Abstract
In vertebrates, the animal–vegetal axis is determined during oogenesis and at ovulation, the egg is radially symmetric. In anamniotes, following fertilization, a microtubule-dependent movement leads to the displacement of maternal dorsal determinants from the vegetal pole to the future dorsal side of the embryo, providing the initial breaking of radial symmetry [Weaver C, Kimelman D (2004) Development 131:3491–3499]. These dorsal determinants induce β-catenin nuclear translocation in dorsal cells of the blastula. Previous work in amphibians has shown that secreted Wnt11/5a complexes, regulated by the Wnt antagonist Dkk-1, are required for the initiation of embryonic axis formation [Cha et al. (2009) Curr Biol 29:1573–1580]. In the current study, we determined that the vegetal maternal dorsal determinant in fish is not the Wnt11/5a complex but the canonical Wnt, Wnt8a. Translation of this mRNA and secretion of the Wnt8a protein result in a dorsal-to-ventral gradient of Wnt stimulation, extending across the entire embryo. This gradient is counterbalanced by two Wnt inhibitors, Sfrp1a and Frzb. These proteins are essential to restrict the activation of the canonical Wnt pathway to the dorsal marginal blastomeres by defining the domain where the Wnt8a activity gradient is above the threshold value necessary for triggering the canonical β-catenin pathway. In summary, this study establishes that the zebrafish maternal dorsal determinant, Wnt8a, is required to localize the primary dorsal center, and that the extent of this domain is defined by the activity of two maternally provided Wnt antagonists, Sfrp1a and Frzb.
Keywords: asymmetry, teleost
Maternally provided dorsal determinants are known to induce a local stabilization and nuclear translocation of the Wnt pathway effector β-catenin protein in the future dorsal region of the blastula (1). In zebrafish, embryos exhibit β-catenin protein in dorsal marginal nuclei after the midblastula transition. However, for a fraction of embryos (15%), it can be observed earlier (2) demonstrating that stimulation of the canonical Wnt pathway does not require transcription of the zygotic genome but depends on the activity of maternal components. Two genes coding for β-catenin have been identified in zebrafish. Only one, β-catenin2, is essential for dorsal axis formation. Reduction in the level of transcription of β-catenin2 in the ichabod mutation results in embryos with severe anterior and dorsal defects (3). This mutation exhibits variable expression with a fraction of embryos completely radialized and lacking in nuclear localization of β-catenin at the dorsal margin in the high and sphere stages (3, 4). Complete radialization is also observed after ablation of the vegetal part of the yolk cell during the first 20 min of development (5), a condition that removes maternal dorsal determinants present at the vegetal pole of the egg. Inhibition of microtubule-dependent transport of these determinants (6–8) results in similar phenotypes. This clearly establishes that the maternal Wnt/β-catenin signaling pathway is activated by dorsal determinants transported from the vegetal pole to the future dorsal margin by a microtubule-dependent mechanism.
In amphibians, the dorsal determinants were initially thought to correspond to intracellular proteins transducing the signal from the canonical Wnt/β-catenin signaling pathway (9). However, this pathway has now been shown to be activated extracellularly in a process that requires Wnt11, Wnt5a, and FRL1 (10). Further studies revealed that Wnt5a and Wnt11 physically interact with each other to activate both canonical and noncanonical Wnt signaling required for Xenopus dorsal axis formation (11). O-sulfation of specific tyrosine residues was found to be necessary for the interaction of Wnt11 with Wnt5a and for enhanced canonical signaling activity (12).
In zebrafish, the identity of the dorsal determinant has been under investigation for a number of years, but it has not been identified yet. In this study, we show that Wnt8a (13), a Wnt ligand known to activate the canonical pathway, is the dorsal determinant in zebrafish. In addition, we establish that two maternally provided Wnt inhibitors, Sfrp1a (14) and Frzb (15), are essential to limit the spatial extent of the maternal Wnt/β-catenin signaling pathway, restricting the nuclear accumulation of β-catenin to the dorsalmost cells.
