Abstract
The ability to learn language is a human trait. In adults and children, brain imaging studies have shown that auditory language activates a bilateral frontotemporal network with a left hemispheric dominance. It is an open question whether these activations represent the complete neural basis for language present at birth. Here we demonstrate that in 2-d-old infants, the language-related neural substrate is fully active in both hemispheres with a preponderance in the right auditory cortex. Functional and structural connectivities within this neural network, however, are immature, with strong connectivities only between the two hemispheres, contrasting with the adult pattern of prevalent intrahemispheric connectivities. Thus, although the brain responds to spoken language already at birth, thereby providing a strong biological basis to acquire language, progressive maturation of intrahemispheric functional connectivity is yet to be established with language exposure as the brain develops.
Keywords: brain activity, newborns, dorsal pathway, ventral pathway
Humans have the unique ability to acquire language. In 1871, Darwin (1) postulated that language is an instinct. The fact that young children acquire language spontaneously when provided with language input already suggests a biological predisposition to acquire language.
Genetic or acquired alteration of the language-relevant neural basis might prevent the normal acquisition and development of language (2–4).
Some support for very early language-related abilities comes from behavioral (5), electrophysiological (6, 7), and optical imaging (8, 9) studies showing that newborns can discriminate between different prosodies and speech sounds already shortly after birth. However, the neuroanatomical basis of these early abilities still needs to be specified.
Neuroimaging with 3-mo-old infants (10, 11) suggests that at this age speech processing is supported by inferior frontal and temporal brain regions similar to adults (12, 13) and, moreover, that in infants these regions are connected by two main fiber bundles: the arcuate fasciculus and the uncinate fasciculus (14). Although Dubois et al. (14) argue for a presence of the arcuate fasciculus at 3 mo, they admit that with their method it was not possible to reconstruct the frontal portion of the tract—that is, the portion that leads into the inferior frontal gyrus (Broca's area). Thus, it is an open issue whether the structural connections between the temporal cortex and Broca's area are present early in life. Structural imaging data from a study of preschool children suggest that this part of the arcuate fasciculus connecting the temporal cortex to Broca's area dorsally matures late and is not adult-like even by the age of 7 y (15).
In adults, a specific neural network supporting the processing of language has been described as involving frontal and temporal brain regions with a clear dominance of the left hemisphere (12, 13, 16). These language-relevant brain areas are connected structurally by major fiber bundles (17, 18) and are, moreover, functionally connected (19, 20). Spoken language, however, carries segmental information such as phonemes and words but also suprasegmental information—namely, prosody indicating sentence intonation. In adults, functional MRI (fMRI) revealed that suprasegmental information is processed predominantly in the right hemisphere, again involving inferior frontal and temporal regions (21, 22). An adult-like left hemispheric specialization for segmental information and an adult-like right hemispheric preference for suprasegmental information has been demonstrated in 4-y-old children using near-infrared spectroscopy (NIRS) (23). NIRS studies on prosody processing in infants are less straightforward, as they report a dominant right hemispheric temporoparietal activation in 3-mo-old infants for normal compared with flattened speech (24), but a right temporal and temporoparietal dominance for flattened compared with normal speech together with a bilateral prefrontal activation by the age of 10 mo (25). This change in the activation pattern is taken to suggest that on its way to adult mechanisms, prefrontal and right temporoparietal regions start to form a functional relationship by the age of 10 mo.
Thus, although both hemispheres are activated during speech processing in the first months of life, it is an open question as to whether these activations reflect an adult-like neural network for language processing, or whether the connectivities between the activated brain regions within this network are still immature at this early stage, in need of language exposure and brain maturation.
Here, we provide functional and structural data from 2-d-old infants, determining the brain basis of speech processing at birth. To do so we used a unique threefold methodological approach: first, a standard fMRI analysis to define the brain activation as a function of the different speech conditions; second, a low-frequency fluctuation (LFF) analysis of the same dataset to define the language default network with its functional connectivities; and third, diffusion tensor imaging (DTI) to determine the language network's structural connectivities. Note, that the first two analyses reflect important information about brain activation. The first analysis explains only a small portion of the overall variance in the fMRI time signal, whereas a large portion of the variance is accounted for by LFFs (20). Thus, both types of analyses are relevant for the description of the brain activation patterns observed.
