Abstract
Historical records show that the A.D. 1783–1784 Laki eruption in Iceland caused severe environmental stress and posed a health hazard far beyond the borders of Iceland. Given the reasonable likelihood of such an event recurring, it is important to assess the scale on which a future eruption could impact society. We quantify the potential health effects caused by an increase in air pollution during a future Laki-style eruption using a global aerosol model together with concentration-response functions derived from current epidemiological studies. The concentration of particulate matter with diameters smaller than 2.5 µm is predicted to double across central, western, and northern Europe during the first 3 mo of the eruption. Over land areas of Europe, the current World Health Organization 24-h air quality guideline for particulate matter with diameters smaller than 2.5 µm is exceeded an additional 36 d on average over the course of the eruption. Based on the changes in particulate air pollution, we estimate that approximately 142,000 additional cardiopulmonary fatalities (with a 95% confidence interval of 52,000–228,000) could occur in Europe. In terms of air pollution, such a volcanic eruption would therefore be a severe health hazard, increasing excess mortality in Europe on a scale that likely exceeds excess mortality due to seasonal influenza.
Keywords: volcanic air pollution, flood lava eruption, health risk, volcanism
Around 25 y ago, epidemiological studies revealed a statistical link between particulate matter (PM) air pollution and premature mortality (e.g., ref. 1). Subsequent research has confirmed a strong link between mortality and both acute and chronic exposure to PM (see reviews in refs. 2 and 3) and has led to the introduction of standards for improving air quality in Europe and other parts of the world. However, policies to improve air quality cannot account for unpredictable natural air pollution events such as volcanic eruptions. The eruptions of the Icelandic Eyjafjöll volcano in 2010 and Grímsvötn volcano in 2011 not only alerted European governments to the risks posed by volcanic ash but also to those that could arise from so-called low-probability, high-impact sulfur-dominated volcanic events such as the A.D. 1783–1784 Laki eruption.
It is well known that volcanic eruptions can increase concentrations of sulfur dioxide (SO2) and other species that affect human health close to the source (e.g., refs. 4–6), although there have been limited attempts to quantify the disease burden associated with exposure to volcanic air pollution (6). However, historical records of some volcanic eruptions and their aftermaths clearly suggest a link between volcanic air pollution and severe negative health effects (e.g., refs. 7 and 8).
In summer A.D. 1783, many countries in the northern hemisphere witnessed an atmospheric phenomenon often referred to as the “great dry fog” or “haze” caused by a widespread sulfuric acid cloud linked to volcanic activity in Iceland (ref. 9 and references therein). The Laki flood lava eruption emitted approximately 122 Tg of SO2 over the course of 8 mo, comparable to global anthropogenic SO2 emissions of approximately 115 Tg during the year 2005 (10). The eruption caused environmental stress and posed a serious health hazard far beyond the borders of Iceland (e.g., refs. 8 and 9). Several historical reports from across Europe, including visibility reductions and the smell of sulfur/hydrogen sulfide, clearly indicate an increase in air pollution. In the Netherlands, the rise in air pollution resulted in “troublesome headaches and respiratory difficulties” and the leaves fell off the trees in such abundance that their appearance was as if it were October or November despite it being June (11). In Iceland itself, around 21% of the human population and 75% of livestock perished (9), and parish records suggest that mortality in England in the summer of 1783 was 10–20% above the 51-y moving mean (8, 12). Several historical accounts of increased mortality rates and/or respiratory disorders are also found in France, the Netherlands, Italy, and Sweden (7, 8, 13–15).
Icelandic records clearly suggest that air pollution contributed directly to increased mortality rates, with northern regions repeatedly exposed to a low-level sulfuric acid haze suffering much higher death rates than other regions (16). However, the contribution of the volcanic air pollution to the excess mortality rates cannot be determined from historical records alone because other factors such as starvation and weather also played a role (15). Moreover, putting such an event into a modern societal context cannot be achieved by simply extrapolating the 1783–1784 mortality rates using current population figures because the modern population is likely to be healthier and more resilient to air pollution exposure than that of the 1780s. Thus, a modeling approach based on modern epidemiological functions is required to estimate the impact of volcanic air pollution on the modern European population.
Records suggest that volcanic events like Laki have occurred four times in Iceland over the past 1,150 y (17), with the size of the eruption ranging from one-quarter to twice the size of Laki. A present-day Laki eruption would provide a very large source of SO2 and acidic aerosol over Europe and could affect a very densely populated area.
