Abstract
Delivery of macromolecules into cells and tissues such as skin is a major challenge. This obstacle poses a particular challenge for the delivery of siRNA where cellular and tissue level transport barriers need to be overcome. siRNAs are potential therapeutics for various dermatological diseases including psoriasis, atopic dermatitis, and cancer; however, their utility is limited by their low absorption across the stratum corneum (SC) and into viable cells of skin. Here, we address this challenge using a peptide identified by phage display termed skin penetrating and cell entering (SPACE) peptide. In vitro studies indicated that the SPACE peptide, when conjugated to cargoes such as small molecules and proteins, was able to facilitate their penetration across the SC into epidermis and dermis. The peptide also exhibited increased penetration into various cells including keratinocytes, fibroblasts, and endothelial cells, likely through a macropinocytosis pathway. The ability of SPACE peptide to deliver siRNA was tested in vivo using two targets, interleukin-10 and GAPDH. Conjugation of the peptide to siRNA led to their enhanced absorption into skin and knockdown of corresponding protein targets.
Keywords: cell-penetrating peptide, dermatology, drug delivery, topical, transdermal
Skin, the largest organ of the human body, is a host to numerous dermatological diseases, which collectively represent a large category of human health conditions (1–3). Accordingly, successful delivery of therapeutics, specifically macromolecules such as siRNA, into skin has become a topic of active research and development (4–6). The goal of topical siRNA delivery, however, is extremely challenging and with some exceptions has been very difficult to accomplish (7, 8). The primary challenge is poor skin penetration of macromolecules (5). Among various physico-chemical methods proposed to enhance penetration of macromolecules (9–12), peptide carriers have emerged as a potential candidate owing to their simplicity of use, diversity, and potential ability to target cellular subtypes within the skin. Several peptides including TAT, polyarginine, meganin, and penetratin, which were initially identified for delivering drugs into the cytoplasm of cells, have been tested for penetration across the stratum corneum (SC) and a few have shown some efficacy in delivering small molecules into epidermis (13–16). In contrast, only one peptide, TD-1, has been specifically discovered to penetrate the SC and possessed the ability to enhance systemic uptake of topically applied drugs (17). Although several peptides are known to penetrate cellular membranes and a few to permeabilize the SC, peptides that simultaneously enhance the penetration of macromolecules across the SC and permeabilize the membrane of viable epidermal and dermal cells have not been reported. Here, we report on the identification and utility of one such peptide.
Results
Peptides that penetrate the SC were identified using in vitro phage display (Fig. 1A). Five rounds of selection led to narrowing down the display library (Fig. 1B). One sequence, AC-TGSTQHQ-CG, appeared in high frequency in higher rounds and was labeled a skin permeating and cell entering (SPACE) peptide. A second sequence (AC-HSALTKH-CG) also appeared in high frequency. In a separate experiment, phage screening was performed to isolate phage that localized in the dermis (SI Materials and Methods). After five rounds of screening, the sequence AC-KTGSHNQ-CG was found to localize specifically in the dermis. Owing to the similarity of this sequence with the SPACE peptide sequence, the SPACE peptide was selected for further studies. Fluorescently labeled phage clones displaying SPACE peptide exhibited small but detectable penetration into skin (Fig. S1A). In contrast phage clones exhibiting a scrambled peptide sequence (AC-THGQTQS-CG) exhibited only superficial penetration (Fig. S1B).
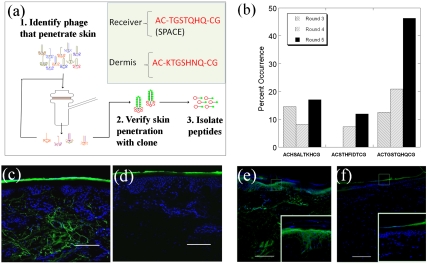
Fig. 1.