Fifteen healthy, full-term, nonsedated Italian newborns participated in the study. Their brain activation was measured shortly after birth (1–3 d) while they listened to a story presented under three different conditions: normal speech with expressive child-directed intonation (normal speech), speech from which the segmental information was removed, leaving the prosody intact (hummed speech), and speech whose prosodic contour was flattened (flattened speech). For details of the stimulus material, see SI Text. This particular paradigm was chosen because it allowed us to investigate the newborns’ brain regions involved in the processing of segmental information (speech sounds) and of suprasegmental information (prosody) for which a comparable study in adults (21) had revealed frontotemporal networks with a left hemispheric dominance for segmental information and a right hemispheric dominance for prosody.
Results
fMRI Results.
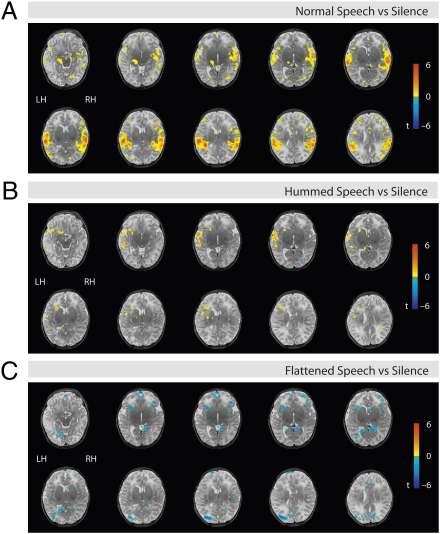
fMRI data from 2-d-old infants in the standard analysis [general linear model (GLM)] showed activation of brain regions known to be involved in speech processing in adults (16, 21). Data analysis revealed a main effect of normal speech vs. silence reflected in bilateral hemispheric activation clusters focused in the superior temporal gyrus (STG) and involving the primary and secondary auditory cortex (transverse temporal gyrus). In the left hemisphere (LH), activations located primarily in the superior temporal gyrus extended to the planum temporale, the inferior frontal gyrus, and the inferior parietal lobule. The hippocampus was activated as well. In the right hemisphere (RH), the activation cluster included the temporal pole, the planum polare, and the planum temporale in the temporal cortex, as well as the inferior frontal gyrus and the inferior parietal lobule (Fig. 1A).
Fig. 1.
Basic fMRI imaging results (n = 15, random effects group analysis, significance threshold P < 0.05 at the voxel level, uncorrected) overlaid on a T2-weighted image from a single newborn (note that the spatial resolution of the functional group data are lower compared with the anatomical image). Regions significantly more active, speech condition compared with silence, are shown in orange/yellow. Axial views, slices plotted from Left to Right present lower z to higher z coordinates. Brain activation of newborns for different speech conditions compared with silence. (A) Normal speech vs. silence. (B) Hummed speech vs. silence. (C) Flattened speech vs. silence.
To understand whether segmental and prosodic information involved different brain regions, we tested the two altered conditions, flattened speech and hummed speech, for specific activations. The comparison of hummed speech vs. silence revealed significant activations in the left inferior frontal cortex, the temporal pole, and the superior temporal gyrus (Fig. 1B). For flattened speech vs. silence, we found a profound reduction of the BOLD signal and significant deactivations located in the inferior frontal cortex, the hippocampus structures, and the posterior cingulate cortex, bilaterally, and in the left inferior parietal lobule (Fig. 1C).
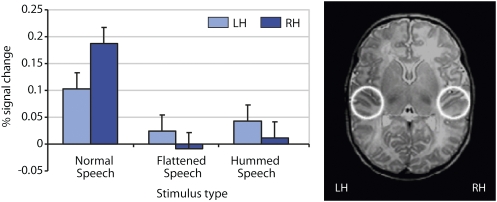
We were also interested in investigating whether any hemispheric predominance in primary and secondary auditory cortex would exist for language. To measure the level of activity in the right and left primary and secondary auditory cortex (RH and LH in Fig. 2), an ROI analysis was conducted on subjects' average percent BOLD signal change for normal speech, hummed speech, and flattened speech vs. silence (SI Text). We found a significant difference in the percentage of signal change between the stimulus types, with stronger activation for the normal speech in the right auditory cortex (Fig. 2 and SI Text).