Results and Discussion
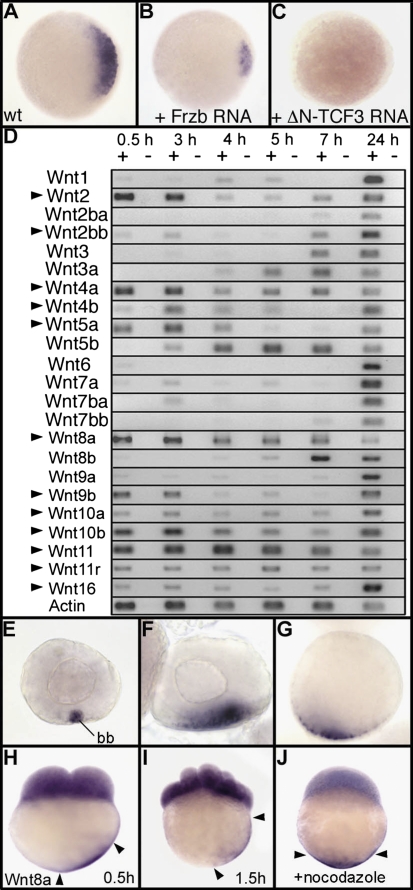
We initially hypothesized that the dorsal determinant in zebrafish is a Wnt ligand, on the basis of analogy with the mechanism described in Xenopus. In support of this hypothesis, we found that overexpression of Frzb, a secreted antagonist of Wnt (16, 17), strongly decreases (Fig. 1 A and B) or even abolishes the expression of chordin, a marker of dorsal tissues. Chordin can also be used as a readout of the maternal β-catenin activity as illustrated with a dominant-negative Tcf-3 construct (DN-Tcf3), which is able to block β-catenin transcriptional activity (18), thereby preventing the expression of chordin (Fig. 1 A and C). To determine whether, in zebrafish, Wnt ligand(s) could be the dorsal determinant(s), we performed a systematic analysis of their expression during early development (Fig. 1D). Among the 23 Wnt genes identified in the zebrafish genome, 12 genes show clear maternally deposited mRNAs (Fig. 1D, arrowheads). Expression of 5 genes (Wnt1, -6, -7a, -8b, and -9a) is barely detectable in the egg, whereas 6 genes (Wnt2ba, -3, -3a, -5b, -7ba, and -7bb) are not expressed maternally. In situ hybridization was performed for all Wnt genes both on ovaries and in early embryos during cleavage stages. Surprisingly, whereas Wnt11 mRNAs are vegetally localized in Xenopus (19), transcripts of this gene are only observed in blastomeres in zebrafish (Fig. S1). We found that Wnt8a is the sole Wnt gene for which transcripts accumulate at the vegetal pole of oocytes and of early zebrafish embryos (Fig. S1). In primary oocytes, strong accumulation of Wnt8a mRNA is observed in the Balbiani body (Fig. 1E), a structure that reveals the first asymmetry in vertebrate oocytes. It is involved in the creation of an early polarity during oogenesis and is a component of a vegetal transport pathway thought to entrap mRNAs and other gene products necessary for germ cell formation and patterning of the embryo (20, 21). Later in oogenesis, during expansion and dispersal of the Balbiani body, Wnt8a mRNAs are observed at the vegetal pole of the oocytes (Fig. 1 F and G). So far, only a very few mRNAs encoding regulators of embryonic patterning, such as Syntabulin, a motor protein linker, have been reported to use the early Balbiani body-mediated vegetal pathway (8).
Fig. 1.
The secreted Wnt ligand Wnt8a is a strong candidate for the dorsal determinant in zebrafish. Expression of chordin at the sphere stage is shown in (A) wild type (WT), (B) an embryo injected with 500 pg of Frzb mRNA, and (C) an embryo injected with 100 pg of mRNA coding for a dominant-negative Tcf-3 (DN-Tcf3) (34). Embryos are in animal pole view with dorsal to the Right. (D) Expression of the 23 Wnt genes present in the zebrafish genome analyzed by RT-PCR at 1- to 2-cell stage (0.5 h), 1,000-cell stage (3 h), sphere stage (4 h), 40% epiboly (5 h), 60% epiboly (7 h), and at 24 hpf. Arrowheads indicate Wnt genes for which mRNAs are maternally deposited in the egg. (E–J) Whole mount in situ hybridization reveals that Wnt8a mRNAs accumulate in the Balbiani body (bb) in stage I oocytes (E) and are localized in a vegetal position in stage II (F) and stage III oocytes (G). Transcripts of Wnt8a are also observed in cleavage-stage embryos both in blastomeres and in the yolk cortical cytoplasm in vegetal position at (H) the 2-cell stage and (I) 16-cell stage. (J) Treatment with nocodazole, which depolymerizes microtubules, prevents transport of Wnt8a mRNA. Embryos are in lateral view with dorsal to the Right. Arrowheads in H–J indicate the limits of Wnt8a mRNA localization in the cortical cytoplasm.