The identification of skin penetrating peptides through in vitro phage display in porcine skin. (A) Phage library was applied in the donor compartment of an FDC. Phage found to penetrate through skin into the receiver compartment were collected, amplified, and used for the subsequent rounds of screening. The skin penetrating ability of individual clones was confirmed through diffusion experiments and confocal microscopy. To confirm the peptide’s ability to penetrate skin, the peptide was isolated from the phage and its penetration into skin was confirmed visually through confocal microscopy. (B) Percentage of occurrence for each high frequency peptide sequence from rounds 3 through 5 of the phage display screen. (C and D) Confocal microscopy images of the skin penetration profiles of SPACE and control peptide into porcine skin, respectively. (E and F) Skin penetration profiles of Alexa Fluor 488 labeled streptavidin conjugated to biotinylated SPACE peptide and Alexa Fluor 488 streptavidin alone, respectively. Insets show zoomed in views of the sections highlighted in the main images. Scale bar: 200 µm.
The SPACE peptide, when removed from the phage, also penetrated into the skin (Fig. 1C). Consistent with the observations made with the entire phage, SPACE peptide was found to localize strongly in the dermis. No significant penetration of the control peptide was observed (Fig. 1D). SPACE peptide was also able to carry macromolecular cargoes across porcine SC. For example, streptavidin, when conjugated to biotinylated SPACE peptide, permeated well beyond the SC and some localization of streptavidin was found in the epidermis and dermis (Fig. 1E). Streptavidin not conjugated to SPACE peptide exhibited minimal penetration into epidermis (Fig. 1F). SPACE peptide-mediated transport appears to be dependent on the cargo size. Specifically, SPACE peptide, when conjugated to streptavidin-coated quantum dots, led to detectable but much smaller transport (Fig. S1C). No significant penetration of quantum dots conjugated to the control peptide was observed (Fig. S1D).
SPACE peptide also permeated across human skin and exhibited penetration comparable to that found in porcine skin (Fig. S1 E and F). When observed from the top, high localization of the SPACE peptide within the corneocytes was found whereas no significant penetration of the control peptide was observed (Fig. S1 G and H). Experiments with isolated human SC revealed that the SPACE peptide binds to corneocyte proteins, most likely to keratin (Fig. S2 A and B). FTIR spectroscopy studies also confirmed the effect of SPACE peptide on keratin. Specifically, SC exposed to SPACE peptide exhibited changes in the FTIR spectrum, indicative of structural changes in keratin (Fig. S2C). The control peptide had no significant effect on protein structure in FTIR compared to that seen in the absence of any peptide (Fig. S2D and Fig. S3). FTIR also showed that the SPACE peptide had no detectable effect on SC lipids (Fig. S2 E and F). Neither a change in the area of the symmetric CH2 stretching peak nor a shift in center frequency was found indicating that the SPACE peptide did not induce extraction or fluidization of SC lipids (18). Consistent with the FTIR data, exposure to SPACE peptide did not induce a significant change in skin’s electrical conductivity (Fig. S4). Specifically, the electrical conductivity of skin increased by about 1.7(+/-0.6)-fold after 24 h incubation with the SPACE peptide. This enhancement, though higher than that observed for the control peptide, was relatively modest. Similarly, coincubation of SPACE peptide with inulin, a large hydrophilic molecule led to only a modest increase in its permeability (Fig. S4), indicating that the SPACE peptide is primarily effective in enhancing permeation of conjugated but not coadministered cargoes.
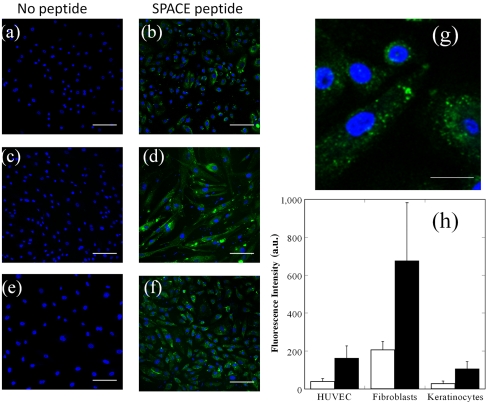
Having confirmed the ability of the SPACE peptide to penetrate the SC, we next investigated its ability to penetrate into viable cells including keratinocytes, fibroblasts, and endothelial cells (HUVECs) in cell cultures and found significant penetration into all cell lines (Fig. 2 A–F and G shows a magnified view of SPACE peptide internalization in keratinocytes). In all cases, the extent of internalization of SPACE peptide was higher than that of control peptide indicating that cellular penetration occurred in a sequence-specific manner (Fig. 2H). SPACE peptide also exhibited internalization in breast cancer cells (MDA-MB-231, Fig. S5 A–C). The ability to penetrate all tested types of cells suggest that the mode of entry into cells for SPACE peptide is through a pathway that is common to all studied cell lines and not because of a particular membrane protein unique to keratinocytes.
