mitochondrial fission precedes and is important for Bax activation.
Fig. 5.
The importance of Drp1 activity for VacA-induced Bax activation. AZ-521 cells were intoxicated with VacA (250 nM) or mock intoxicated, in the presence or absence of mdivi-1 (50 μM). After 18 h, the cells were analyzed for activated Bax. The data were combined from three independent experiments.
Bax Is Not Essential for Drp1-Dependent Mitochondrial Fission in VacA-Intoxicated Cells.
Studies to evaluate the essentiality of cellular Bax for mitochondrial network fragmentation revealed clearly visible fragmentation in both bax+/+ and bax−/− mouse embryonic fibroblasts (MEFs) that had been incubated with VacA (Fig. S5D). These results suggest that Bax is not required for Drp1-dependent mitochondrial fission within VacA-intoxicated cells.
Drp1-Dependent Fission Is Important for VacA-Induced MOMP.
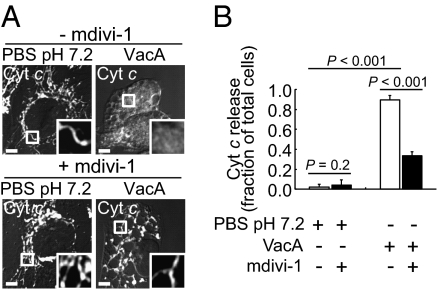
Within VacA-intoxicated cells, Bax activation is associated with MOMP, as manifested by release of mitochondrial cytochrome c (Cyt c) into the cytosol (19). Studies to evaluate the relationship between MOMP and Drp1-dependent mitochondrial fission revealed that the fraction of AZ-521 cells with Cyt c released from mitochondria into the cytosol had not increased after 1-h incubation with VacA (Fig. S5E), although mitochondrial fragmentation was evident at this same time point (Fig. S2D). However, there was a significant increase in the fraction of cells with Cyt c released from mitochondria into the cytosol by 2 h, and the fraction continued to increase through 8 h (Fig. S5E), similar to the kinetics of Bax activation (Fig. S5C), indicating that VacA-mediated mitochondrial fragmentation precedes MOMP. Cyt c release was significantly inhibited in the presence of the Drp1 inhibitor mdivi-1 (Fig. 6 A and B). In addition, Cyt c release in response to VacA was visibly reduced in cells overexpressing EGFP-DN-Drp1 (K38A) (Fig. S5F). These results suggest that enhanced mitochondrial fission promotes MOMP within VacA-intoxicated cells.
Fig. 6.
The importance of Drp1 activity for VacA-induced Cyt c release. AZ-521 cells were incubated with VacA (250 nM) or PBS, in the presence or absence of mdivi-1 (50 μM) (A and B). After 8 h, cells were evaluated for Cyt c cellular localization. (A) The images are representative of those collected over the course of three independent experiments. (Scale bar, 5 μm.) (B) The data were combined from three independent experiments.
VacA-Mediated Drp1 Activation Is Important for Toxin-Dependent Cell Death.
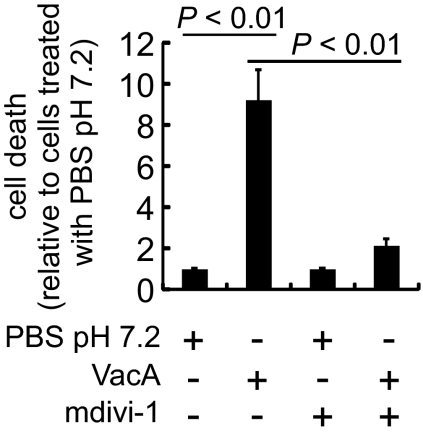
VacA-dependent MOMP precedes and is necessary for cell death (16, 19). Studies to evaluate the importance of Drp1 revealed that cell death was significantly reduced in AZ-521 (Fig. 7) or AGS cells (Fig. S6) that were incubated with VacA in the presence of mdivi-1. These results indicate that Drp1-dependent fission is important for VacA-induced cell death and suggest that the disruption of cellular mitochondrial dynamics plays an important role in activation of the cell death mechanism following Hp infection.
Fig. 7.
The importance of Drp1 activity for VacA-induced cell death. AZ-521 cells were intoxicated with VacA (250 nM) or PBS, in the presence or absence of Drp1 inhibitor, mdivi-1 (50 μM). After 24 h, relative cell death was determined by combining the data from three independent experiments.