In this study, we assess the impact of a future Laki-style eruption on European air quality using the Global Model of Aerosol Processes (GLOMAP) (ref. 18; the SI Text contains further details). We simulate the course of the eruption (i.e., the magnitude, altitude, and timing of the release of the volcanic SO2) as reconstructed for A.D. 1783–1784 (9, 19) but under present-day atmospheric conditions (see SI Text for details). The eruption commenced on June 8, 1783 and was most vigorous during the first 1.5 mo, with the volcanic activity declining thereafter until it ended on February 7, 1784. The eruption featured 10 episodes during which volcanic gases were injected into the upper troposphere and lower stratosphere (9, 19). Volcanic ash was only a minor constituent of the emissions (i.e., 0.4 km3 or 2.6% of the total erupted volume) (19), thus the scope of our study is to assess the potential health effects arising from an increase in particulate matter air pollution due to the sulfate (SO4) aerosol formed during such an event.
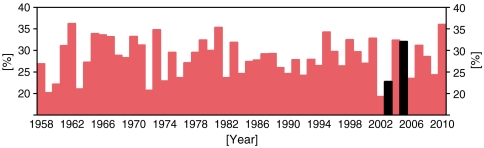
The transport of volcanic pollutants into mainland Europe is dependent on the meteorological situation and, in particular, the prevalence of northwesterly air flow from Iceland to the United Kingdom and continental Europe. To examine the interannual variability in northwesterly air flow, we analyzed data from European Center for Medium-Range Weather Forecasts reanalyses (20, 21). Fig. 1 summarizes the prevalence of northwesterly air flow from Iceland to the United Kingdom for the period 1958 to 2010 (see SI Text for details). For our model analysis of air quality over Europe, we simulate the eruption in a year with a low frequency of northwesterly air flow (2003) and a year with a high frequency of northwesterly air flow (2005). Reconstruction of the meteorological situation in 1783 suggests the Laki eruption itself occurred in a year with a low frequency of northwesterly air flow (22) (see Fig. S1). The simulations were run for 1 y following the onset of the eruption in June. We calculate the concentration of particulate matter with diameters smaller than 2.5 µm (PM2.5) over Europe for two scenarios: control runs for the years 2003 and 2005 including natural aerosol emissions and anthropogenic emissions, and volcanically perturbed runs for the same years including the Laki emissions. In Results and Discussion, we refer to the June 2003 to May 2004 simulation as “2003” and to the June 2005 to May 2006 simulation as “2005” because the major eruption episodes occur in those years.
Fig. 1.
Percentage positive (i.e., northwesterly air flow) Iceland to UK flow index shown as annual values at 500 hPa for the years 1958–2010 using European Center for Medium-Range Weather Forecasts reanalyses (20, 21). The years 2003 and 2005 used for the simulations are highlighted in black.
To quantify the negative health effects associated with a long-lasting, Laki-style air pollution event, we use concentration-response (C-R) functions appropriate for PM2.5 (23, 24). Generally, C-R functions are derived from epidemiological studies and link changes in pollutant concentrations to changes in a given health risk. Given the dominance of SO2 released during the eruption and the fact that the composition of the background atmosphere is representative of present-day air pollution, we regard it reasonable to apply the epidemiological evidence to the scenario presented here. Following the onset of the eruption, the PM2.5 mass will exhibit a higher contribution from SO4 when compared to the volcanically unperturbed situation.
Results
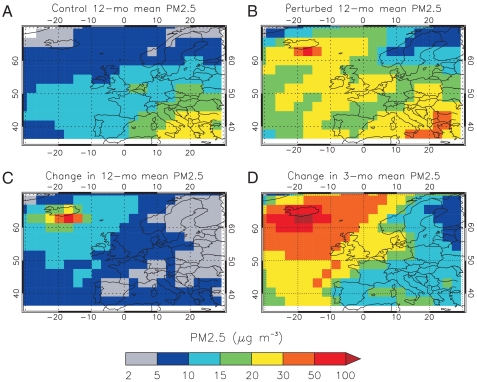
In the control runs, GLOMAP simulates annual European-mean surface PM2.5 concentrations of about 12 μg/m3 with only minor differences arising from using 2003 and 2005 meteorology. Averaged for the 2003 and 2005 simulations, background peak concentrations reach 29 μg/m3 over polluted regions in southern Europe (Fig. 2A). European-mean PM2.5 concentrations predicted by the model agree within 14% of multiyear observations across Europe (see Fig. S2). Averaged over the first 3 mo of the eruption, European-mean PM2.5 concentrations increase by 23 μg/m3 (Fig. 2D), or 120%. The impact of the eruption on PM2.5 concentrations varies spatially depending on the meteorology used to drive the model—a high frequency of northwesterly air flow (in 2005) results in higher 3-mo mean PM2.5 concentrations over Norway and Sweden when compared to a low frequency year (i.e., ca. 25 μg/m3 using 2003 versus ca. 29 μg/m3 using 2005 meteorology). Averaged over the first 3 mo, PM2.5 concentrations increase by up to approximately 320% over northern Europe and up to approximately 60% over southern Europe because of the eruption. Annual European-mean PM2.5 concentrations increase by 8 μg/m3 due to the eruption, with a peak change of 62 μg/m3 close to the source region in Iceland (Fig. 2C).