Fig. 2.
Cellular penetration of SPACE peptide into various cell lines. (A, C, and E) Confocal images of cells treated with no peptide. (B, D, and F) Cells incubated with fluorescently labeled SPACE peptide for 24 h. (A and B) Human keratinocytes, (C and D) human fibroblasts, and (E and F) HUVEC. (G) Magnified image of SPACE peptide internalization in human keratinocytes. (H) Average fluorescence intensity of control peptide (open bars) and SPACE peptide (closed bars) internalization after 24 h in HUVEC, fibroblasts, and keratinocytes. Error bars indicate SD (N≥30). Scale bar: 100 µm (A–F) and 20 μm (G).
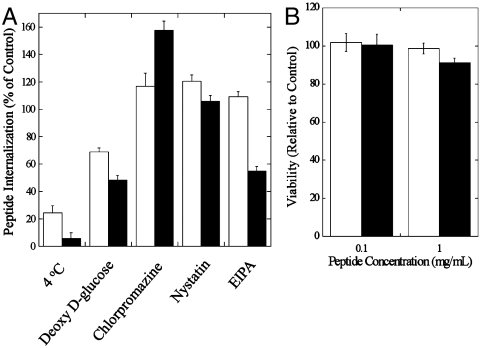
To determine the potential mechanism of cellular penetration for SPACE peptide, the effect of several endocytosis inhibitors including incubation at 4 °C on internalization was tested in human keratinocytes (Fig. 3A). Incubation at 4 °C significantly reduced internalization of SPACE peptide (about 5% uptake compared to that at 37 °C) as well as the control peptide indicating that both enter cells through an active mechanism. The potential mechanism for cellular penetration was further confirmed by the use of deoxy-d-glucose, which also resulted in the reduction of internalization of both peptides (∼52%). To further assess the nature of the active uptake, cells were incubated with the clathrin-mediated endocytosis inhibitor chlorpromazine and the caveolae-mediated endocytosis inhibitor nystatin. Neither of them reduced the cellular internalization of SPACE peptide or the control peptide. Finally, we tested the effect of a macropinocytosis inhibitor 5-(N-ethyl-N-isopropyl) amiloride (EIPA). Exposure of cells to EIPA resulted in approximately 50% reduction in SPACE internalization. In contrast, EIPA had no effect on control peptide internalization. Collectively, these results suggest that macropinocytosis plays a major role in the internalization of SPACE peptide, a conclusion that is shared by other cell-penetrating peptides in the literature (19, 20). Studies have reported that cargoes that are internalized by macropinocytosis are often not colocalized with endo/lysosomes implying that their entry into degrading lysosomal compartment can be potentially avoided (21, 22). MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, assays on keratinocyte cultures revealed that neither SPACE peptide nor control peptide exhibited significant toxicity to cells at the concentration range studied here (0.1–1.0 mg/mL, Fig. 3B).
Fig. 3.
Cellular mechanism and toxicity studies. (A) Peptide internalization (percent of control) for control peptide and SPACE peptide at 4 °C and with the endocytosis inhibitors deoxy-d-glucose, chlorpromazine, nystatin, and EIPA in human keratinocytes. (B) Cell proliferation of human keratinocytes in the presence of control peptide (open bars) or SPACE peptide (closed bars) at 0.1 mg/mL and 1.0 mg/mL. Error bars indicate SD (N = 4).