Fig. 2.
ROI analysis, including primary and secondary auditory cortex, was conducted on subjects' average percent BOLD signal change for normal speech, hummed speech, and flattened speech vs. silence. Percent signal change in activation in the left hemisphere (LH) and right hemisphere (RH) are displayed for the three auditory conditions (normal speech, flattened speech, and hummed speech). The histograms show the percent BOLD signal change measured in each ROI during each of the three stimulus types.
Low-Frequency Fluctuations (LFF) Results.
To test whether the crucial difference between the newborn and adult network lies in the functional connectivity of the different brain regions involved, we conducted an LFF analysis of the data of the newborns that participated in the present fMRI study. We compared the newborn data to adult data from previous language studies (20). In adults, such analyses across different language studies revealed a default language network involving left inferior frontal and temporal regions, which are highly correlated intrahemispherically (20).
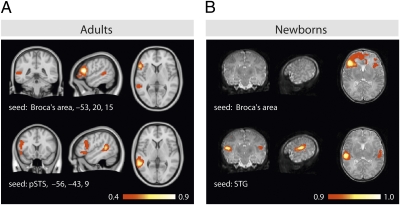
LFF analyses of brain activations observed in the present newborn study across the different conditions indicate weak intrahemispheric connectivities, but strong connectivities between the two hemispheres when seeded in the relevant inferior frontal and temporal brain regions, respectively (Fig. 3).
Fig. 3.
Functional connectivity results. Correlation value of low-pass–filtered residuals of language experiments in (A) adults and (B) newborns with seeds in Broca's area (Upper) and in the posterior superior temporal sulcus (pSTS) and superior temporal gyrus (STG). For adults, Talairach coordinates are given. As no such coordinates are available for newborns’ brains, the neuroanatomical location is given.
Diffusion-Weighted Imaging (DWI) Results.
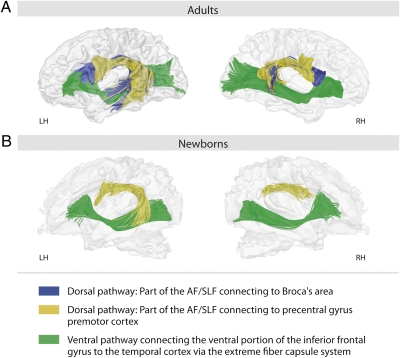
To investigate whether these functional connectivity findings in newborns can be backed up by structural connectivity data, we also conducted a DWI protocol with the same group of newborns. Data from this group were compared with a group of adults specified in Methods. Our analysis focused on the fiber tracts known to connect language-relevant brain areas in adults (for a recent review, see ref. 26). Several studies suggest ventral and dorsal pathways connecting these brain regions. For adults, a ventral pathway has been identified that connects the ventral inferior frontal gyrus with the anterior and middle portions of the superior temporal cortex via the extreme capsule (27–29). A dorsal pathway connecting the temporal and the prefrontal cortex, the arcuate fasciculus, merging into the superior longitudinal fasciculus, has repeatedly been described (17, 18, 27, 28). Interestingly, some of the studies emphasize the dorsal pathway to connect the temporal cortex to the premotor cortex (28), whereas others argue for a dorsal pathway that connects the temporal cortex to Broca's area (30). A third view provides evidence for two dorsal connections: a direct one connecting the temporal cortex and Broca's area and an indirect one connecting the temporal cortex via the parietal cortex to a more posterior region in the prefrontal cortex (possibly the premotor cortex) (31). The two dorsal pathways may serve different functions, with the latter supporting auditory-to-motor mappings crucial during early stages of language acquisition (18, 32), and the former supporting the processing of syntactically complex sentences relevant during later stages of language development (15, 26).
