Abstract
The peritrophic matrix (PM) forms a layer composed of chitin and glycoproteins that lines the insect intestinal lumen. This physical barrier plays a role analogous to that of mucous secretions of the vertebrate digestive tract and is thought to protect the midgut epithelium from abrasive food particles and microbes. Almost nothing is known about PM functions in Drosophila, and its function as an immune barrier has never been addressed by a genetic approach. Here we show that the Drosocrystallin (Dcy) protein, a putative component of the eye lens of Drosophila, contributes to adult PM formation. A loss-of-function mutation in the dcy gene results in a reduction of PM width and an increase of its permeability. Upon bacterial ingestion a higher level of expression of antibacterial peptides was observed in dcy mutants, pointing to an influence of this matrix on bacteria sensing by the Imd immune pathway. Moreover, dcy-deficient flies show an increased susceptibility to oral infections with the entomopathogenic bacteria Pseudomonas entomophila and Serratia marcescens. Dcy mutant flies also succumb faster than wild type upon ingestion of a P. entomophila toxic extract. We show that this lethality is due in part to an increased deleterious action of Monalysin, a pore-forming toxin produced by P. entomophila. Collectively, our analysis of the dcy immune phenotype indicates that the PM plays an important role in Drosophila host defense against enteric pathogens, preventing the damaging action of pore-forming toxins on intestinal cells.
Keywords: gut, innate immunity, insect immunity, entomopathogens
Because the gut epithelium is in contact with microorganisms, it must be armed with efficient systems for microbial recognition and control (1). This is also true for insects such as Drosophila, which live on decaying matter such as rotting fruits and ingest large quantities of microbes. Some studies have started to investigate the mechanisms underlying the gut defense to bacterial infection in Drosophila. These studies have indicated that (i) reactive oxygen species (ROS) production through the enzyme Duox, (ii) production of antibacterial peptides through the Imd pathway, and (iii) maintenance of gut homeostasis through regulation of stem cell activity are all essential elements of the gut defense to infection (2).
Oral ingestion of bacteria induces the rapid synthesis of ROS in the Drosophila gut by an NADPH oxidase called Duox (3). Ingested bacteria were shown to persist throughout the intestinal tract of Duox RNAi flies, which indicates a predominant role of ROS in the elimination of ingested microbes. Complementary to this ROS response, several antimicrobial peptides (e.g., Diptericin) are produced in the gut under the control of the Imd pathway (4). This local immune response is triggered by the recognition of Gram-negative peptidoglycan by the pattern recognition receptor PGRP-LC (peptidoglycan recognition protein LC) (5) and was shown to contribute to host survival upon intestinal infection with several pathogenic bacteria (6–8). Finally, efficient and rapid recovery from bacterial infection is possible only when clearance of bacteria from the gut is coordinated with epithelium renewal to repair damage caused by infection. Epithelium renewal of the Drosophila gut is stimulated by the release of the cytokine Upd3 from damaged enterocytes, which then activates the JAK/STAT pathway in intestinal stem cells to promote both their division and differentiation, establishing a homeostatic regulatory loop (9, 10). Interestingly, both Imd pathway activity and epithelium renewal are also stimulated at a basal level by the indigenous gut microbiota (10).
The peritrophic matrix (PM) forms a layer composed of chitin and glycoproteins that lines the insect midgut lumen (11, 12). Although structurally different, it plays a role analogous to that of mucous secretions of the vertebrate digestive tract and is thought to protect the midgut epithelium from abrasive food particles and microbes. Studies in insects have suggested that the PM plays a role in the defense against ingested pathogens. However, most of these studies are based on indirect evidence, such as insect survival analysis after ingestion of corrosive agents that disrupt the PM (13, 14). Diptera such as Drosophila have a type II PM that is continuously produced by specific cells of the cardia, a specialized organ at the anterior of the midgut (15). As the PM grows posteriorly, it encloses the food passing through it all along the digestive tract. To date almost nothing is known about PM functions in Drosophila, and specifically its function as an immune barrier has never been addressed by a genetic approach. In this study, we show that a protein with a chitin-binding domain, Drosocrystallin (Dcy) contributes to PM formation in Drosophila adults. A loss-of-function mutation in dcy results in a reduction of PM width and renders flies more susceptible to infections with the entomopathogenic bacteria Pseudomonas entomophila. We show that this lethality is due in part to an increased deleterious action of Monalysin, a pore-forming toxin produced by P. entomophila. Collectively, our analysis of the dcy mutation indicates that the PM plays an important role in Drosophila host defense against intestinal pathogens by preventing the action of toxins on gut cells.
Results
Dcy Is a PM Protein Induced upon Oral Bacterial Infection.
A microarray analysis indicated that a gene named drosocrystallin (dcy) encoding a chitin-binding protein is induced in the Drosophila adult gut upon oral infection with the Gram-negative bacterium Erwinia carotovora 15 (Ecc15) (16). Real-time quantitative PCR (qPCR) analysis confirmed that dcy is up-regulated soon after infection and reaches its maximum 4 h after infection, at a level approximately sevenfold higher than in unchallenged condition (Fig. 1A). A previous microarray study indicated that dcy expression is not controlled by the Imd pathway (16). In agreement with FlyAtlas (17), we found that dcy mRNA is strongly enriched in the midgut and heads of adult flies (Fig. 1B).