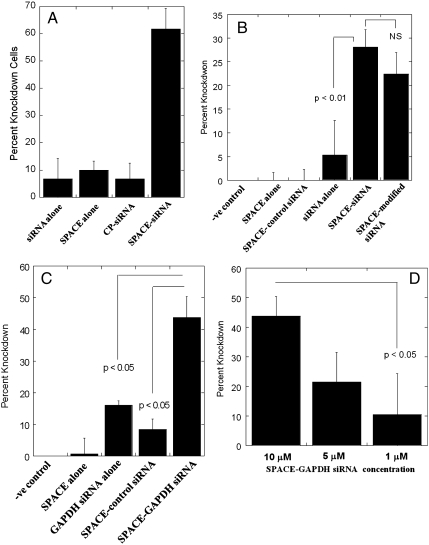
The ability of SPACE peptide to penetrate into a variety of cells makes it an excellent candidate for siRNA delivery. This possibility was explored using GFP-expressing endothelial cells as a model cell line in vitro. SPACE peptide-conjugated siRNA induced significant knockdown of GFP (Fig. 4A). In contrast, no significant knockdown was observed with siRNA alone, SPACE alone, SPACE conjugated to a control siRNA, or control peptide conjugated to siRNA. To determine whether siRNA conjugation to SPACE peptide had an adverse effect on the potency of siRNA, both unconjugated siRNA and SPACE-siRNA were complexed with Lipofectmine™ and knockdown was assessed. In both cases, knockdown was significant compared to control, that is, no siRNA treatment (Fig. S5 D–F).
Fig. 4.
Delivery of siRNA. (A) Percentage knockdown of GPF in GFP-expressing endothelial cells. Error bars indicate SD (N≥30). (B) Percentage knockdown of IL-10 protein levels in mice 24 h after treatment. Error bars indicate SE (N≥3); NS, not significantly different (p > 0.15). (C) Percentage knockdown of GAPDH protein levels in mice 72 h after treatment. Error bars indicate SE (N≥3). (D) Dose dependence of GAPDH knockdown upon topical siRNA application. Error bars indicate SE (N≥3).
SPACE peptide also penetrated into mouse skin in vivo at levels significantly higher than control peptide (Fig. S6 A–F, Fig. S7 A–F). Application of the SPACE peptide on mouse skin for 30 min resulted in penetration, and application for 2 h resulted in significant penetration into the skin and localization in the deep dermis, consistent with that seen in porcine and human skin.
The ability of SPACE peptide to enhance dermal penetration of IL-10 siRNA was next assessed. This siRNA was selected because of its potential for treating atopic dermatitis, a major dermatological disease. Because of the insignificant knockdown seen with control peptide and the lack of skin penetration in vivo when compared to SPACE peptide, the control peptide was not assessed in the in vivo siRNA studies. Application of IL-10 siRNA alone without the peptide produced minimal effect on IL-10 levels compared to mice that received no treatment, SPACE peptide alone, or SPACE conjugated to luciferase siRNA (control siRNA). In contrast, animals treated with SPACE conjugated to IL-10 siRNA and SPACE conjugated to 2-O-methyl modified IL-10 siRNA showed significant reduction in IL-10 levels (Fig. 4B).
As another example, SPACE peptide was conjugated to GAPDH siRNA and its effect on skin GAPDH levels was assessed. This target was chosen because GAPDH is a common housekeeping protein and provides an example of a common siRNA target. Animals treated with SPACE-GAPDH siRNA conjugate induced significant reduction in protein levels compared to controls (no treatment, siRNA alone, SPACE peptide alone, and SPACE conjugated to control siRNA, Fig. 4C). Knockdown of GAPDH in skin was dose dependent; 43% knockdown was observed at 10 μM, 21% knockdown at 5 μM, and 10% knockdown at 1 μM (Fig. 4D). Knockdown was also dependent on application time with longer application times resulting in higher knockdown (Fig. S8).
Discussion
Penetration of phage across the SC is quite unexpected given its size (23–25). Large solutes (typically molecular mass > 500 Da) exhibit poor skin penetration and measurement of their transdermal permeation is often limited by the sensitivity of their detection. This limitation can be addressed by using phage. The M13 phage used in this study is a long filamentous particle, approximately 8 nm in width and 900 nm in length. High donor concentration (∼2 × 1011 pfu/mL), low detection limit (∼1 pfu), and the potential for amplification facilitated assessment of dermal penetration of phage. The measured permeability of phage across porcine skin was very low; although the permeability of phage displaying SPACE peptide was higher than that of phage without the peptide library (see SI Materials and Methods for details). Permeation of phage, though much smaller than that of low molecular weight solutes, was significant and unexpected. Most importantly, penetration of phage was sequence-specific (see SI Materials and Methods for details).
Diffusion through intercellular lipids represents the classical mechanism for transdermal permeation of molecules. This mechanism, however, is generally limited to small, lipophilic molecules. Permeation of large, hydrophilic molecules is relatively less studied. Transdermal transport of such solutes is attributed to two pathways; (i) polar or porous pathways and (ii) appendages (follicles). Mathematical models have been described in the literature to describe contributions of both pathways to transdermal permeation (26, 27). Applications of these models to phage transport and their comparison with experimental observations is presented in SI Materials and Methods.