Based on our findings, we focused on three possible fiber tracts in the present study: a ventral one [the extreme capsule fiber system (ECFS)] and two dorsal ones [one connecting the temporal cortex with the premotor cortex and one connecting the temporal cortex with the inferior frontal gyrus (Broca's area)]. DTI analyses for the newborns participating in the present study demonstrated that the ventral fiber tract connecting the ventral portion of the inferior frontal gyrus via the ECFS to the temporal cortex was clearly present at birth, as was the dorsal fiber tract connecting the temporal cortex and the premotor cortex. However, in contrast to adults, the dorsal tract connecting the temporal cortex and Broca's area was not yet detectable in newborns (Fig. 4). Thus, the comparison between adults and 2-d-old infants suggests that there are two parallel dorsal pathways from the temporal cortex to the prefrontal cortex via the arcuate fasciculus/superior longitudinal fasciculus (AF/SLF) that mature with a different time course, one terminating in the premotor cortex, developing early as shown here in newborns, and one terminating in Broca's area, developing late. Interestingly, the fiber tracking results suggest that in infants the AF/SLF connecting to the premotor cortex differs between the hemispheres. In the RH it primarily connects to the parietal cortex, whereas in the LH it clearly goes to the temporal cortex.
Fig. 4.
Structural connectivity results. Fiber tracking of diffusion tensor imaging data for (A) adults and (B) newborns for speech-relevant regions with seed in Broca's area and seed in the precentral gyrus/premotor cortex. Two dorsal pathways are present in adults—one connecting the temporal cortex via the arcuate fasciculus (AF) and the superior longitudinal fasciculus (SLF) to the inferior frontal gyrus, i.e., Broca's area (blue), and one connecting the temporal cortex via the AF and SLF to the precentral gyrus, i.e., premotor cortex (yellow). In newborns, only the pathway to the precentral gyrus can be detected. The ventral pathway connecting the ventral inferior frontal gyrus via the extreme capsule to the temporal cortex (green) is present in adults and newborns.
Discussion
Our results demonstrate that brain regions that are known to be part of the auditory language network in adults (32, 33) and in infants (10, 11, 34)—in particular, the left and right temporal cortices and the left inferior frontal cortex—are also activated in newborns, as a function of speech input. The present fMRI data are the first to show in 2-d-old newborns that the brain regions triggered by speech are similar to those observed somewhat later in life in infants (10, 11, 34), children (35), and adults (12, 13, 21, 33). The relative involvement of the left and the right hemispheric regions appears to be less lateralized in newborns than in adults. At birth, we even found a preponderance in the right primary and secondary auditory cortex for speech input, as shown by the ROI analysis.
The present study reveals that these brain regions were strongly activated in normal speech and to a lesser extent also in hummed speech (carrying only prosodic information; see Fig. 1 A and B, SI Text, and Fig. S1B for direct comparisons), whereas they were not activated in flattened speech (Fig. 1C). The partial overlap in activations between normal speech and hummed speech suggest that newborns are primarily processing phonological information i.e., phonemic and prosodic information both available in normal speech and the latter available in hummed speech, rather than lexical or syntactic information available in normal speech and flattened speech. These fMRI data are, in principle, compatible with findings from studies indicating that newborns have the capacity to distinguish different phonemes (6, 7) and pitch contours (5). The strong overlap of activations for normal and hummed speech in newborns suggests a lack of a hemispheric specialization observed in children and adults, with a left hemispheric dominance for segmental information (phonemes, syllables, morphemes, and words) and a right hemispheric dominance for suprasegmental (prosodic) information (21, 23). In fact, the whole-brain fMRI analysis indicates that newborns process hummed speech and normal speech in a similar neural network, even if less extended for hummed speech. One possible explanation for the present data are that hummed speech is still processed as a human voice/articulatory product, but is perceived to be less natural, thereby leading to reduced activations in the left hemisphere.
In addition to the whole-brain analysis discussed so far, an ROI analysis focusing on the primary and secondary auditory cortices in the left and the right hemisphere was conducted (see ROI analysis and Fig. 2). This analysis revealed that specifically for the processing of normal speech, more activation was present in the right compared with the left auditory cortex. This is an interesting finding, because although a bilateral activation with an extended left and right hemispheric involvement was observed in the whole-brain analysis, the ROI analysis indicates that the right primary and secondary auditory cortex is recruited more strongly than the left auditory cortex. This finding suggests a higher reliance on prosodic than segmental information during speech processing in newborns. Such an interpretation would be compatible with optical imaging data reported for 3-mo-olds (24); moreover, it is reminiscent of a comparable right-predominant activation in primary and secondary auditory cortex when newborns listen to music (36). Together, our results suggest that very early in life, speech processing and music processing rely partially on the same neural substrates in the right auditory cortex.