Fig. 1.
Dcy expression is induced in the midgut upon oral bacterial infection. (A) Dcy expression upon Ecc15 oral infection in wild-type and dcy mutant flies. Dcy mRNA was measured by real-time qPCR in whole flies at indicated time points, and results are shown as a relative Dpt/rpL32 ratio. (B) Real-time qPCR analysis of dcy mRNA expression from the indicated tissues of wild-type Drosophila. Note that adult carcasses do not include gut. Data are representative of three (A) or two (B) independent experiments (shown are error bars). (C) Transversal sections of wild-type or dcy adult anterior midgut were analyzed by immunostaining with an anti-Dcy serum. Arrows indicate the PM (Scale bars, 50 μm.)
The dcy gene encodes one transcript for a 477-aa protein with a predicted molecular mass of 55.9 kDa. This protein was initially named Drosocrystallin because of its strong expression in the Drosophila compound eye, where it is thought to be a structural component of the corneal lens (18, 19). Dcy contains a signal peptide and a chitin-binding domain. Its strong expression in the midgut suggested that Dcy could be a component of the PM. To address this hypothesis, we examined the localization of Dcy in the midgut with an anti-Dcy serum. The immunostaining analysis showed that a signal is observed on the PM with the anti-Dcy serum in wild-type intestines, suggesting that Dcy protein is a component of the PM in adult Drosophila (Fig. 1C).
Loss of dcy Compromises PM Permeability.
To gain insight into the physiological role of dcy, we used a Drosophila strain, dcyMB08319 (referred to as dcy1), with a Minos transposon inserted in the first intron of dcy (Fig. S1A). The amount of dcy mRNA in dcy1 homozygous flies or in flies carrying the dcy1 allele over a deficiency [Df(2L)Exel6030] was less than 10% of that of wild-type flies (Fig. S1B). Dcy1 flies fail to induce any dcy expression after oral infection with Ecc15 (Fig. 1A). In addition, immunostaining of midgut sections from dcy1 adults with the anti-Dcy serum gave only a faint signal in the PM (Fig. 1C). These results indicate that dcy1 is a strong loss-of-function mutation of dcy. We also generated a precise excision line of dcy1 by remobilization of the Minos transposable element. This line, referred to as dcyRev, expressed a normal level of dcy and was used in addition to the Oregon R strain as a wild-type control strain in the following experiments.
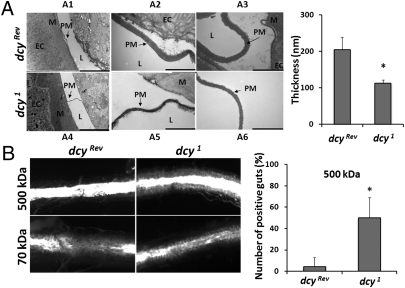
Dcy1 homozygous mutants are viable, fertile, and do not show any external morphological defect. dcy1 mutants are apparently not blind because they can orient themselves according to the light (Fig. S1C). Nevertheless, the dcy1 have a shorter lifespan, starting to die 3 to 4 wk after emergence (Fig. S1D). Interestingly, transmission electron microscopy of sections of anterior midguts revealed that the thickness of the PM in dcy1 adults is only approximately half that of control flies (Fig. 2A). We next investigated the effect of the dcy1 mutation on the PM permeability by feeding adults with FITC-labeled dextran molecules of different sizes as described in ref. 13. The occurrence of FITC signals in close contact with the intestinal epithelium was interpreted as the result of molecules crossing the PM into the ectoperitrophic space (between the PM and epithelium). Fig. 2B and Fig. S2 show that ingested 70- and 150-kDa FITC-labeled dextran molecules were observed in contact with the epithelial cells of wild-type flies, whereas 500-kDa and almost all 250-kDa dextran molecules remained in the lumen because of their incapacity to cross the PM. Interestingly, we observed that in dcy1 mutants, 250-kDa and a large proportion of 500-kDa molecules were found in close vicinity to the gut epithelium (Fig. 2B, Right, and Fig. S2). These results indicate that the PM of dcy1 mutants tends to be more permeable than that of wild-type flies.
Fig. 2.
The dcy mutation induces PM defects. (A) Left: Ultrathin sections of adult anterior midguts derived from wild-type or dcy1 mutant flies were observed by transmission electron microscopy. A2 and A5 are magnified views of A1 and A4. A4 and A6 show another section at high magnification. Arrows indicate the PM. M, mucus; EC, enterocytes; L, lumen with ingested food. (Scale bars, 10 μm in A1 and A4, 1 μm in A2, A3, A5, and A6.) Right: Quantitative measurements of the thickness of the PM in wild-type or dcy1 flies. Data show means and SEs from six and nine different midgut sections for the control and the mutant, respectively. (B) Dextran-feeding assay of wild-type or dcy1 flies. Adult flies were fed with 70-kDa or 500-kDa FITC-labeled dextran beads. Guts were dissected and examined under a fluorescence microscope. The FITC signal is retained in the lumen if the dextran beads cannot pass through the PM. The FITC signal is observed in contact with epithelial cells (indicated as positive) if beads can cross the PM. Bar graph shows the number of “positive” guts for each genotype when 500-kDa molecules were fed. Means and SEs from four independent experiments are shown. *P < 0.05.