Deep penetration and retention of SPACE peptide in skin is very peculiar. A BLAST search revealed some matches to the SPACE peptide sequence including a complete match of the seven amino acid sequence to a protein in Leishmania major and a partial match (GSTQHQ) to a surface protein of skin-resident Staphylococcus epidermidis. The latter is of particular interest because the surface proteins dictate binding to extracellular matrix (ECM) components (28) and proteins that interact with ECM components may also interact with intermediate filaments such as keratin, a major component of skin (29). A few previous studies have used phage display to identify peptides that penetrate biological barriers including skin, oral mucosa, and lung epithelium. Chen et al. identified a peptide, TD-1 (AC-SSSPSKH-CG), based on in vivo phage display on mouse skin (17). In another study, Kang et al. identified a sequence C-SKSSDYQ-C that enhances oral absorption after oral delivery of the phage library (30). More recently, Morris et al. studied translocation of phage library in alveolar epithelial cultures and identified a sequence C-TSGTHPR-C that enables translocation (31). The sequence of SPACE peptide shares some resemblance with the intestine-permeating peptide (note that T and S are similar). The SPACE peptide also shares some resemblance with the airway epithelium-penetrating peptide, especially in terms of the TGST motif.
The results presented here demonstrate the ability of the SPACE peptide to deliver siRNA across the SC and into skin cells at levels required to produce a therapeutic effect. Although several important siRNA therapeutic targets in skin have been identified, the delivery of siRNA into skin has proved challenging. This limitation can be potentially addressed through the studies reported here. Increased purity and optimization of the SPACE peptide-siRNA complex along with further studies focused on the assessment of therapeutic efficacy, cost-effectiveness and safety may open up unique opportunities in topical siRNA therapies. Compared to existing physical and chemical enhancers, peptides offer unique opportunities because of their diversity and ability to perform multiple tasks including targeting skin layers, conjugation to protein drugs, and ability to deliver drugs into cells.
Microscopy studies indicated that the peptide exhibited strong association with corneocytes whereas no significant extraction and/or disruption of the lipids was observed based on FTIR studies. Conjugation of SPACE peptide with the cargo was essential to observe enhancement of skin penetration. Collectively, these studies suggest that the primary effect of SPACE peptide is to enhance partitioning into the SC, primarily corneocytes, which subsequently enhances the ability of SPACE and solutes conjugated to SPACE to cross the skin barrier. An additional effect of the peptide on permeation may also be potentially expected because of its ability to impact keratin structure.
The ability of the SPACE peptide to deliver siRNA across the SC and viable cell membranes opens up the possibility for the treatment of various skin diseases. Atopic dermatitis, a skin disease characterized by a loss in skin barrier functioning and chronic inflammation, affects approximately 20% of the general population (32, 33). An imbalance of TH2 versus TH1 cytokines in addition to reduction in filaggrin expression have been shown to be major factors in the development of atopic dermatitis (34). In particular, studies have shown that an increase in the production of IL-10, a TH2 cytokine, is thought to play a role in the development of atopic dermatitis through the inhibition of IFN-γ and to hinder immunity to pathogens resulting in an increased susceptibility to infections (32). The ability of the SPACE peptide to deliver IL-10 siRNA and reduce the overall level of the cytokine may offer unique therapeutic opportunities in the treatment of atopic dermatitis and other dermatological diseases.
Materials and Methods
Phage Display.
To screen for skin penetrating peptides in vitro, the Ph.D.-C7C Phage Display Peptide Library (New England Biolabs) was used. Screening studies were performed on porcine skin in Franz diffusion cells (FDCs, PermeGear). The phage display library was placed in the donor compartment of the FDC. After 24 h, phage which had penetrated through the skin were collected from the receiver compartment, amplified, and again placed in the donor compartment for an additional round of screening to further narrow down the library (see SI Materials and Methods for more details).
Phage Cloning.
To verify the ability of the phage to penetrate the skin, the Ph.D. Peptide Display Cloning System (M13KE vector, New England Biolabs) was used to create phage which displayed specific peptide sequences of interest. See SI Materials and Methods for detailed protocol of cloning procedure.