However, the full brain activation pattern for speech in newborns (present study) shows similarities and differences from the pattern reported for newborns while hearing music (36). Crucially, in both studies, the activations at birth were not confined to primary and secondary auditory cortices but extended toward higher associative brain areas, for music being associated with a highly predominant overall right hemispheric activation (36), and for language showing an extended bilateral hemispheric involvement (present study).
A recent study with 2-mo-olds comparing speech and music processing directly (34), also reported differences in the neural networks processing speech and music, but with a left hemispheric dominance for speech and bilateral activation for music (34). It may well be that the more clearly expressed left hemispheric involvement in 2-mo-old infants compared with newborns is due to developmental age. An increase in language lateralization toward the left hemisphere as a function of age from childhood to adolescence has been also reported in earlier studies (35, 37–39).
Alterations of speech characteristics as in the present fMRI experiment for flattened speech did not lead to significant activations, suggesting that the newborn's brain is not sensitive to speech that lacks the fully fledged set of characteristics of normal spoken language, thereby rendering it biologically invalid. Pitch violations and sensory dissonance in music excerpts, as in Perani et al. (36), similarly led to a profound reduction of BOLD signal changes. This finding is noteworthy because it corresponds with the finding that a newborn's brain can adapt its neurophysiology to specific biological input, which makes learning from experiences possible (40). This adaptation is arguably crucial for language acquisition.
Both our functional and structural connectivity analyses of 2-d-old infants suggest a strong interhemispheric connectivity between the left and the right temporal region and between the left and the right frontal regions, respectively. This strong functional interhemispheric connectivity pattern in newborns contrasts with the strong intrahemispheric functional connectivities between left frontal and temporal regions observed in adults using an identical analysis (20). The present observation is in line with the results of recent studies on the resting functional architecture of the infant brain (41–43) compared with the adult brain (42). Using resting-state fMRI data, it has been shown that, in contrast to adults, the functional network in infants includes primarily local sensorimotor, auditory, and visual networks rather than distributed networks involving long-range connectivities (42). Here, we show that interhemispheric connections exist and function during language processing from birth, guaranteeing interplay between the two hemispheres’ neural competences. The intrahemispheric functional connections, in contrast, are not yet well developed. Thus, the interhemispheric interactions might be a primary and important facet of the developing brain, allowing the activity generated by both hemispheres to be coordinated and integrated, possibly leading to the adult pattern of hemispheric lateralization.
Our analyses of the structural connectivities with the same group of newborns focused on the fiber tracts that connect language-relevant brain areas identified in adults (11, 18, 26, 28)—namely, one ventral pathway connecting the ventral inferior frontal gyrus with the anterior and middle portions of the superior temporal cortex via the extreme capsule, and two parallel dorsal fiber tracts via the arcuate fasciculus and the superior longitudinal fasciculus (17, 18, 27, 28), one connecting the mid-to-posterior superior temporal cortex with the premotor cortex and one connecting the temporal cortex with Broca's area. It has been discussed that the latter two may serve different functions, with the former supporting auditory-to-motor mapping (18, 29, 31) and the latter supporting the processing of sentence syntax (15, 17). The present DTI analyses of newborns show that the ventral fiber tract is clearly present at birth, as is a dorsal fiber tract connecting the temporal cortex and the premotor cortex. However, in contrast to adults, a dorsal pathway connecting the temporal cortex and Broca's area is not yet detectable in newborns (Fig. 4). These findings are in line with the assumption that there are two dorsal pathways in the adult, with the dorsal pathway that connects the temporal cortex to the inferior frontal gyrus (Broca's area) maturing late. The present data are in agreement with DTI studies with infants aged 1–4 mo (14, 44), showing that the arcuate fasciculus develops late. These studies suggest that at the age of 2 d, the myelinized fibers of the arcuate fasciculus bundle do not extend to the inferior frontal gyrus, but only to the premotor cortex. This latter connection allows sensory-to-motor mapping, which is most relevant for early language development because it guarantees sensory-motor feedback during the infant's babbling phase during the first months of life.