Fig. 2.
Modeled PM2.5 mass concentrations at the surface (shown as a mean of the 2003 and 2005 simulations). Twelve-month mean PM2.5 mass concentrations (microgram per cubic meter) for (A) control run without Laki emissions and (B) perturbed run including the Laki emissions, with (C) showing the absolute change (perturbed minus control run) in 12-mo mean PM2.5 concentrations, and (D) showing the absolute change in 3-mo mean PM2.5 concentrations calculated for first 3 mo following the onset of a Laki-style eruption on June 8.
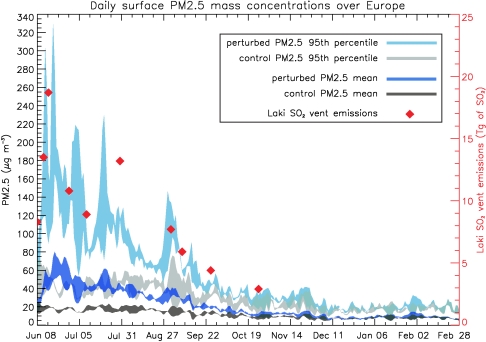
Daily surface PM2.5 concentrations are temporally highly variable in the months following the onset of the eruption (Fig. 3). Without the volcanic eruption, European-mean PM2.5 concentrations range between 4.4 and 25.5 μg/m3, with the highest concentrations occurring during the summer months. In the volcanically perturbed run, European-mean PM2.5 concentrations exceed 25 μg/m3 throughout the summer with a peak European-mean value of 80.9 μg/m3 in mid-June for the 2005 simulation and 62.1 μg/m3 in mid-July for the 2003 simulation. Although the two years with very different air flow result in differences in day-to-day variability in pollution, the main driver of pollution episodes over Europe is clearly the vigor of the eruption. The temporal pattern of PM2.5 concentrations also closely mimics the changes in the haze opacity reconstructed for the summer of 1783 (9).
Fig. 3.
Time series of surface PM2.5 mass concentrations averaged over the European domain starting on June 8 and ending on February 28 for the control runs (black) and the perturbed runs (dark blue), including the 95th percentiles for the control runs (gray) and perturbed runs (light blue). The time and magnitude of the SO2 emissions during the 10 Laki eruption episodes are depicted by red diamonds and the y axis on the right-hand side.
The 95th percentile of the 24-h mean PM2.5 concentration exceeds 100 μg/m3 during most of June, and episodically during July and August—each several days after major eruption episodes (red diamonds in Fig. 3). After the end of October, the European-mean PM2.5 concentrations steadily revert to background concentrations reflecting the decline in eruption intensity and occurrence of explosive phases.
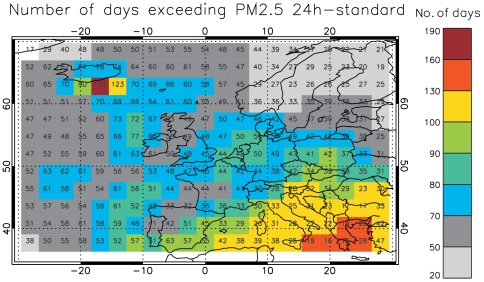
The model dataset allows us to estimate the number of days (out of the 266 considered) that exceed the current World Health Organization (WHO) 24-h mean PM2.5 guideline of 25 μg/m3 (Fig. 4). Over land areas of Europe (excluding Iceland), the mean number of exceedances increases by 36 d (range 14–63 d) when averaged for the 2003 and 2005 simulations compared to a background mean of 38 d. The highest number of exceedance days is simulated for Iceland (175 d with an absolute change of 172 d due to the eruption).
Fig. 4.
Total number of days out of the 266 d considered exceeding the current WHO 24-h PM2.5 guideline of 25 μg/m3 (shown as a mean of the 2003 and 2005 simulations). Colored boxes depict the perturbed situation (including Laki emissions). Numbers in grid boxes denote the absolute change (i.e., perturbed minus control run).