VacA Anion Channel Activity Is Important for Mitochondrial Fragmentation.
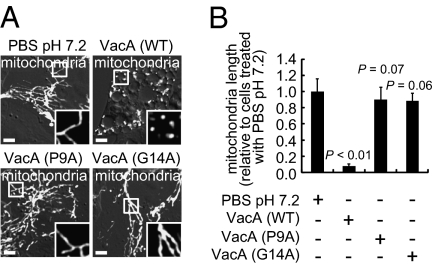
Anion-selective, membrane channels formed by VacA were earlier reported to be important for toxin-dependent MOMP and cell death (16). Studies to evaluate whether VacA channel activity is also required for toxin-dependent disruption of mitochondrial dynamics revealed the absence of detectable mitochondrial fragmentation in cells exposed to two mutant forms of toxin (Fig. 8 A and B), VacA (P9A) or VacA (G14A) (39), each of which had been previously shown to be attenuated in membrane channel-forming activity (40). Additionally, ectopic expression of the amino-terminal p34 domain of VacA, which contains the residues required for VacA channel activity (40), directly within the cytosol of transiently transfected AZ-521 cells, resulted in morphological changes, including apparent fragmentation in the cellular mitochondrial network (Fig. S7A). Finally, we confirmed that there was significantly less Bax activation (Fig. S7B) and cell death (Fig. S7C) in AZ-521 monolayers exposed to VacA (P9A) or VacA (G14A) than cells exposed to wild-type toxin. These data indicate that VacA membrane channel activity is important for the activation of Drp1-dependent mitochondrial fission within intoxicated cells.
Fig. 8.
The importance of VacA anion channel function for mitochondrial fragmentation. AZ-521 cells previously transfected with pDsRed2-Mito were incubated for 4 h with Hp culture filtrates (HPCFs) (0.05 mg/mL) containing wild-type VacA, VacA (P9A), VacA (G14A), or with PBS. (A) (Scale bar, 5 μm.) (B) The data were combined from three independent experiments.
Discussion
Mitochondrial health is closely linked to cell viability, as these organelles produce the energy required for cellular function while at the same time functioning as regulators of programmed cell death (41). Accordingly, mitochondrial dysfunction has been increasingly linked to several human pathologies, including those associated with cancer (42), inflammatory disorders (43), and degenerative diseases (44).
Here, we demonstrated that Hp infection of gastric epithelial cells disrupts the morphological dynamics of mitochondria by a mechanism dependent on the mitochondrial acting exotoxin, VacA. Whereas mitochondrial fragmentation has been previously observed in cells infected with Hp (23) or intoxicated with VacA (29), our studies revealed that Drp1-mediated mitochondrial fission precedes and is important for induction of VacA-dependent cell death. For Hp, an increase in cell death within the gastric mucosa may alter the host niche in several ways, including the loss of specialized cells, such as gastric parietal cells, but also increased cellular proliferation and gastric atrophy that precedes metaplasia, dysplasia, and ultimately cancer (45, 46).
A causal link between Drp1 and the cellular apoptotic machinery has been reported within diverse organisms such as yeast (47) and Drosophila (48). In contrast, down-regulation of Drp1 expression within HeLa or COS-7 cells did not prevent Bax-dependent apoptosis induced by UV radiation or actinomycin D (49). In addition, a recent report indicated that cell death does not result from the perturbation of mitochondrial dynamics in cells infected with Listeria monocytogenes (50), indicating that cell death is not an obligate outcome of disrupting mitochondrial dynamics. Thus, potential roles for Drp1 in mitochondrial-dependent cell death may ultimately be dictated by factors such as cell/tissue type or the nature of the prodeath stimulus.
Our results indicate that Drp1-mitochondrial localization and GTPase activity is required for activation of Bax in VacA-intoxicated cells (Fig. 5). Recent studies have demonstrated that functional crosstalk between Drp1 and Bax exists in some cases (51). One study identified Drp1 as the factor within rat brain extracts that stimulates Bax activity and mitochondrial Cyt c release, but in a manner that is independent of its GTPase activity (52), suggesting fundamental differences with Drp1-dependent Bax activation in VacA-intoxicated cells, which requires Drp1 GTPase activity (Fig. 5). On the other hand, Bax was reported to colocalize with Drp1 at mitochondrial scission sites (37) and promote sumoylation of Drp1, which is required for stable association of Drp1 with mitochondria (38). Furthermore, Bax was reported to influence redistribution of Drp1 to mitochondria through release of the mitochondrial effector DDP/TIMM8a (53). Our data indicate that Bax is not required for Drp1-mediated fission, suggesting that within VacA-intoxicated cells, crosstalk between Drp1 and Bax may be unidirectional (e.g., Drp1-dependent fission as a trigger for Bax-mediated MOMP). Currently, we are investigating the mechanism by which Drp1-dependent fission results in Bax activation and MOMP within VacA-intoxicated cells.