The present multidimensional approach provides a unique view on the functional neuroanatomical prerequisites of human language faculty. Our results show that at birth, the infant is equipped with a brain in which regions in the frontal and temporal cortex are activated as a function of language input. The early propensities in the way the auditory nervous system processes sound information may antedate birth and are also influenced by external auditory input during the gestational period (45).
The temporal cortex processes speech, but only when the acoustic parameters are in a biological valid form—that is, when speech is normal but not when artificially flattened. The temporal cortices in the left and the right hemispheres perform this process in concert, as indicated by the functional connectivity data. The frontal cortices are also functionally interconnected, but it is not yet clear what they contribute to the process of speech perception. Structurally, the temporal cortex is connected to the premotor cortex, stronger in the left hemisphere than in the right hemisphere, providing a good basis for sensory-to-motor mappings as needed for later language acquisition (32). The lack of a structural connection between the temporal cortex and Broca's area, as well as the lack of functional connectivity between these regions in the left hemisphere in newborns compared with adults, suggests that language acquisition might depend to a large extent on the development of intrahemispheric functional connections between language-relevant brain regions, which cooccurs with the maturation of crucial connecting fiber tracts and exposure to language. Thus, even though the basic components of the neural language substrate, involving inferior frontal and temporal cortices interconnected across hemispheres, are present and active at birth, further development of functional connectivity and maturation of intrahemisperic fiber bundles are necessary to fully establish a highly specialized language system, crucially including lexical and syntactic competence once matured.
Beyond this fundamental finding, our results provide evidence useful for understanding developmental language disorders that might be caused by the disconnection of language-relevant brain regions and the consequent lack of proper functional connectivity during brain maturation.
Methods
Participants.
Newborns.
Fifteen healthy, full-term, nonsedated newborns (seven girls, eight boys; Apgar score ≥8) within the first 3 d of life participated in the study. Gestation and birth histories were normal for all subjects. Subjects’ immediate family members were predominantly right-handed (80% right-handed) (46), with no history of learning disabilities or psychiatric and neurological disorders, and of monolingual Italian background. Parents gave written consent in accordance with the procedures approved by the Ethical Committee of the San Raffaele Scientific Institute, and by the Ethics Review Board of the New and Emerging Science and Technology in the Sixth Framework Program (FP6) of the European Union.
Adult control group.
For details concerning the adult control group for LFF and DTI analysis, consult respective sections below.
Stimuli.
A fairy tale (Goldilocks, adapted for preschoolers) served as stimulus material in this study. A digital recording of a female native Italian speaker was made with a high-quality microphone (Neumann KMS104) in an anechoic chamber. The story was presented to the newborns in three different conditions: either as continuous speech, with expressive, child-directed intonation (set 1, normal speech), with the formants not audible (set 2, hummed speech), or with the variations in the fundamental frequency excluded (set 3, flattened speech). See SI Text for details.
fMRI Analysis.
See SI Text for fMRI procedure, fMRI preprocessing analysis, and fMRI group analysis.
A block design was used to maximize statistical power with 21-s blocks alternating between voice conditions and silence in a pseudorandom order, so that two versions of the same excerpt never followed each other, for a total scan time of 8 min and 3 s. Two identical 8-min and 3-s sequences were presented. Images were processed within the framework of the GLM in AFNI (http://afni.nimh.nih.gov/) (47).
LFF Analysis.
LFF analysis was done using the software package LIPSIA (48). See SI Text for details.
DTI Analysis.
Newborn group.
Diffusion MR images were acquired after the fMRI scans in the same imaging session using a DTI echo planar imaging (EPI) sequence with a voxel size of 1.406 × 1.406 × 2 mm3 covering the whole brain (40 axial slices).
Adult control group.
DTI data from nine healthy students were acquired on a whole-body 3T Magnetom Trio scanner (Siemens). See SI Text for complete details.