Peptide Synthesis.
The peptide sequence for the SPACE peptide was ACTGSTQHQCG and the peptide sequence for the control peptide (CP) was ACTHGQTQSCG with the formation of the disulfide bond between the cysteines to produce a cyclic peptide. Fluorescein isothiocyanate (FITC) or 5-carboxyfluorescein (5-FAM) conjugated peptides were synthesized by ChinaTech Peptide Co. and RS Synthesis. The dye was placed on the N terminus of the peptide. Biotinylated versions of both peptides were synthesized by ChinaTech Peptide Co. and the peptides with no modifications were synthesized by RS Synthesis.
Peptide and Macromolecule Penetration in Skin.
Full thickness porcine skin was obtained from the lateral abdominal region of Yorkshire pigs. Full thickness human skin was obtained from the National Disease Research Interchange. The skin was stored at -80°C and defrosted immediately prior to use. The conductivity of the skin was measured to ensure the integrity of the skin barrier. Skin samples with a resistivity above 50 kΩ were used for experiments. For fluorescently labeled peptides, 200 μL of a 1 mg/mL solution was placed in the donor compartment of the FDC. In the case of studying macromolecule delivery into skin, the peptide was first conjugated to the macromolecule (see SI Materials and Methods) and then the peptide-macromolecule complex was placed into the donor compartment of the FDC. After 24 h the remaining solution in the donor compartment was removed and the FDC was dismantled. The skin sample was retrieved and rinsed with deionized water to remove excess peptide or peptide complex on the surface of the skin. Skin samples were then prepared for imaging with confocal microscopy (see details in SI Materials and Methods).
Cell Penetration Studies.
For cell penetration studies, 1.2 × 104 cells were seeded on poly-d-lysine-coated glass bottom culture dishes (MatTek). For human umbilical vein endothelial cells (HUVEC) cells, the culture dishes were coated with 1% gelatin prior to seeding with cells. After incubation at 37 °C for 4 h, the media was removed and 20 μL of a 1 mg/mL fluorescent peptide solution was added to 180 μL of media and subsequently added to the cell culture dish. For the control, an equivalent amount of PBS was added in place of a peptide solution. After addition of the peptide, cell cultures were incubated at the appropriate condition for studying cellular penetration (4 °C or 37 °C) and incubated for either 6 or 24 h. Cells were prepared for imaging with confocal microscopy (see SI Materials and Methods for details).
Cell proliferation was measured using the Vybrant MTT Cell Proliferation Assay Kit (Invitrogen). Cells were incubated with various peptide solutions and cell proliferation was measured after 24 h.
Cell Penetration Mechanism Studies.
For cell mechanism studies, cells were incubated with various endocytosis inhibitors or at 4 °C for 1 h prior to the addition of fluorescently labeled peptides (see SI Materials and Methods). Cells were incubated with fluorescently labeled peptide for 3 h and then harvested for analysis using flow cytometry.
siRNA Delivery in Vitro.
GFP-expressing endothelial cells (American Type Culture Collection) were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. GFP siRNA, 5′-GAC GUA AAC GGC CAC AAG UUC N6-3′ (Dharmacon), was conjugated to fluorescently labeled peptide (containing a free carboxyl group) through N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) chemistry (see SI Materials and Methods for details).
The peptide-siRNA complex was added to the appropriate cell culture media to obtain a final concentration of 1 μM siRNA. The media along with peptide-siRNA was then added to the cells and allowed to incubate for 48 h. Cells were imaged using confocal microscopy and image analysis was performed using ImageJ to determine the overall fluorescence intensity for each cell. Knockdown was determined as the percent of cells in the test case that possess intensity at least 30% lower than the mean intensity observed for the population in the control case (no treatment).
siRNA Delivery in Vivo.