Given the increase in air pollution, we now apply the epidemiological evidence to our scenario. Current evidence indicates that both short-term (i.e., days) and long-term (i.e., months to years) exposure to PM2.5 is associated with all-cause and cardiopulmonary mortality (3). Several studies also find strong associations between SO4 air pollution and premature mortality (e.g., refs. 2 and 23). Given the strength of the evidence for PM2.5 relative to one of its constituents, SO4, we have used C-R functions for PM2.5. However, the existing evidence suggests that, on a mass basis, sulfates are likely to have larger effects per microgram per cubic meter than other aerosol components (2). It should be noted that we consider only a subset of the adverse health outcomes associated with exposure to PM. Dozens of studies have linked ambient PM to a wide range of morbidity outcomes including hospital admissions, emergency room visits, nonfatal heart attacks, exacerbation of asthma, and work and school loss (2).
We calculate excess mortality resulting from both short-term exposure and from long-term exposure. These outcomes should not be added together because the effects of the long-term exposures likely include those from the short-term as well. However, these two averaging times represent alternative assumptions about the biological mechanisms associated with exposures to volcanic air pollution.
To estimate the effect of long-term exposure, we use gridded 12-mo mean PM2.5 model data. Although PM2.5 concentrations vary substantially during the 12 mo, this is also the case with the background air pollution on which the epidemiological evidence is based, thus justifying C-R functions that examine the impacts of long-term exposures. In addition, Puett et al. (25) demonstrate that significant effects of cumulative exposure will be experienced during the first year. Thus, there is evidence for a very short latency period after the exposure. To calculate cardiopulmonary excess mortality due to long-term exposure to PM2.5, we apply the results of the American Cancer Society cohort study reported by Pope et al. (23) to our European PM2.5 dataset. However, this study uses a linear exposure function, which may not be appropriate for the very high concentrations that would occur after the eruption. Consequently, we use an adjusted function which assumes that mortality depends on the logarithm of exposure (24), thus the C-R function flattens out at the higher concentrations. The long-term mortality effect (excess mortality per year) is expressed as
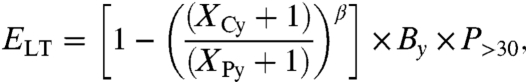 |
[1] |
where XCy is the annual mean PM2.5 mass concentration (microgram per cubic meter) in the control run; XPy is the annual mean PM2.5 mass concentration (microgram per cubic meter) in the volcanically perturbed run; the parameter β is an empirical parameter for cardiopulmonary mortality: 0.1551 (95% C.I. = 0.05624, 0.2541); By is the baseline incidence rate {deaths per person per year} for the health effect under study; and P>30 represents the exposed population—that is, persons who are older than 30 y.
To calculate excess all-cause mortality due to short-term exposure to PM2.5, we follow the methods of Ostro (24) using our European gridded daily mean PM2.5 data between June 8, 2003 (2005) and February 28, 2004 (2006). We assume there is a constant pool of susceptible individuals over the period of the eruption, most likely individuals with preexisting cardiovascular or respiratory disease. By utilizing the results of four recent time-series studies that link acute exposure to PM2.5 with all-cause mortality (26–29) (see table 2 in ref. 3), we obtain a mean effect by calculating the inverse-variance mean (see SI Text). In our study, a 10 μg/m3 change in PM2.5 mass concentrations is associated with a 0.96% increase in daily all-cause mortality. The effect in terms of fatalities per day is given by
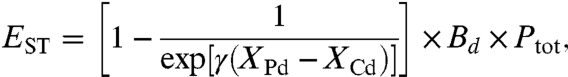 |
[2] |
where the variables have the same meaning as in Eq. 1, except that they refer to daily means rather than annual means. The parameter γ is the excess mortality risk per unit change in daily mean PM2.5: 0.00096 (95% C.I. = 0.00079, 0.00113) (see Table S1 for percentage increase in mortality per 10 μg/m3), and Ptot represents the exposed population; that is, all ages. Wong et al. (30) show that the C-R function for short-term exposure is basically linear without a threshold, even if the PM mass concentrations are very high as observed in Asian cities. It is therefore reasonable to apply such a linear relationship in our study.
Baseline incidence rates for all-cause mortality and cardiopulmonary mortality in Europe are obtained for the year 2004 from the WHO (31) and data on the total exposed population (see Fig. S3) from the History Database of the Global Environment (HYDE 3.1) (32).