DTI Fiber Tracking.
Anatomical connectivity in brain white matter was investigated by fiber tracking to compute the connectivity between cortical brain areas from the diffusion tensor maps (27). Mean DTI data averaged for the group of newborns and adults were examined by whole-brain deterministic fiber tracking. Therefore, the preprocessed diffusion images for each group were aligned by nonlinear registration (49) implemented in LIPSIA (48) and combined to one dataset. A diffusion tensor was fitted to the combined data, resulting in one averaged diffusion tensor of each voxel in each group. In this way, the averaging was integrated implicitly into the tensor fitting procedure to avoid averaging of diffusion tensors. The fiber tracking algorithm used the entire diffusion tensor to deflect the estimated fiber trajectory as implemented in MedINRIA according to Fillard et al. (50). See SI Text for complete details.
Supplementary Material
Acknowledgments
We thank the parents who graciously agreed to have their infants participate in the study. This work was supported by a grant from the Fondazione Mariani (to D.P.) and by the European Union Project BrainTuning (FP6-2004 NEST-PATH-028570).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102991108/-/DCSupplemental.
References
- 1.Darwin CR. The Descent of Man, and Selection in Relation to Sex. London: John Murray; 1871. [Google Scholar]
- 2.Bishop DV. Genes, cognition, and communication: Insights from neurodevelopmental disorders. Ann N Y Acad Sci. 2009;1156:1–18. doi: 10.1111/j.1749-6632.2009.04419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 4.Hécaen H. Acquired aphasia in children and the ontogenesis of hemispheric functional specialization. Brain Lang. 1976;3:114–134. doi: 10.1016/0093-934x(76)90009-2. [DOI] [PubMed] [Google Scholar]
- 5.Mehler J, et al. A precursor of language acquisition in young infants. Cognition. 1988;29:143–178. doi: 10.1016/0010-0277(88)90035-2. [DOI] [PubMed] [Google Scholar]
- 6.Cheour-Luhtanen M, et al. Mismatch negativity indicates vowel discrimination in newborns. Hear Res. 1995;82:53–58. doi: 10.1016/0378-5955(94)00164-l. [DOI] [PubMed] [Google Scholar]
- 7.Dehaene-Lambertz G, Pena M. Electrophysiological evidence for automatic phonetic processing in neonates. Neuroreport. 2001;12:3155–3158. doi: 10.1097/00001756-200110080-00034. [DOI] [PubMed] [Google Scholar]
- 8.Peña M, et al. Sounds and silence: An optical topography study of language recognition at birth. Proc Natl Acad Sci USA. 2003;100:11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gervain J, Nespor M, Mazuka R, Horie R, Mehler J. Bootstrapping word order in prelexical infants: A Japanese-Italian cross-linguistic study. Cognit Psychol. 2008;57:56–74. doi: 10.1016/j.cogpsych.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 11.Dehaene-Lambertz G, et al. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc Natl Acad Sci USA. 2006;103:14240–14245. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perani D, et al. Brain processing of native and foreign languages. Neuroreport. 1996;7:2439–2444. doi: 10.1097/00001756-199611040-00007. [DOI] [PubMed] [Google Scholar]
- 13.Binder JR, et al. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- 14.Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: A feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage. 2006;30:1121–1132. doi: 10.1016/j.neuroimage.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Brauer J, Anwander A, Friederici AD. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb Cortex. 2011;21:459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- 16.Friederici AD, Alter K. Lateralization of auditory language functions: A dynamic dual pathway model. Brain Lang. 2004;89:267–276. doi: 10.1016/S0093-934X(03)00351-1. [DOI] [PubMed] [Google Scholar]
- 17.Friederici AD, Bahlmann J, Heim S, Schubotz RI, Anwander A. The brain differentiates human and non-human grammars: functional localization and structural connectivity. Proc Natl Acad Sci USA. 2006;103:2458–2463. doi: 10.1073/pnas.0509389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saur D, et al. Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. Neuroimage. 2010;49:3187–3197. doi: 10.1016/j.neuroimage.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Tyler LK, Marslen-Wilson W. Fronto-temporal brain systems supporting spoken language comprehension. Philos Trans R Soc Lond B Biol Sci. 2008;363:1037–1054. doi: 10.1098/rstb.2007.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohmann G, et al. Setting the frame: The human brain activates a basic low-frequency network for language processing. Cereb Cortex. 2010;20:1286–1292. doi: 10.1093/cercor/bhp190. [DOI] [PubMed] [Google Scholar]