Dcy Is Required for the Defense Against Oral Infection with Entomopathogenic Bacteria.
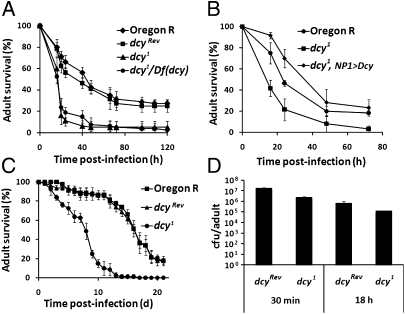
We next used dcy1 flies to analyze the contribution of the PM to the protection against oral bacterial infection. P. entomophila is a natural bacterial pathogen of Drosophila (20). Oral infection with P. entomophila at a high dose induces a strong local and systemic immune response in Drosophila but is still highly pathogenic because it quickly induces a blockage of food uptake and irreversible gut damage (10). Fig. 3A shows that dcy1 mutants exhibit a higher susceptibility than wild-type to oral infection with a lethal dose of P. entomophila. The dcy mutants even succumb to the infection with a fourfold-diluted P. entomophila solution, which is not lethal for wild-type flies (Fig. S3A). Several lines of evidence indicate that this increased susceptibility is indeed due to the dcy mutation and not to the genetic background. First, revertant dcyRev flies do not show the increased susceptibility, and Df(dcy)/dcy1 flies exhibit the same phenotype as the homozygous dcy1 mutants (Fig. 3A). Second, another genetic background, a y,w, Diptericin-lacZ, Drosomycin-GFP; dcy1 line, has the same susceptibility as dcy1 flies (Fig. S3B). Third, an in vivo RNAi silencing of dcy in the intestine using a midgut-specific GAL4 driver (genotype: NP1-GAL4; UAS-dcy-IR) also results in higher susceptibility to P. entomophila (Fig. S3C). Fourth, overexpression of dcy in the midgut using the NP1-GAL4 driver (genotype: dcy1/dcy1; NP1-GAL4/UAS-dcy) rescued the dcy1 susceptibility phenotype (Fig. 3B). Fig. 3C shows that the dcy1 mutants also succumb more rapidly than wild-type when orally infected with Serratia marcescens Db11, another entomopathogenic bacterium (7). Finally, dcy1 mutant flies have a wild-type resistance to Ecc15, a nonlethal Gram-negative bacterium, upon septic injury (Fig. S3D), indicating that the survival phenotype of the dcy1 mutant upon oral bacterial infection is not caused by a general weakness of dcy1 mutant flies.
Fig. 3.
Dcy is required for protection against oral infection with entomopathogenic bacteria. (A) Survival analysis of wild-type, dcyRev, homozygous dcy1, and dcy1/Df(dcy) hemizygous flies upon oral infection of P. entomophila. Means and SEs of four independent experiments are shown (P < 0.0001, log–rank test). (B) Survival analysis of wild-type, homozygous dcy1, and the dcy1 mutants expressing the dcy gene in the midgut upon oral infection of P. entomophila. Means and SEs of three independent experiments are shown (P < 0.0002, log–rank test). (C) Survival analysis of wild-type, dcyRev, and homozygous dcy1 flies upon oral infection with S. marcescens Db11. Graphs show the means of 60 flies, bars show the SE. This experiment was repeated three times and yielded similar results (P < 0.0001, log–rank test). (D) Bacterial persistence in wild-type and dcy1 flies. Bacterial persistence in dcy1 and dcyRev at 30 min and 18 h upon oral infection with P. entomophila (OD600 = 100), as the number of cfu per fly. No difference was observed between the two strains. Each histogram corresponds to the average of three independent experiments.
Both resistance and tolerance mechanisms contribute to maintain gut integrity upon bacterial infection. Resistance mechanisms involve the activation of various immune responses that directly target pathogens, whereas tolerance mechanisms improve the capacity to survive infection without acting on bacterial elimination. (21). We observed that the number of P. entomophila detected in the gut 18 h after infection (when flies start to die) was roughly similar in dcy1 and wild-type (Fig. 3D). This result indicates that the dcy1 mutation does not affect bacterial clearance but rather the tolerance of flies to P. entomophila infection. This is consistent with a role of the PM in protecting the gut epithelium from lethal infection. This also indicates that the dcy1 phenotype was not caused by a feeding defect because dcy1 and wild-type flies ingested the same quantity of P. entomophila, as determined by cfu assay at 30 min after ingestion (Fig. 3D). Moreover, the triglyceride and glycogen stores of the dcy1 unchallenged mutants were not significantly different from wild-type (Fig. S4), indicative of normal digestive capacity.