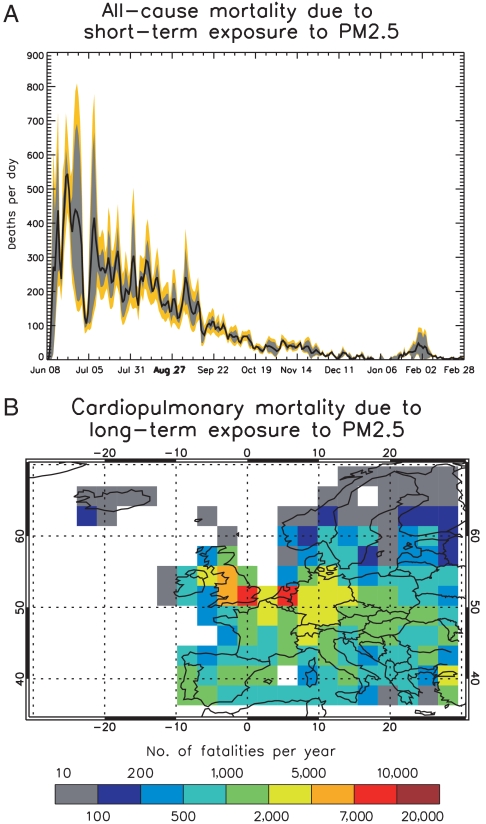
Using 2004 population data, we estimate between 27,500 (95% C.I. 22,600–32,200) (using 2003 meteorology) and 30,100 (95% C.I. 24,800–35,300) (using 2005 meteorology) additional all-cause fatalities from short-term exposure to PM2.5 over the course of 266 d following the onset of the eruption. Generally, daily all-cause mortality rates remain in excess of 100 deaths per day until mid-September, coinciding with the most vigorous stages of the eruption (Fig. 5A).
Fig. 5.
Number of excess fatalities in Europe resulting from short- and long-term exposure to PM2.5. A) Time-series of daily all-cause excess mortality due to short-term exposure to PM2.5 starting with the onset of the Laki-style eruption on June 8 and ending on February 28 (shown as range for the 2003 and 2005 simulations). The mean of the 2003 and 2005 simulations is shown as the black line and the 95% confidence interval is shown in orange. B) Number of excess cardiopulmonary fatalities in the year following the onset of a Laki-style due to long-term exposure to PM2.5 (shown as a mean of the 2003 and 2005 simulations).
If we base our estimates on the likelihood of longer-term exposure to PM2.5, we obtain around 139,000 (95% C.I. 51,000–224,000) excess cardiopulmonary deaths from long-term exposure to PM2.5 during 2003, and 144,000 (95% C.I. 53,000–232,000) during 2005 (Fig. 5B). The long-term mortality rates vary spatially depending on the co-location of PM2.5 and exposed population. Independent of the meteorological conditions, we obtain highest excess cardiopulmonary fatalities (Fig. 5B) per year for the Netherlands and Belgium (8,400 and 8,800 fatalities for 2003 and 2005, respectively). Averaged for 2003 and 2005, we obtain a total of 142,000 (95% C.I. 52,000–228,000) excess fatalities in the European domain with 7,400 fatalities in the southeast of the United Kingdom (total of 21,200 in whole of the United Kingdom), 19,000 in Germany, and 13,000 in France.
Discussion
Our results show that a future Laki-style volcanic air pollution event would be a severe and spatially widespread health hazard. In fact, the results highlight that the impacts occur as far downwind of the eruption site as southern and eastern Europe. The results also highlight that only slight differences arise in total excess mortality rate under different meteorological conditions. A predominantly westerly to northwesterly air flow favors transport into less-populated regions such as Norway, thus, although the pollutant transport to Europe is greater, there is only a slight difference in the total European excess mortality rate.
Next we compare the estimated excess mortality rates occurring in the United Kingdom, Ireland, and parts of western Europe with baseline fatalities reported by the WHO for the year 2004 (31). Depending on the meteorological conditions, excess cardiopulmonary mortality in the year following a Laki-style eruption equates to an increase of approximately 8.2% (95% C.I. 3.0–13.1) for 2003 and approximately 8.6% (95% C.I. 3.2–13.8) for 2005 when considering the Netherlands, Belgium, United Kingdom, Ireland, Germany, and France. For 2003, that corresponds to an increase of 77,500 (95% C.I. 28,600–124,700) fatalities and, for 2005, to an increase of 81,900 (95% C.I. 30,300–131,600) fatalities from a baseline of 950,000 all-cause cardiopulmonary fatalities reported by the WHO for the year 2004 (31). Assuming only short-term exposure is relevant, we find an increase in all-cause mortality between 0.6% and 0.7% when compared to the number of people who died in 2004 in the Netherlands, Belgium, United Kingdom, Ireland, Germany, and France. For the years 2003 and 2005, that corresponds to an increase of 14,300 and 16,200 fatalities, respectively, simulated from 2.24 million baseline all-cause fatalities reported by the WHO (31) for the aforementioned countries.