The mechanism by which VacA induces Drp1-dependent mitochondrial fission is not clear. Our data indicate that VacA channel activity is required for toxin-dependent mitochondrial fragmentation (Fig. 8), but the cellular site at which toxin channel activity is required for fragmentation has not been identified. Because ectopic expression of p34-EGFP directly within the cytosol of AZ-521 cells alters mitochondrial morphology (Fig. S7A), it is unlikely that VacA membrane channels formed on the surface of mammalian cells (54) are required for toxin-mediated activation of Drp1-dependent mitochondrial fission. Alternatively, VacA might act directly at mitochondria, as several previous studies reported that a portion of VacA taken up from the cell surface localizes to this organelle (16, 29, 55). Preliminary studies to address a possible relationship between the location of intracellular VacA and the perturbation of mitochondrial dynamics revealed that VacA localization to mitochondria (Fig. S8) is evident within AZ-521 cells at 30 min, before the earliest time (60 min) that visible mitochondrial fragmentation was detected after exposure to toxin (Fig. S2E). Whereas these data are consistent with the idea that VacA may act directly at mitochondria to induce Drp1-mediated fission, we cannot currently rule out the possibility that VacA-mediated mitochondrial fragmentation is triggered independently of toxin localization to mitochondria. In support of this latter possibility, a recent study reported that localization of VacA to mitochondria is delayed within MEFs lacking both Bax and the related effector Bcl-2 homologous antagonist/killer (Bak) (55), although our data clearly indicate that Bax is not required for VacA-induced Drp1-mediated mitochondrial fission (Fig. S5D).
Preliminary studies to address the relationship between mitochondrial dysfunction and fission within VacA-intoxicated cells revealed that Drp1 activity is not required for VacA-mediated dissipation of mitochondrial transmembrane potential (ΔΨm) (Fig. S9), suggesting that fission in VacA-intoxicated cells is not likely the trigger for mitochondrial dysfunction. However, it remains possible that VacA-induced ΔΨm dissipation induces Drp1-dependent fission. Support for this idea comes from unrelated studies that demonstrated that mitochondrial depolarization in HeLa cells induced a sustained cytosolic calcium rise, followed by calcineurin-mediated dephosphorylation of Drp1 at Ser637 as a mechanism to drive Drp1 translocation to mitochondria (56). VacA is sufficient to induce ΔΨm dissipation (19) and increases in cytosolic calcium (57), but a potential link between elevated cellular calcium or calcineurin action and Drp1-dependent mitochondrial fission within VacA-intoxicated cells remains to be evaluated.
In summary, these results demonstrate that apoptosis of gastric epithelial cells during Hp infection is triggered by VacA-induced disruption of mitochondrial morphological dynamics through Drp1-mediated fission, which both precedes and is required for Bax-dependent remodeling of the mitochondrial outer membrane. Future work will be required to reveal whether other pathogens also promote host cell death by targeting the morphological dynamics of mitochondria.
Materials and Methods
The sources of reagents, cell lines, bacterial strains, and plasmids used in this study are provided in SI Materials and Methods. In addition, a detailed description of experimental methods is provided in SI Materials and Methods. Finally, statistical analyses of the data are described in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Marina Jendrach (Goethe University) for pEGFP-Drp1 and pEFGP-DN-Drp1 (K38A), Dr. Wei-Xing Zong (Stony Brook University) for bax+/+ and bax−/− MEFs, and Dr. Joachim Rassow (Ruhr-Universität Bochum) for the plasmid encoding p34-EGFP (30). We also thank Drs. Barbara Pilas and Bernard Montez (University of Illinois R. J. Carver Biotechnology Center Flow Cytometry Facility) for technical expertise and assistance. This work was supported by Grant R01 AI045928 from the National Institutes of Health (to S.R.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105175108/-/DCSupplemental.