Together, our results show that expression of dcy in the midgut is required for defense against oral infection with entomopathogenic bacteria.
Enhanced Activation of the Imd Pathway in the dcy Mutant.
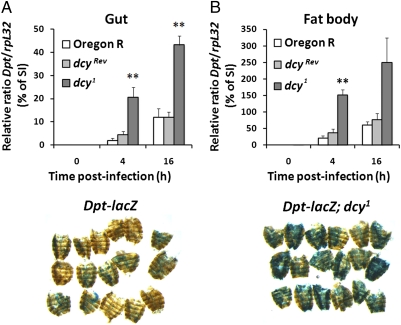
The Imd pathway regulates antimicrobial peptide production in the gut and plays a role in the resistance against both P. entomophila and S. marcescens (6–8). This prompted us to analyze the effect of the dcy1 mutation on the Imd pathway. For this, we compared the expression of Diptericin (Dpt), an antibacterial peptide gene tightly controlled by the Imd pathway, in dcy1 and wild-type flies. Real-time qPCR revealed that dcy1 flies show a stronger induction of Dpt in the gut upon oral infection with P. entomophila (Fig. 4A), notably at early time points of infection. This higher induction was not specific to P. entomophila because similar results were obtained when flies were fed with the nonlethal strain Ecc15 (Fig. S5). P. entomophila leads not only to a local but also to a strong systemic fat body immune response (20). Both real-time qPCR and X-gal staining with flies carrying a Dpt-lacZ reporter gene revealed higher levels of systemic Dpt expression in dcy1 mutant flies compared with wild-type upon P. entomophila infection (Fig. 4 A and B). The observation that the dcy1 mutation leads to a stronger overall activation of the Imd pathway in infected flies indicates that the PM influences bacterial sensing in the gut.
Fig. 4.
Dpt expression upon oral bacterial infection is higher in dcy1 flies compared with wild-type flies. (A) Dpt expression in the midgut (Left) or the fat body (Right) of wild-type and dcy1 flies upon oral infection with P. entomophila was measured by real-time qPCR at the indicated time points. Data are the mean of four independent experiments, and error bars show the SE. **P < 0.01 vs. dcyRev. Results are shown as a percentage of the Dpt/rpL32 ratio normalized to the levels observed in wild-type flies collected 8 h after septic injury (SI) with P. entomophila. (B) β-Galactosidase staining reveals lacZ gene expression in the fat body of wild-type or dcy1 flies carrying a Dpt-lacZ reporter. Flies were collected 4 h after oral infection with P. entomophila.
Dcy Mutation Affects Neither Resistance to ROS Nor Gut Repair Mechanisms.
Recent studies have demonstrated that the activation of epithelium renewal is required in the gut to compensate for damage caused by infectious agents. (9, 10, 22). Along this line, infection with high doses of P. entomophila leads to a loss of gut integrity, suggesting that virulence factors of this entomopathogen disrupt epithelium renewal through excessive damage to the gut. This raised the possibilities that the dcy mutant might be more sensitive to the oxidative burst associated with infection and/or has a compromised gut repair ability, either of which could explain the susceptibility of the dcy mutant to P. entomophila. To examine these possibilities, we orally administrated paraquat, a potent inducer of ROS, or bleomycin, a DNA-damaging agent that damages the gut, thus increasing stem cell proliferation (10, 23). Both compounds have molecular sizes (paraquat, 0.257 kDa; bleomycin, 1.4 kDa) small enough to easily cross the PM. We observed that dcy1 flies show survival rates similar to wild-type flies upon feeding with either paraquat or bleomycin (Fig. S6 A and B).
To determine whether the dcy1 mutation impacts gut repair, we quantified the level of epithelial renewal in flies upon infection with the strain Ecc15. We choose this strain because, unlike P. entomophila, oral infection with Ecc15 triggers a high level of epithelial renewal without affecting flies’ viability. We first examined the level of epithelium renewal itself upon Ecc15 oral infection by counting the number of dividing cells along the midgut using an anti-phosphohistone H3 (anti-PH3) antibody as an indicator of mitotic activity (10). No difference was observed between wild-type and dcy1 flies (Fig. S7A). Signals from both JAK-STAT and EGFR pathways control stem cell proliferation and thereby gut repair and homeostasis after oral infection (16). We monitored the activation of these pathways by quantifying transcripts for upd3, Socs36E (JAK-STAT pathway), and Keren and argos (EGFR pathway) upon oral infection with P. entomophila. Fig. S7B shows that the dcy mutants express wild-type levels of these genes in response to Ecc15 infection.
These results indicate that the dcy1 mutation does not induce a higher sensitivity to oxidative burst and does not affect the ability to repair the gut upon damage.
Increased Susceptibility of dcy Mutants to Ingested Bacterial Toxin.