Our estimates of percentage excess fatalities due to exposure to volcanic air pollution in a modern setting can be compared with historical records of the aftermath of the Laki eruption. Grattan et al. (8) find a 10–20% increase in summer mortality in 1783 in England. We find the United Kingdom to be one of the worst affected areas in Europe (after the Netherlands and Belgium), with an increase in mortality of the modern population of 3.5% (total of 20,900 additional cardiopulmonary fatalities due to eruption on top of 595,800 all-cause deaths in the United Kingdom in 2004). Our predicted total excess mortality rates due to exposure to PM2.5 in Iceland are very low (less than 400 fatalities in total) compared to the estimated 21% death toll in 1783–1784 (9).
There are several possible explanations for the lower relative death rates in the modern setting. First, there are other factors such as starvation associated with the eruption that certainly added to the number of deaths in Iceland. Second, due to a lack of C-R functions, our estimates neglect the adverse health effects of exposure to elevated SO2 concentrations, which is likely to exacerbate asthma (e.g., ref. 33). Third, the modern population is likely to be healthier and more resilient to air pollution exposure than in the 1780s, so modern C-R functions are unlikely to extrapolate well to historical events. Fourth, we have considered only a subset of the adverse health outcomes associated with exposure to PM. Fifth, there is uncertainty regarding the precise C-R functions to apply. Although several recent studies generate much higher C-R functions for mortality (ref. 3 and references therein), we used the study of Pope et al. (23) because it involves the largest cohort and includes a wide range of individual city and urban characteristics. Lastly, there may be inaccuracies in the model predictions for such a large event, although multimodel intercomparisons of normal anthropogenic SO4 pollution suggest that modeled SO4 mass concentrations at the surface are generally predicted within 20% of the observations (34).
We note that the time of year of the eruption as well as the magnitude and duration of individual eruption episodes will influence the overall health outcome, with a summertime eruption most likely maximizing the total mortality rates due to more effective photochemical processing compared to a wintertime eruption. There are also uncertainties in the baseline of PM2.5 exposure. If we base our calculations on the extreme assumption that the entire European population is exposed to a higher baseline in PM2.5 concentrations, which is typically observed in urban environments in Europe, then the long-term excess mortality rate within the European domain is, according to Eq 1, reduced from 142,000 to 96,000 (see SI Text for details).
Concluding Remarks
Given the historic records of the Laki eruption and the probability of a recurrence of such an event, we consider it crucial to assess the scale on which volcanic air pollution due to a future Laki-style eruption could impact society. Our work agrees with historical records of the potential of Icelandic flood lava events to impact air quality and subsequently human health in Europe. Given the complex socioeconomic framework we live in, it is important to further investigate the societal impacts of such “low-probability, high-impact” events. We also note that a future Laki-style eruption will not only affect human health but also has the potential to affect commercial aviation due to high SO2 and SO4 mass concentrations in upper parts of the atmosphere. Moreover, such an event has a huge potential to affect the environment in terms of, for instance, crop failure.
To put our results in perspective, as part of the WHO Global Burden of Disease assessment, Cohen et al. (35) estimated that globally 800,000 deaths in the year 2000 could be attributed to urban outdoor air pollution, measured as PM2.5. Of this total, 23,000 deaths (95% C.I. 10,000–43,000) would occur in western and central Europe. Thus, our results are significant given that we only consider Europe, where a total of around 142,000 additional deaths could occur. Furthermore, between 250,000 and 500,000 persons die worldwide per year as a consequence of seasonal influenza (36) including an estimated 41,000 per year in the United States, estimated to be associated with around 72 billion dollars economic burden (37). Equivalent data are not available for Europe, although a direct scaling with population would equate to about 66,000 deaths. Thus, a large and long-lasting Icelandic eruption like Laki, potentially causing around 142,000 additional deaths in Europe in the first year, would constitute a major health hazard.
Materials and Methods
For the calculation of excess mortality, the predicted number of deaths depends on (i) the change in PM2.5 concentrations from baseline; (ii) the population exposed; (iii) the excess risks from exposure (i.e., percent increase in mortality per microgram per cubic meter of PM2.5); and (iv) the baseline mortality rates.