References
- 1.Blanke SR. Micro-managing the executioner: Pathogen targeting of mitochondria. Trends Microbiol. 2005;13:64–71. doi: 10.1016/j.tim.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Rudel T, Kepp O, Kozjak-Pavlovic V. Interactions between bacterial pathogens and mitochondrial cell death pathways. Nat Rev Microbiol. 2010;8:693–705. doi: 10.1038/nrmicro2421. [DOI] [PubMed] [Google Scholar]
- 3.Moss SF, Calam J, Agarwal B, Wang S, Holt PR. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones NL, et al. Enhanced disease severity in Helicobacter pylori-infected mice deficient in Fas signaling. Infect Immun. 2002;70:2591–2597. doi: 10.1128/IAI.70.5.2591-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peek RM, Jr, et al. Helicobacter pylori alters gastric epithelial cell cycle events and gastrin secretion in Mongolian gerbils. Gastroenterology. 2000;118:48–59. doi: 10.1016/s0016-5085(00)70413-6. [DOI] [PubMed] [Google Scholar]
- 6.Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320–332. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 7.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salama NR, Otto G, Tompkins L, Falkow S. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect Immun. 2001;69:730–736. doi: 10.1128/IAI.69.2.730-736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujikawa A, et al. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat Genet. 2003;33:375–381. doi: 10.1038/ng1112. [DOI] [PubMed] [Google Scholar]
- 10.Kuck D, et al. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect Immun. 2001;69:5080–5087. doi: 10.1128/IAI.69.8.5080-5087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cover TL, Krishna US, Israel DA, Peek RM., Jr Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951–957. [PubMed] [Google Scholar]
- 12.Gupta VR, et al. Sphingomyelin functions as a novel receptor for Helicobacter pylori VacA. PLoS Pathog. 2008;4:e1000073. doi: 10.1371/journal.ppat.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta VR, Wilson BA, Blanke SR. Sphingomyelin is important for the cellular entry and intracellular localization of Helicobacter pylori VacA. Cell Microbiol. 2010;12:1517–1533. doi: 10.1111/j.1462-5822.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yahiro K, et al. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase beta. J Biol Chem. 1999;274:36693–36699. doi: 10.1074/jbc.274.51.36693. [DOI] [PubMed] [Google Scholar]
- 15.Yahiro K, et al. Protein-tyrosine phosphatase alpha, RPTP alpha, is a Helicobacter pylori VacA receptor. J Biol Chem. 2003;278:19183–19189. doi: 10.1074/jbc.M300117200. [DOI] [PubMed] [Google Scholar]
- 16.Willhite DC, Blanke SR. Helicobacter pylori vacuolating cytotoxin enters cells, localizes to the mitochondria, and induces mitochondrial membrane permeability changes correlated to toxin channel activity. Cell Microbiol. 2004;6:143–154. doi: 10.1046/j.1462-5822.2003.00347.x. [DOI] [PubMed] [Google Scholar]
- 17.Galmiche A, et al. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19:6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foo JH, et al. Both the p33 and p55 subunits of the Helicobacter pylori VacA toxin are targeted to mammalian mitochondria. J Mol Biol. 2010;401:792–798. doi: 10.1016/j.jmb.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 19.Yamasaki E, et al. Helicobacter pylori vacuolating cytotoxin induces activation of the proapoptotic proteins Bax and Bak, leading to cytochrome c release and cell death, independent of vacuolation. J Biol Chem. 2006;281:11250–11259. doi: 10.1074/jbc.M509404200. [DOI] [PubMed] [Google Scholar]
- 20.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: The bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho DH, Nakamura T, Lipton SA. Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol Life Sci. 2010;67:3435–3447. doi: 10.1007/s00018-010-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandemange S, Herzig S, Martinou JC. Mitochondrial dynamics and cancer. Semin Cancer Biol. 2009;19:50–56. doi: 10.1016/j.semcancer.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Ashktorab H, et al. Bax translocation and mitochondrial fragmentation induced by Helicobacter pylori. Gut. 2004;53:805–813. doi: 10.1136/gut.2003.024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura M, et al. Vacuolating cytotoxin purified from Helicobacter pylori causes mitochondrial damage in human gastric cells. Microb Pathog. 1999;26:45–52. doi: 10.1006/mpat.1998.0241. [DOI] [PubMed] [Google Scholar]
- 25.Willhite DC, Cover TL, Blanke SR. Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J Biol Chem. 2003;278:48204–48209. doi: 10.1074/jbc.M304131200. [DOI] [PubMed] [Google Scholar]
- 26.Vinion-Dubiel AD, et al. A dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J Biol Chem. 1999;274:37736–37742. doi: 10.1074/jbc.274.53.37736. [DOI] [PubMed] [Google Scholar]