After fertilization, during early cleavage stages, Wnt8a transcripts are asymmetrically localized in the cortical cytoplasm on one side of the yolk cell and appear to move progressively to a more animal position (Fig. 1 H and I). This movement is abolished (Fig. 1J) by treatment with nocodazole, an agent that is known to disrupt microtubules and to prevent the transport of vegetally localized dorsal determinants (6).
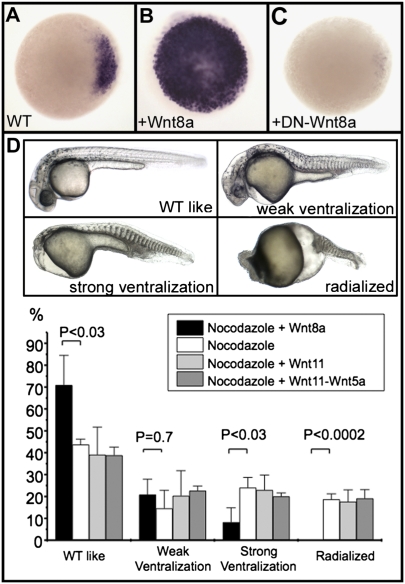
Altogether, accumulation of Wnt8a mRNA in the Balbiani body during oogenesis, its vegetal localization in mature oocytes, and its movement toward the animal pole during early cleavage stages strongly suggest that Wnt8a is the maternal dorsal determinant in fish. In agreement with this hypothesis, we found that overexpression of Wnt8a strongly induces expression of chordin at the early blastula stage (Fig. 2B) and overexpression of a dominant-negative form of Wnt8a (DN-Wnt8a) abolishes expression of this dorsal molecular marker (Fig. 2C). In addition, localized injection of Wnt8a mRNA into an animal pole blastomere at the 64-cell stage results in ectopic expression of dharma, a direct target of the maternal β-catenin signaling (4, 22), revealing activation of this pathway at the animal pole of injected embryos (Fig. S2A). This activation is prevented by overexpression of DN-Wnt8a (Fig. S2B).
Fig. 2.
Wnt8a acts as the initial dorsal determinant in zebrafish. (A–C) Expression of chordin, analyzed by in situ hybridization, at the sphere stage in a wild-type (WT) embryo (A), in an embryo overexpressing Wnt8a (100 pg Wnt8a mRNA) (B), and in an embryo overexpressing a dominant-negative form of Wnt8a (500 pg of mRNA coding for DN-Wnt8a ORF2) (C). Embryos are in animal pole view, dorsal to the Right. (D) Nocodazole treatment generates embryos displaying various ventralization phenotypes ranging from weak (lacking notochord), strong (additionally missing the head), and radialized (lacking any dorsal territories). Injection of 25 pg of Wnt8a mRNA into one marginal blastomere at the 64-cell stage efficiently rescues radialized embryos and strongly reduces the frequency of strongly ventralized embryos; injections of 400 pg of Wnt11 mRNA or of a mix of 400 pg of Wnt11 mRNA and 400 pg of Wnt5a mRNA fails to rescue these phenotypes as shown in the graph. Embryos are presented in lateral view with anterior to the Left. Means and error bars (SD) were obtained from multiple experiments (three to five) with at least 50 embryos for each experimental condition. Comparisons between experiments were undertaken using unpaired Student's t tests. P value of <0.05 was considered statistically significant.
Activation of the maternal β-catenin pathway by Wnts, whose mRNAs are maternally supplied to the egg, appears specific to Wnt8a. The other canonical Wnts that display strong maternal expression (Wnt2, Wnt9b, and Wnt10b), and all noncanonical Wnts (Wnt4a, Wnt4b, Wnt5a, Wnt5b, Wnt11, and Wnt11r), are unable to induce dharma expression at the animal pole, even with injection of 20 times more mRNA than was used for Wnt8a (Fig. S3). Similarly, coinjection into one animal pole blastomere at the 64-cell stage of mRNAs coding for the two Wnts, Wnt11 and Wnt5a, known to be responsible for the activation of the maternal β-catenin signaling pathway in amphibians (10–12), fails to induce dharma expression at the animal pole (Fig. S3L) and to induce nuclear localization of β-catenin in animal pole blastomeres (Fig. S4). This strongly suggests that the Wnt11/5a complex, which acts as a dorsal determinant in frog, is not the maternal determinant that defines the dorsal center in fish.