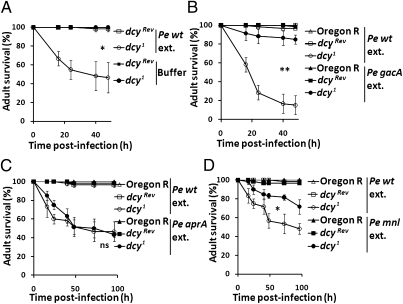
It has been proposed that the PM is a barrier protecting the midgut epithelium from secreted bacterial virulence factors such as toxins or proteases (12). We therefore speculated that toxic compounds secreted by P. entomophila could have a more damaging effect in the dcy1 mutant owing to the higher permeability of the PM. Membrane-filtered extracts from sonicated P. entomophila cells were fed to wild-type and dcy1 adults. We found that up to 80% of the dcy1 mutants succumbed to this treatment, whereas no lethality was observed in wild-type flies fed with the same extract (Fig. 5A). Virulence factors of P. entomophila required for Drosophila infection include a secreted metalloprotease (AprA) that protects against antimicrobial peptides, and a pore-forming toxin named Monalysin that participates in the damage to intestinal cells (6, 24). Both AprA and Monalysin are regulated by the GacS-GacA two-component system that regulates the production of secreted proteins and metabolites (6). Fig. 5B shows that a P. entomophila extract from a gacA mutant no longer kills the dcy1 mutant flies, whereas extracts from an aprA mutant retain their wild-type pathogenicity on dcy1 flies (Fig. 5C). Interestingly, extracts from the monalysin (mnl) mutant P. entomophila less efficiently kill dcy1 mutant flies (Fig. 5D), implying that this pore-forming toxin is partially responsible for the killing activity of the extract. The observations that (i) Monalysin contributes to the killing activities of the extract and (ii) the dcy1 mutation increases the susceptibility to the P. entomophila extract indicate that the PM provides protection against ingested toxins, especially pore-forming toxins.
Fig. 5.
Dcy1 flies succumbed to ingestion of a P. entomophila extract. (A) Survival analysis of dcyRev and dcy1 flies upon feeding of the extract from P. entomophila (Pe extract) or buffer (PBS 1% Triton X-100). (B) Survival analysis of wild-type, dcyRev, and dcy1 flies upon feeding with extracts derived from wild-type and gacA P. entomophila derivatives. (C) Survival analysis of wild-type, dcyRev, and dcy1 flies upon feeding with extracts derived from wild-type and AprA P. entomophila derivatives. (D) Survival analysis of wild-type, dcyRev, and dcy1 flies upon feeding with extracts derived from wild-type and monalysin (mnl) P. entomophila derivatives. In A–D, graphs show the means of 60 flies, and bars show the SE. These experiments were repeated three times and yielded similar results (*P < 0.0001, **P < 0.005, log–rank test. ns, not significant).
Discussion
Proteomic analyses have revealed the complexity of the insect PM that is composed of chitin microfibrils embedded in a matrix of proteins and glycoproteins (12, 25, 26). PM-associated proteins include mucins and peritrophin proteins. The Drosophila genome contains approximately 25 genes encoding chitin-binding domain proteins and 36 genes encoding mucin-like proteins (27). To date, none of these genes have been studied in the context of the PM, and little is known about the role of mucus and the PM in Drosophila gut homeostasis and immunity. Here we show that the Dcy protein, a putative component of the eye lens of Drosophila, contributes to adult PM formation. Although, we cannot exclude that Dcy also exist as free molecules in the gut, four lines of evidences support that Dcy is an integral component of the PM: (i) the presence of a chitin-binding domain, suggesting that Dcy associates with chitin fibrils of the PM; (ii) the staining of the PM using an anti-Dcy antibody; (iii) the thinner PM observed in dcy1 mutants; and (iv) the higher permeability of the PM in dcy1 mutants. Because dcy is expressed in the midgut and not in the cardia, it is likely that this protein is directly incorporated to the PM after its synthesis. The observation that a strong loss-of-function mutation in dcy reduces the PM width by half and increases its permeability to larger molecules indicates that despite the high number of structural proteins associated with the PM, Dcy is an essential component of the PM. Dcy cannot be considered sensu stricto as a peritrophin, owing to the absence of characteristic cystein arrangement in its chitin-binding domain. The dual function of this protein in both eye and gut is intriguing. Of note, no clear Dcy paralog is encoded by the Drosophila genome. In addition, no homolog can be found outside the Drosophilidae family, suggesting that Dcy has evolved to fulfill a function specific to this clade.