- 27.McClain MS, et al. A 12-amino-acid segment, present in type s2 but not type s1 Helicobacter pylori VacA proteins, abolishes cytotoxin activity and alters membrane channel formation. J Bacteriol. 2001;183:6499–6508. doi: 10.1128/JB.183.22.6499-6508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González-Rivera C, et al. Reconstitution of Helicobacter pylori VacA toxin from purified components. Biochemistry. 2010;49:5743–5752. doi: 10.1021/bi100618g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oldani A, et al. Helicobacter pylori counteracts the apoptotic action of its VacA toxin by injecting the CagA protein into gastric epithelial cells. PLoS Pathog. 2009;5:e1000603. doi: 10.1371/journal.ppat.1000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domańska G, et al. Helicobacter pylori VacA toxin/subunit p34: Targeting of an anion channel to the inner mitochondrial membrane. PLoS Pathog. 2010;6:e1000878. doi: 10.1371/journal.ppat.1000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 32.Santel A, Frank S. Shaping mitochondria: The complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life. 2008;60:448–455. doi: 10.1002/iub.71. [DOI] [PubMed] [Google Scholar]
- 33.Ingerman E, et al. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukushima NH, Brisch E, Keegan BR, Bleazard W, Shaw JM. The GTPase effector domain sequence of the Dnm1p GTPase regulates self-assembly and controls a rate-limiting step in mitochondrial fission. Mol Biol Cell. 2001;12:2756–2766. doi: 10.1091/mbc.12.9.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassidy-Stone A, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 37.Karbowski M, et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye D, Blanke SR. Mutational analysis of the Helicobacter pylori vacuolating toxin amino terminus: Identification of amino acids essential for cellular vacuolation. Infect Immun. 2000;68:4354–4357. doi: 10.1128/iai.68.7.4354-4357.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClain MS, et al. Essential role of a GXXXG motif for membrane channel formation by Helicobacter pylori vacuolating toxin. J Biol Chem. 2003;278:12101–12108. doi: 10.1074/jbc.M212595200. [DOI] [PubMed] [Google Scholar]
- 41.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 42.Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncogene. 2006;25:4647–4662. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- 43.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 44.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimura T, et al. Gastric mucosal inflammation and epithelial cell turnover are associated with gastric cancer in patients with Helicobacter pylori infection. J Clin Pathol. 2000;53:532–536. doi: 10.1136/jcp.53.7.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia HH, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: Implications in gastric carcinogenesis. Am J Gastroenterol. 2001;96:16–26. doi: 10.1111/j.1572-0241.2001.03447.x. [DOI] [PubMed] [Google Scholar]
- 47.Fannjiang Y, et al. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 2004;18:2785–2797. doi: 10.1101/gad.1247904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goyal G, Fell B, Sarin A, Youle RJ, Sriram V. Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster. Dev Cell. 2007;12:807–816. doi: 10.1016/j.devcel.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parone PA, et al. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stavru F, et al. Listeria monocytogenes transiently alters mitochondrial dynamics during infection. Proc Natl Acad Sci USA. 2011;104:13467–13472. doi: 10.1073/pnas.1100126108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Autret A, Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell. 2009;36:355–363. doi: 10.1016/j.molcel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Montessuit S, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnoult D, et al. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol. 2005;15:2112–2118. doi: 10.1016/j.cub.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 54.Szabò I, et al. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 1999;18:5517–5527. doi: 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calore F, et al. Endosome-mitochondria juxtaposition during apoptosis induced by H. pylori VacA. Cell Death Differ. 2010;17:1707–1716. doi: 10.1038/cdd.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cereghetti GM, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Bernard M, et al. The Helicobacter pylori VacA cytotoxin activates RBL-2H3 cells by inducing cytosolic calcium oscillations. Cell Microbiol. 2005;7:191–198. doi: 10.1111/j.1462-5822.2004.00446.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.