The siRNA sequences used in the in vivo studies are the following: IL-10, 5′-GAA UGA AUU UGA CAU CUU CUU N6-3′; GAPDH, 5′-GUG UGA ACC ACG AGA AAU AUU N6-3′; and luciferase (control), 5′-UAA GGC UAU GAA GAG AUA CUU N6-3′. The 2-O-methyl modification was placed on all bases for IL-10. All siRNAs were purchased from Dharmacon.
siRNA delivery was performed in female Balb/C mice (Charles River Laboratories) between six and eight-wk old according to protocols approved by the Institutional Animal Care and Use Committee. Mice were placed under anesthesia (1–2% isofluorane) and the hair on their back was lightly shaved. Two hundred microliters of a 10 μM of peptide-siRNA solution or corresponding controls were topically applied over a 3 cm2 area on the back of the animal. The solution was then covered with sterile gauze and a breathable bandage. After 24 h for IL-10 experiments and 72 h for GAPDH experiments, the mice were euthanized using CO2 and skin samples were immediately taken using a 4 mm biopsy punch. Two 4 mm biopsies were randomly taken from the treatment area and immediately frozen in liquid nitrogen. The skin was then placed in a surfactant combination of 0.5% (wt/vol) 3-(Decyl dimethyl ammonio) propane sulfonate (DPS) and Brij 30 and homogenized (IKA disperser) on ice for 1 min to extract the proteins from the skin samples. The homogenate was then centrifuged at 10,000 rpm for 5 min and the supernatant was collected. The total protein concentration was determined using the Micro Bicinchoninic Acid Protein Assay Kit (Pierce), IL-10 levels were determined using a mouse IL-10 ELISA (Raybiotech), and GAPDH levels were measured using the Kdalert™ GAPDH Assay kit (Ambion).
Supplementary Material
Acknowledgments.
Authors acknowledge Fred Tan and Jordanne Gregorio for technical assistance. This work was supported by the National Center for Research Resources shared instrumentation Grant 1S10RR017753-01.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016152108/-/DCSupplemental.
References
- 1.Nichols K, Cook-Bolden F. Allergic skin disease: Major highlights and recent advances. Med Clin North Am. 2009;93:1211–1224. doi: 10.1016/j.mcna.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Koh H, Geller A. Melanoma control in the United States: Current status. Recent Results Cancer Res. 1995;139:215–224. doi: 10.1007/978-3-642-78771-3_16. [DOI] [PubMed] [Google Scholar]
- 3.Fleischer AJ, Feldman S, Rapp S. Introduction. The magnitude of skin disease in the United States. Dermatol Clin. 2000;18:15–21. doi: 10.1016/s0733-8635(05)70163-7. [DOI] [PubMed] [Google Scholar]
- 4.Geusens B, Sanders N, Prow T, Van Gele M, Lambert J. Cutaneous short-interfering RNA therapy. Expert Opin Drug Delivery. 2009;6:1333–1349. doi: 10.1517/17425240903304032. [DOI] [PubMed] [Google Scholar]
- 5.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guy R. Handb Exp Pharmacol. 2nd Ed. New York: Marcel Dekker, Inc.; 2010. Transdermal drug delivery; pp. 399–410. [DOI] [PubMed] [Google Scholar]
- 7.Kigasawa K, et al. Noninvasive delivery of siRNA into the epidermis by iontophoresis using an atopic dermatitis-like model rat. Int J Pharm. 2010;383:157–160. doi: 10.1016/j.ijpharm.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Ritprajak P, Hashiguchi M, Azuma M. Topical application of cream-emulsified CD86 siRNA ameliorates allergic skin disease by targeting cutaneous dendritic cells. Mol Ther. 2008;16:1323–1330. doi: 10.1038/mt.2008.91. [DOI] [PubMed] [Google Scholar]
- 9.Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269:850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 10.Guy RH. Iontophoresis—recent developments. J Pharm Pharmacol. 1998;50:371–374. doi: 10.1111/j.2042-7158.1998.tb06875.x. [DOI] [PubMed] [Google Scholar]
- 11.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Delivery Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Karande P, Jain A, Mitragotri S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat Biotechnol. 2004;22:192–197. doi: 10.1038/nbt928. [DOI] [PubMed] [Google Scholar]
- 13.Rothbard J, et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6:1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 14.Lopes L, et al. Comparative study of the skin penetration of protein transduction domains and a conjugated peptide. Pharm Res. 2005;22:750–757. doi: 10.1007/s11095-005-2591-x. [DOI] [PubMed] [Google Scholar]