For long-term exposure, the relative risk (RR) is calculated as follows:
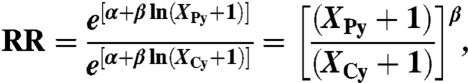 |
[3] |
where XCy and XPy are the annual mean PM2.5 mass concentrations (microgram per cubic meter) in the control and perturbed runs, respectively; β is an empirical parameter for cardiopulmonary mortality: 0.1551 (95% C.I. = 0.05624, 0.2541).
The attributable fraction (AF) is calculated as follows:
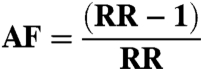 |
[4] |
and the total long-term mortality effect (ELT) is given by
| [5] |
which simplifies to Eq. 1 in the main text. In Eq. 5, By represents the baseline incidence rate {deaths per person per year} for the health effect under study, and P>30 represents the exposed population; that is, persons who are older than 30 y.
For short-term exposure, the RR is calculated as follows:
| [6] |
where XCd and XPd are the daily mean PM2.5 mass concentrations (microgram per cubic meter) in the control and perturbed runs, respectively; the parameter γ is the excess mortality risk per unit change in daily mean PM2.5: 0.00096 (95% C.I. = 0.00079, 0.00113). The AF is calculated according to Eq. 4, and the total short-term mortality effect (EST) is given by
| [7] |
which simplifies to Eq. 2 in the main text. In Eq. 7, Bd represents the baseline incidence rate {deaths per person per day} for the health effect under study, and Ptot represents the exposed population; that is, all ages.
Supplementary Material
Acknowledgments.
We thank the anonymous reviewers for their detailed comments helping to improve the manuscript. A.S. was funded through a University of Leeds PhD Research Scholarship. G.W.M. is supported by the Natural Environment Research Council through the National Centre for Atmospheric Science. Model development for this project was supported by the United Kingdom Chemistry and Aerosol (UKCA) project. Part of the development of UKCA is supported by Department of Energy and Climate Change/Defra Grant GA01101.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108569108/-/DCSupplemental.
References
- 1.Ostro B. A search for a threshold in the relationship of air-pollution to mortality—a reanalysis of data on London winters. Environ Health Perspect. 1984;58:397–399. doi: 10.1289/ehp.8458397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pope CA, Dockery DW. Health effects of fine particulate air pollution: Lines that connect. J Air Waste Manage Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 3.Brook RD, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 4.Baxter PJ. Impacts of eruptions on human health. In: Sigurdsson H, Houghton BF, McNutt SR, Rymer H, Stix J, editors. Encyclopaedia of Volcanoes. London: Academic; 2000. pp. 1035–1043. [Google Scholar]
- 5.Delmelle P, Stix J, Baxter PJ, Garcia-Alvarez J, Barquero J. Atmospheric dispersion, environmental effects and potential health hazard associated with the low-altitude gas plume of Masaya volcano, Nicaragua. Bull Volcanol. 2002;64:423–434. [Google Scholar]
- 6.Hansell A, Oppenheimer C. Health hazards from volcanic gases: A systematic literature review. Arch Environ Health. 2004;59:628–639. doi: 10.1080/00039890409602947. [DOI] [PubMed] [Google Scholar]
- 7.Durand M, Grattan J. Extensive respiratory health effects of volcanogenic dry fog in 1783 inferred from European documentary sources. Environ Geochem Health. 1999;21:371–376. [Google Scholar]
- 8.Grattan J, Durand M, Taylor S. Illness and elevated human mortality in Europe coincident with the Laki fissure eruption. In: Oppenheimer C, Pyle DM, Barclay J, editors. Volcanic Degassing. Vol. 213. London: Geological Soc; 2003. pp. 401–414. Special Publications. [Google Scholar]
- 9.Thordarson T, Self S. Atmospheric and environmental effects of the 1783–1784 Laki eruption: A review and reassessment. J Geophys Res Atmos. 2003;108:4011. [Google Scholar]
- 10.Smith SJ, et al. Anthropogenic sulfur dioxide emissions: 1850–2005. Atmos Chem Phys. 2011;11:1101–1116. [Google Scholar]
- 11.van Swinden SP. Observations on the cloud (dry fog) which appeared in June 1783. In: Hemmer J, König C, editors. Emphemerides Societatis Meteorologicae Palatinae, Observations Anni 1783. Mannheim, Germany: Fr. Scwan; 1783. pp. 679–688. [Google Scholar]