However, another canonical Wnt, Wnt3a, coexpressed with Wnt8a at the gastrula margin and in the tail bud, and required, as is Wnt8a, for posterior mesoderm formation (23), is also able to activate the maternal β-catenin pathway. Injection of Wnt3a mRNA into an animal pole blastomere of the 64-cell stage embryo results in induction of dharma expression at the animal pole (Fig. S3B) and nuclear accumulation of β-catenin in animal pole blastomeres (Fig. S4 E and F). The dominant-negative form of Wnt8a is also able to prevent activation of the canonical β-catenin pathway by Wnt3a (Fig. S4 G and H), but the absence of detectable Wnt3a mRNA by RT-PCR before late blastula stage (Fig. 1) excludes this Wnt from being the physiological ligand that stimulates the maternal β-catenin pathway.
This last observation raises a question about the specificity of the dominant-negative effect of DN-Wnt8a. However, injection into the yolk stream at the 8- to 16-cell stage of mRNA coding for DN-Wnt8a induces phenotypes highly similar to Wnt8a mutants or morphants (24) (Fig. S2 E and F). When expressed under this condition (1–1.5 h after fertilization), DN-Wnt8a fails to block the activation of the maternal β-catenin but efficiently interferes with the zygotic β-catenin pathway, inducing a posteriorization of the embryo. This is characterized by a strong posterior enlargement of the midbrain and forebrain at late gastrula stage (Fig. S2 C and D) and by posteriorly truncated embryos at 24 h postfertilization (hpf) (Fig. S2E), which are very similar to Wnt8a morphants (Fig. S2F). These phenotypes are clearly different from single loss-of-function phenotypes of noncanonical Wnts such as Wnt11, characterized by cyclopia and other midline defects in the head (25) or from phenotypes of multiple loss of function of Wnt4a, Wnt11, and Wnt11r (26). This demonstrates that DN-Wnt8a does not interfere with the activity of these noncanonical Wnts and supports the conclusion that Wnt8a is the only Wnt antagonized by DN-Wnt8a during early cleavage stages.
We further tested the hypothesis that Wnt8a is the maternal dorsal determinant by performing rescue experiments on embryos defective for the transport of dorsal determinants by injecting Wnt mRNAs into one marginal blastomere at the 64-cell stage. Depolymerization of microtubules by nocodazole treatment during the first 10 min after laying results in embryos eliciting a range of ventralization phenotypes with 25% of embryos being completely radialized (Fig. 2D). These embryos, resembling a strong ichabod mutant phenotype, are likely to be defective in the initial induction of the maternal Wnt/β-catenin signaling pathway. Injection of Wnt8a efficiently rescued these ventralization phenotypes with a complete disappearance of radialized embryos and a statistically significant reduction in the number of embryos that are strongly ventralized (Fig. 2D). Moreover, almost all embryos coinjected with Wnt8a developed a hatching gland derived from the injected blastomeres (Fig. S5). This organ is a prechordal plate derivative that corresponds to the more anterior dorsal mesendoderm and derives from cells of the dorsal organizer. This result demonstrates that Wnt8a, when applied locally, is able to compensate for the absence of vegetally derived dorsal determinants. Conversely, injection of Wnt11 or of a mix of Wnt11 and Wnt5a mRNAs is unable to rescue nocodazole-treated embryos, even for injection of 16 times more mRNA than was used for Wnt8a. Moreover, Wnt11- injected embryos do not display a hatching gland derived from the injected clone (Fig. S6). Therefore, whereas Wnt11 and Wnt5a, two noncanonical Wnts, acting together are able to stimulate the canonical Wnt signaling pathway during early embryonic development in amphibians (10–12), they are unable to do so in fish. Our results demonstrate that Wnt8a, which activates the canonical Wnt signaling pathway (27), is the maternal dorsal determinant in zebrafish.