- 15.Sawant R, Torchilin V. Intracellular transduction using cell-penetrating peptides. Mol BioSyst. 2010;6:628–640. doi: 10.1039/b916297f. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, Ludovice P, Prausnitz M. Transdermal delivery enhanced by magainin pore-forming peptide. J Controlled Release. 2007;122:375–383. doi: 10.1016/j.jconrel.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, et al. Transdermal protein delivery by a coadministered peptide identified via phage display. Nat Biotechnol. 2006;24:455–460. doi: 10.1038/nbt1193. [DOI] [PubMed] [Google Scholar]
- 18.Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci USA. 2005;102:4688–4693. doi: 10.1073/pnas.0501176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakase I, et al. Cellular uptake of arginine-rich peptides: Roles for macropinocytosis and actin rearrangement. Mol Ther. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Patel LN, Zaro JL, Shen WC. Cell penetrating peptides: Intracellular pathways and pharmaceutical perspectives. Pharm Res. 2007;24:1977–1992. doi: 10.1007/s11095-007-9303-7. [DOI] [PubMed] [Google Scholar]
- 21.Tamaru M, Akita H, Fujiwara T, Kajimoto K, Harashima H. Leptin-derived peptide, a targeting ligand for mouse brain-derived endothelial cells via macropinocytosis. Biochem Biophys Res Commun. 2010;394:587–592. doi: 10.1016/j.bbrc.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Walsh M, et al. Evaluation of cellular uptake and gene transfer efficiency of pegylated poly-L-lysine compacted DNA: Implications for cancer gene therapy. Mol Pharm. 2006;3:644–653. doi: 10.1021/mp0600034. [DOI] [PubMed] [Google Scholar]
- 23.Potts RO, Guy RH. Predicting skin permeability. Pharm Res. 1992;9:663–669. doi: 10.1023/a:1015810312465. [DOI] [PubMed] [Google Scholar]
- 24.Magnusson B, Pugh W, Roberts M. Simple rules defining the potential of compounds for transdermal delivery or toxicity. Pharm Res. 2004;21:1047–1054. doi: 10.1023/b:pham.0000029295.38564.e1. [DOI] [PubMed] [Google Scholar]
- 25.Mitragotri S. Modeling skin permeability to hydrophilic and hydrophobic solutes based on four permeation pathways. J Controlled Release. 2003;86:69–92. doi: 10.1016/s0168-3659(02)00321-8. [DOI] [PubMed] [Google Scholar]
- 26.Tang H, Mitragotri S, Blankschtein D, Langer R. Theoretical description of transdermal transport of hydrophilic permeants: Application to low-frequency sonophoresis. J Pharm Sci. 2001;90:545–568. doi: 10.1002/1520-6017(200105)90:5<545::aid-jps1012>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Peck KD, Ghanem AH, Higuchi WI. Hindered diffusion of polar molecules through and effective pore radii estimates of intact and ethanol treated human epidermal membrane. Pharm Res. 1994;11:1306–1314. doi: 10.1023/a:1018998529283. [DOI] [PubMed] [Google Scholar]
- 28.Otto M. Staphylococcus epidermidis—the accidental pathogen. Nat Rev Microbiol. 2009;7:555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birkenfeld P, Haratz N, Klein G, Sulitzeanu D. Cross-reactivity between the EBNA-1 p107 peptide, collagen, and keratin: Implications for the pathogenesis of rheumatoid arthritis. Clin Immunol Immunopathol. 1990;54:14–25. doi: 10.1016/0090-1229(90)90002-8. [DOI] [PubMed] [Google Scholar]
- 30.Kang SK, et al. Identification of a peptide sequence that improves transport of macromolecules across the intestinal mucosal barrier targeting goblet cells. J Biotechnol. 2008;135:210–216. doi: 10.1016/j.jbiotec.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Morris CJ, Smith MW, Griffiths PC, McKeown NB, Gumbleton M. Enhanced pulmonary absorption of a macromolecule through coupling to a sequence-specific phage display-derived peptide. J Controlled Release. 2011;151:83–94. doi: 10.1016/j.jconrel.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Leung DY. Atopic dermatitis: The skin as a window into the pathogenesis of chronic allergic diseases. J Allergy Clin Immunol. 1995;96:302–318. doi: 10.1016/s0091-6749(95)70049-8. [DOI] [PubMed] [Google Scholar]
- 33.Palmer CN, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 34.Howell MD, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124(Suppl 2):R7–R12. doi: 10.1016/j.jaci.2009.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.