- 12.Wrigley EA, Schofield RS. Cambridge, UK: Cambridge Univ Press; 1989. The population history of England 1541–1871: A reconstruction; 794 pp. [Google Scholar]
- 13.Durand M, Grattan J. Effects of volcanic air pollution on health. Lancet. 2001;357:164–164. doi: 10.1016/S0140-6736(00)03586-8. [DOI] [PubMed] [Google Scholar]
- 14.Grattan J. Pollution and paradigms: Lessons from Icelandic volcanism for continental flood basalt studies. Lithos. 2005;79:343–353. [Google Scholar]
- 15.Witham CS, Oppenheimer C. Mortality in England during the 1783–1784 Laki craters eruption. Bull Volcanol. 2004;67:15–26. [Google Scholar]
- 16.Hálfdánarson G. Mannfall í Móðuharðindum. In: Einarsson T, et al., editors. Skaftáreldar 1783-84: Ritgerdir og Heimildir. Reykjavík, Iceland: Mál og Menning; 1984. pp. 139–162. [Google Scholar]
- 17.Thordarson T, Larsen G. Volcanism in Iceland in historical time: Volcano types, eruption styles and eruptive history. J Geodyn. 2007;43:118–152. [Google Scholar]
- 18.Mann GW, et al. Description and evaluation of GLOMAP-mode: A modal global aerosol microphysics model for the UKCA composition-climate model. Geosci Model Dev. 2010;3:519–551. [Google Scholar]
- 19.Thordarson T, Self S. The Laki (Skaftár Fires) and Grímsvötn eruptions in 1783–1785. Bull Volcanol. 1993;55:233–263. [Google Scholar]
- 20.Uppala SM, et al. The ERA-40 Reanalysis. Q J R Meteorol Soc. 2005;131:2961–3012. [Google Scholar]
- 21.Dee DP, et al. The ERA-interim reanalysis: Configuration and performance of the data assimilation system. Q J R Meteorol Soc. 2011;137:553–597. [Google Scholar]
- 22.Kington JA. The Weather of the 1780s over Europe. Cambridge, UK: Cambridge Univ Press; 1988. [Google Scholar]
- 23.Pope CA, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J Am Med Assoc. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostro B. Geneva: WHO; 2004. Outdoor air pollution: Assessing the environmental burden of disease at national and local levels. (WHO Environmental Burden of Disease Series, No. 5). [Google Scholar]
- 25.Puett RC, et al. Chronic particulate exposure, mortality, and coronary heart disease in the nurses’ health study. Am J Epidemiol. 2008;168:1161–1168. doi: 10.1093/aje/kwn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klemm RJ, Mason R. Revised Analyses of Time-Series of Air Pollution and Health. Boston: Health Effects Inst; 2003. Replication of reanalysis of Harvard six-city mortality study. [Google Scholar]
- 27.Ostro B, Broadwin R, Green S, Feng W-Y, Lipsett M. Fine particulate air pollution and mortality in nine California counties: Results from calfine. Environ Health Perspect. 2006;114:29–33. doi: 10.1289/ehp.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2006;17:279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- 29.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: A national analysis. Environ Health Perspect. 2009;117:898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong C-M, et al. Public health and air pollution in Asia (PAPA): A multicity study of short-term effects of air pollution on mortality. Environ Health Perspect. 2008;116:1195–1202. doi: 10.1289/ehp.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathers C, et al. The Global Burden of Disease: 2004 Update. Geneva: WHO; 2008. pp. 1–150. [Google Scholar]
- 32.Klein Goldewijk K, Beusen A, Janssen P. Long term dynamic modeling of global population and built-up area in a spatially explicit way, HYDE 3.1. Holocene. 2010;20:565–573. [Google Scholar]
- 33.US Environmental Protection Agency Integrated Science Assessment (ISA) for Sulfur Oxides—Health Criteria (Final Report) Washington, DC: US EPA; 2008. EPA/600/R-08/047F. [Google Scholar]
- 34.Barrie LA, et al. A comparison of large-scale atmospheric sulphate aerosol models (COSAM): Overview and highlights. Tellus Ser B. 2001;53:615–645. [Google Scholar]
- 35.Cohen AJ, et al. Urban air pollution. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative Quantification of Health Risks, Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Vol. 2. Geneva: WHO; 2004. Chap 17. [Google Scholar]
- 36.World Health Organization. Influenza, 2003. 2003. Available at http://www.who.int/mediacentre/factsheets/2003/fs211/en/. Accessed December 13, 2010.
- 37.Molinari N-AM, et al. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine. 2007;25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.