Using the dcy1 mutation, we were able to indirectly assess a role for the PM in Drosophila host defense. Our observation that dcy mutants are highly susceptible to infection with P. entomophila and S. marcescens points to a protective role of the PM in host defense against entomopathogenic bacteria. We observed that ingestion of a P. entomophila extract is sufficient to induce lethality in dcy1 mutant flies but not in wild-type flies. This supports a role of the PM in limiting the diffusion of a bacterial toxin. Interestingly, a P. entomophila extract from the pore-forming toxin monalysin-deficient strain seems less toxic to dcy flies. This indicates that the PM provides an effective protection against the action of this pore-forming toxin. β-Pore-forming toxins such as Monalysin have the ability to multimerize into circular polymers, a step required for pore formation in targeted cells (28). This suggests that the PM could function as a sieve blocking the action of this class of toxins. A role of the PM in the protection against pore-forming toxins is also supported by studies in other insects. Hayakawa et al. (29) showed that Bombyx mori is sensitive to the Cry1Aa toxin and resistant to Cry1Ac, both insecticidal toxins of Bacillus thuringiensis. This difference correlates with the capacity of Cry1Aa to pass through the PM faster than Cry1Ac in an in vitro assay. It was also reported that the activity of Cry1Ac toxin on Helicoverpa armigera larvae is enhanced by B. thuringiensis Enhancin, a metalloprotease that degrades PM-associated mucins (30). The involvement of a pore-forming toxin in P. entomophila virulence, together with the well-characterized action of B. thuringiensis cytotoxin Cry, have recently led to the notion that pore-forming toxins constitute an efficient arm to promote bacterial colonization of the insect gut (24, 31). Our present studies suggest that the PM provides an important barrier to counteract the action of this class of toxins.
The observation that dcy as well as several peritrophin genes are induced upon ingestion of bacteria (16) also points to the existence of active mechanisms that reinforce the role of the PM barrier during infection. This indicates that the PM is not just a passive physical barrier but can be remodeled during gut infection. However, our study does not address whether PM protection against P. entomophila pore-forming toxin is mediated by its impermeability to Monalysin or by its capacity to bind and sequester this toxin. In support of the second hypothesis, Abedi and Brown (32) discovered that the PM excreted by Aedes aegypti larvae that are resistant to dichlorodiphenyltrichloroethane was laden with the insecticide. This finding led to the notion that this matrix may play a role in sequestering and possibly detoxifying ingested xenobiotics.
Production of ROS in the gut by Duox in response to bacteria inflicts damage to the intestinal epithelium that is repaired through stem cell proliferation (10). Although some of these ROS compounds could be inactivated by antioxidant enzymes (33), it has been proposed that the PM could serve as a “sacrificial antioxidant” through the scavenging of ROS (34, 35). In opposition to this idea, we did not observe a higher susceptibility of dcy mutants to ROS produced by paraquat. This does not rule out a role of the PM as antioxidant but suggests that in our infection model the main defensive role of the PM is to limit the action of bacterial toxins. Moreover, the gut repair capacity through epithelium renewal is not affected by the dcy mutation in flies orally infected with Ecc15 or flies that ingested Bleomycin, thus reinforcing the idea that the PM protection is specific for a certain type of threat, such as that linked to pore-forming toxins.
Recent studies in Drosophila have revealed that multiple regulatory mechanisms are required to precisely control the level of Imd pathway activity in the gut. These include Pirk, a protein interacting with PGRP-LC and with amidase PGRPs, which both restrict the activation of the immune pathway by indigenous flora (5, 36). Here, we observed that disruption of the PM affects the level of Imd pathway activity in response to infection. Dcy1 mutants exhibit enhanced gut and systemic immune responses to Gram-negative bacteria. This indicates a role of the PM in the fine-tuning of Imd pathway activity during bacterial infection. An attractive hypothesis is that the PM could limit the diffusion from the gut lumen to epithelial cells of peptidoglycan, the bacterial elicitor recognized by the Imd pathway.
In conclusion, our studies ascribe important functions to the PM in Drosophila host defense against bacteria by limiting the effect of bacterial toxins and reducing Imd pathway activity. The importance of the PM function is even underestimated in our study because the dcy1 mutation reduces but does not eliminate the PM. Our study is also in line with those in vertebrates that revealed the key role of mucus in gut homeostasis and mucosal immunity (37). Indeed, deletion of the large gel-forming mucin Muc2 in mice allows the direct contact of bacteria with the epithelia cells, thus provoking colon inflammation. It now seems that both mucus in mammals and PM in insects provide an important protection against the action of pathogens and influence the immune reactivity of the digestive tract. A better comprehension of the physiological role of the PM is essential to understand insect gut homeostasis. Moreover, we must keep in mind that the PM is an attractive target for insect pest management strategies (11, 12). We expect that this study will open the route for a genetic dissection of PM function in Drosophila that could be useful in other insects of economic or global health importance.
Materials and Methods
Fly Stocks.
Oregon R flies were used as wild-type flies. Dcy1 (MB08319, Fig. S1) and Df(2L)Exel6030 were obtained from the Bloomington Drosophila Stock Center. Canton S, w1118, RelishE20 (RelE20), NP1-Gal4, and Diptericin-LacZ fly lines are described in ref. 16. The UAS-CG16963-IR RNAi line from the Vienna Drosophila RNAi Stock Center and UAS-yellow (Bloomington Center) or w1118 were used as control. A full-length cDNA of dcy (CG16963_cDNA gold RH66281 from the Drosophila Genomics Resource Center) was inserted in the pENTR Gateway entry clone (Invitrogen) and then subcloned in the pTW transgenesis vector used for generating transgenic flies according to standard procedures. Fly line carrying the transgene on the third chromosome was established and used as UAS-dcy. F1 progeny carrying both the UAS construct and the Gal4 driver were transferred to 29 °C at late pupal stage for optimal efficiency of the UAS/Gal4 system. To obtain a revertant of dcy1, the Minos-element MB08319 was mobilized by a Minos transposase source (38), and precise excision line (referred to as dcyrev) was isolated. Drosophila stocks and crosses were maintained at 25 °C in tubes containing standard fly medium (maize flour, dead yeast, agar, and fruit juice) devoid of living yeast.