- 21.Meyer M, Alter K, Friederici AD, Lohmann G, von Cramon DY. fMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Hum Brain Mapp. 2002;17:73–88. doi: 10.1002/hbm.10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandour J, et al. Hemispheric roles in the perception of speech prosody. Neuroimage. 2004;23:344–357. doi: 10.1016/j.neuroimage.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Wartenburger I, et al. The processing of prosody: Evidence of interhemispheric specialization at the age of four. Neuroimage. 2007;34:416–425. doi: 10.1016/j.neuroimage.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Homae F, Watanabe H, Nakano T, Asakawa K, Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neurosci Res. 2006;54:276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Homae F, Watanabe H, Nakano T, Taga G. Prosodic processing in the developing brain. Neurosci Res. 2007;59:29–39. doi: 10.1016/j.neures.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Friederici AD. Pathways to language: Fiber tracts in the human brain. Trends Cogn Sci. 2009;13:175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knösche TR. Connectivity-based parcellation of Broca's area. Cereb Cortex. 2007;17:816–825. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- 28.Saur D, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly C, et al. Broca's region: Linking human brain functional connectivity data and non-human primate tracing anatomy studies. Eur J Neurosci. 2010;32:383–398. doi: 10.1111/j.1460-9568.2010.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- 32.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;5:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 33.Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- 34.Dehaene-Lambertz G, et al. Language or music, mother or Mozart? Structural and environmental influences on infants’ language networks. Brain Lang. 2010;114:53–65. doi: 10.1016/j.bandl.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Brauer J, Friederici AD. Functional neural networks of semantic and syntactic processes in the developing brain. J Cogn Neurosci. 2007;19:1609–1623. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- 36.Perani D, et al. Functional specializations for music processing in the human newborn brain. Proc Natl Acad Sci USA. 2010;107:4758–4763. doi: 10.1073/pnas.0909074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holland SK, et al. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- 38.Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brauer J, Neumann J, Friederici AD. Temporal dynamics of perisylvian activation during language processing in children and adults. Neuroimage. 2008;41:1484–1492. doi: 10.1016/j.neuroimage.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fifer WP, et al. Newborn infants learn during sleep. Proc Natl Acad Sci USA. 2010;107:10320–10323. doi: 10.1073/pnas.1005061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fransson P, Åden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 2010;21:145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- 42.Fair DA, et al. Functional brain networks develop from a “local to distributed” organization. PLOS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Homae F, et al. Development of global cortical networks in early infancy. J Neurosci. 2010;30:4877–4882. doi: 10.1523/JNEUROSCI.5618-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubois J, et al. Asynchrony of the early maturation of white matter bundles in healthy infants: Quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum Brain Mapp. 2008;29:14–27. doi: 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Draganova R, Eswaran H, Murphy P, Lowery C, Preissl H. Serial magnetoencephalographic study of fetal and newborn auditory discriminative evoked responses. Early Hum Dev. 2007;83:199–207. doi: 10.1016/j.earlhumdev.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 46.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 47.Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 48.Lohmann G, et al. LIPSIA—a new software system for the evaluation of functional magnetic resonance images of the human brain. Comput Med Imaging Graph. 2001;25:449–457. doi: 10.1016/s0895-6111(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 49.Thirion J-P. Image matching as a diffusion process: An analogy with Maxwell's demons. Med Image Anal. 1998;2:243–260. doi: 10.1016/s1361-8415(98)80022-4. [DOI] [PubMed] [Google Scholar]
- 50.Fillard P, Pennec X, Arsigny V, Ayache N. Clinical DT-MRI estimation, smoothing, and fiber tracking with log-Euclidean metrics. IEEE Trans Med Imaging. 2007;26:1472–1482. doi: 10.1109/TMI.2007.899173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.