Bacterial Stocks and P. entomophila Protein Extracts.
P. entomophila L48 (20) was grown in LB for all experiments. P. entomophila mutated for the gacA, aprA, and mnl are described elsewhere (6, 24). The Ecc15 strain was described previously (20). They were grown at 29 °C and allowed to reach the stationary phase. Cells were then concentrated at OD600 = 200 except when indicated. The solution was added. S. marcescens strain Db11 (7) was grown at 37 °C and used as pellets of OD600 = 200. Pellets were not washed before use. For P. entomophila extracts, stationary phase cultures of wild-type and mutant P. entomophila were concentrated by centrifugation. The cell pellet was washed with PBS and adjusted to OD600 = 200 in PBS with 1% Triton X-100. The pellets were sonicated, recentrifuged, and filtered with a 0.22-μm membrane (Millipore).
Infection and Survival Assays.
Septic injuries were performed by pricking adults in the thorax with a thin needle dipped into a concentrated bacterial pellet. For oral infection, female flies were starved for 2 h at 29 °C and then placed in a fly vial with food solution. The food solution was obtained by mixing a pellet of bacteria (OD600 = 200, corresponding to 1.3 × 1011 bacteria/mL), solution of paraquat (10–50 mM, Sigma), or 500 μg/mL bleomycin (Sigma) with a solution of 5% sucrose (1:1), added to a filter disk that completely covered the surface of standard fly medium. Flies were maintained at 29 °C, and mortality was monitored as at different time points. Survival assays were performed with 60–80 flies for each genotype.
Antibodies, Immunohistochemistry, and X-Gal Staining.
Anti-PH3 staining was performed as previously described (16). An Anti-Dcy antibody was raised by immunizing rats with the carboxyl end of Dcy protein (amino acid positions 402–477), which was expressed in Escherichia coli as a fusion protein with GST and affinity-purified. Immunohistochemistry, electron microscopy, and X-gal staining are described in SI Materials and Methods.
Dextran Feeding Assay.
FITC-dextran beads (Sigma) were dissolved in 2.5% sucrose and filtrated with Sephadex G-10 (GE Healthcare) and used for feeding experiments. Female flies were starved for 2 h at 29 °C in an empty vial and then placed in a normal fly vial covered with a filter paper soaked with the dextran solution. After 15 min at 29 °C, guts were dissected in PBS, and FITC signal was observed under a fluorescent microscope (Zeiss). Images were captured with a Leica DFC300FX camera and the Leica Application Suite.
Real-Time qPCR.
Total RNA was extracted from whole flies, cuticles of flies as fat bodies, dissected midguts, or other tissues by using TRIzol (Invitrogen). Real-time qPCR was performed using SYBR Green I (Roche) on a LightCycler 2.0 as described previously (16). The amount of mRNA detected was normalized to rpL32 control values. Primers used to monitor mRNA quantification can be obtained on request.
Statistics.
Statistical analyses were done by Student t test or log–rank test, and P values of <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank our colleagues J. P. Boquete and Danielle Brandalise for technical assistance; Nichole Broderick for helpful comments on the manuscript; D. Ferrandon (Strasbourg) for the S. marcescens Db11; the Bloomington Stock Center, the Vienna Drosophila Research Center, and the National Institute of Genetics for fly stocks; and S. Rosset and G. Knott (Ecole Polytechnique Fédérale de Lausanne BioEM Facility) for assistance with histological analysis. The B.L. laboratory is supported by a European Research Council Advanced Investigators grant, the Bettencourt-Schueller foundation, and Swiss National Science Foundation Grant 3100A0-12079/1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105994108/-/DCSupplemental.
References
- 1.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–964. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 2.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 3.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 4.Tzou P, et al. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity. 2000;13:737–748. doi: 10.1016/s1074-7613(00)00072-8. [DOI] [PubMed] [Google Scholar]
- 5.Zaidman-Rémy A, et al. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity. 2006;24:463–473. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Liehl P, Blight M, Vodovar N, Boccard F, Lemaitre B. Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog. 2006;2:e56. doi: 10.1371/journal.ppat.0020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nehme NT, et al. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 2007;3:e173. doi: 10.1371/journal.ppat.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryu JH, et al. An essential complementary role of NF-kappaB pathway to microbicidal oxidants in Drosophila gut immunity. EMBO J. 2006;25:3693–3701. doi: 10.1038/sj.emboj.7601233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehane MJ. Peritrophic matrix structure and function. Annu Rev Entomol. 1997;42:525–550. doi: 10.1146/annurev.ento.42.1.525. [DOI] [PubMed] [Google Scholar]
- 12.Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol. 2009;54:285–302. doi: 10.1146/annurev.ento.54.110807.090559. [DOI] [PubMed] [Google Scholar]
- 13.Edwards MJ, Jacobs-Lorena M. Permeability and disruption of the peritrophic matrix and caecal membrane from Aedes aegypti and Anopheles gambiae mosquito larvae. J Insect Physiol. 2000;46:1313–1320. doi: 10.1016/S0022-1910(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Granados RR. Calcofluor disrupts the midgut defense system in insects. Insect Biochem Mol Biol. 2000;30:135–143. doi: 10.1016/s0965-1748(99)00108-3. [DOI] [PubMed] [Google Scholar]
- 15.King DG. Cellular organization and peritrophic membrane formation in the cardia (proventriculus) of Drosophila melanogaster. J Morphol. 1988;196:253–282. doi: 10.1002/jmor.1051960302. [DOI] [PubMed] [Google Scholar]
- 16.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 18.Janssens H, Gehring WJ. Isolation and characterization of drosocrystallin, a lens crystallin gene of Drosophila melanogaster. Dev Biol. 1999;207:204–214. doi: 10.1006/dbio.1998.9170. [DOI] [PubMed] [Google Scholar]
- 19.Komori N, Usukura J, Matsumoto H. Drosocrystallin, a major 52 kDa glycoprotein of the Drosophila melanogaster corneal lens. Purification, biochemical characterization, and subcellular localization. J Cell Sci. 1992;102:191–201. doi: 10.1242/jcs.102.2.191. [DOI] [PubMed] [Google Scholar]
- 20.Vodovar N, et al. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc Natl Acad Sci USA. 2005;102:11414–11419. doi: 10.1073/pnas.0502240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider DS, Ayres JS. Two ways to survive infection: What resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatterjee M, Ip YT. Pathogenic stimulation of intestinal stem cell response in Drosophila. J Cell Physiol. 2009;220:664–671. doi: 10.1002/jcp.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opota O, et al. Monalysin, a novel β-pore-forming toxin from the Drosophila pathogen Pseudomonas entomophila, contributes to host intestinal damage and lethality. PLoS Pathog. 2011 doi: 10.1371/journal.ppat.1002259. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tellam RL, Wijffels G, Willadsen P. Peritrophic matrix proteins. Insect Biochem Mol Biol. 1999;29:87–101. doi: 10.1016/s0965-1748(98)00123-4. [DOI] [PubMed] [Google Scholar]
- 26.Dinglasan RR, et al. The Anopheles gambiae adult midgut peritrophic matrix proteome. Insect Biochem Mol Biol. 2009;39:125–134. doi: 10.1016/j.ibmb.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Syed ZA, Härd T, Uv A, van Dijk-Härd IF. A potential role for Drosophila mucins in development and physiology. PLoS ONE. 2008;3:e3041. doi: 10.1371/journal.pone.0003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez MR, Bischofberger M, Pernot L, van der Goot FG, Frêche B. Bacterial pore-forming toxins: The (w)hole story? Cell Mol Life Sci. 2008;65:493–507. doi: 10.1007/s00018-007-7434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayakawa T, Shitomi Y, Miyamoto K, Hori H. GalNAc pretreatment inhibits trapping of Bacillus thuringiensis Cry1Ac on the peritrophic membrane of Bombyx mori. FEBS Lett. 2004;576:331–335. doi: 10.1016/j.febslet.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Fang S, et al. Bacillus thuringiensis bel protein enhances the toxicity of Cry1Ac protein to Helicoverpa armigera larvae by degrading insect intestinal mucin. Appl Environ Microbiol. 2009;75:5237–5243. doi: 10.1128/AEM.00532-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallet-Gely I, Lemaitre B, Boccard F. Bacterial strategies to overcome insect defences. Nat Rev Microbiol. 2008;6:302–313. doi: 10.1038/nrmicro1870. [DOI] [PubMed] [Google Scholar]
- 32.Abedi ZH, Brown AWA. Peritrophic membrane as vehicle for DDT and DDE excretion in Aedes aegypti larvae. Ann Entomol Soc Am. 1961;54:539–542. [Google Scholar]
- 33.Ha EM, et al. An antioxidant system required for host protection against gut infection in Drosophila. Dev Cell. 2005;8:125–132. doi: 10.1016/j.devcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Barbehenn RV, Stannard J. Antioxidant defense of the midgut epithelium by the peritrophic envelope in caterpillars. J Insect Physiol. 2004;50:783–790. doi: 10.1016/j.jinsphys.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Summers CB, Felton GW. Peritrophic envelope as a functional antioxidant. Arch Insect Biochem Physiol. 1996;32:131–142. [Google Scholar]
- 36.Lhocine N, et al. PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signaling. Cell Host Microbe. 2008;4:147–158. doi: 10.1016/j.chom.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Johansson ME, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metaxakis A, Oehler S, Klinakis A, Savakis C. Minos as a genetic and genomic tool in Drosophila melanogaster. Genetics. 2005;171:571–581. doi: 10.1534/genetics.105.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.