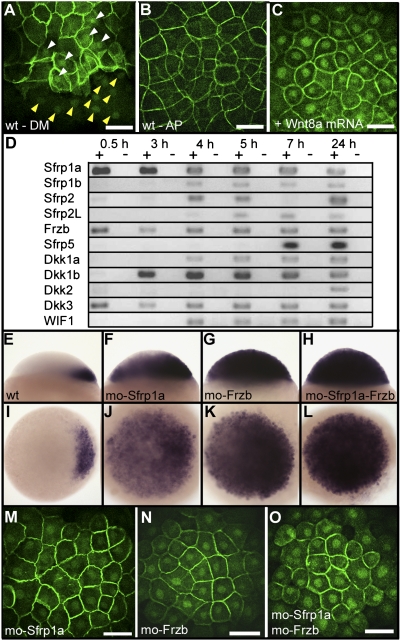
Whereas Wnt8a transcripts are present at the vegetal pole of the embryo, a large amount of Wnt8a mRNA is also observed in the blastomeres. In addition, 11 other Wnt genes are also maternally expressed (Fig. 1D) and show transcripts present in these blastomeres. Altogether, translation and secretion of these Wnt mRNAs may result in the stimulation of the pathway. Given this localization, why is β-catenin not stabilized and translocated into the nucleus of all blastomeres? Two hypotheses have been considered. First, one or more element(s) essential for the canonical Wnt signaling pathway may be missing in blastomeres at early cleavage stages. We probed this hypothesis by performing extensive analyses of the expression of Wnt receptors and known downstream components of the canonical Wnt signaling pathway. We found that all components tested are present as maternally deposited mRNAs in the egg (Fig. S7), making this first hypothesis rather unlikely. In addition, overexpression of Wnt8a by injection of in vitro synthesized mRNA at the one-cell stage results in the activation of the canonical pathway and in the translocation of β-catenin into the nucleus of all blastomeres (Fig. 3C). This demonstrates that all components necessary for the transduction of the canonical Wnt signal are present in all cells of the embryo.
Fig. 3.
Negative regulation of the canonical Wnt pathway by Sfrp1a and Frzb. Immunofluorescence localization of β-catenin is shown at 3 hpf (high stage) (A), at the dorsal margin (DM), and (B) at the animal pole (AP) of a wild-type embryo or (C) at the animal pole of an embryo overexpressing Wnt8a (10 pg Wnt8a mRNA). White and yellow arrowheads in A point to the accumulation of β-catenin in blastomeres and yolk syncytial layer nuclei, respectively. (D) Analysis by RT-PCR of the expression of zebrafish Wnt inhibitors during early development. Sfrp (TLC), expressed only during gastrulation (35), is not presented here. Expression of chordin at the sphere stage in lateral view (Upper) and animal pole view (Lower) in (E and I) wild-type embryos, (F and J) Sfrp1a morphants, (G and K) Frzb morphants, and (H and L) Sfrp1a–Frzb double morphants. (M–O) Immunofluorescence localization of β-catenin in nuclei of the animal pole of embryos at the high stage in (J) Sfrp1a, (K) Frzb, and (L) Sfrp1a–Frzb morphants. Embryos are in dorsal view (A), lateral view (E–H), and in animal pole view (B, C, and I–O). (Scale bar, 100 μM.)
The alternative hypothesis is that the activity of Wnts expressed in blastomeres may be repressed by specific Wnt inhibitors. Analysis of the expression of the known Wnt antagonists in zebrafish at early developmental stages (Fig. 3D) reveals that Sfrp1a (14), Dkk-3 (28), and Frzb (15) are maternally expressed and their mRNAs deposited in the egg. Loss-of-function analysis of Dkk-3 has been reported (28) and does not affect early embryonic development. Dkk-3 is therefore not a suitable candidate to antagonize Wnt8a at early stages of development. We then tested Sfrp1a and Frzb for their possible contribution to the regulation of Wnt8a, using loss-of-function studies. We performed single and double morpholino knockdowns of Sfrp1a and Frzb and examined their effect on the induction of dorsal tissues, checking for chordin expression (Fig. 3 E–H). Both Sfrp1a and Frzb loss of function result in a strong increase in the extent of chordin expression at the blastula stage. Their double knockdown was even more effective, resulting in expression of chordin in all cells of the embryo at the sphere stage (Fig. 3H). This massive induction of the canonical Wnt pathway in the entire embryo was confirmed by examining the nuclear localization of β-catenin (Fig. 3 M–O). Loss of function of Sfrp1a and Frzb clearly mimics the effect of overexpression of Wnt8a (Fig. 3C). This occurs in the presence of Dkk-3, further supporting the conclusion that Dkk-3 has no or only a negligible role in the control of Wnt8a activity.
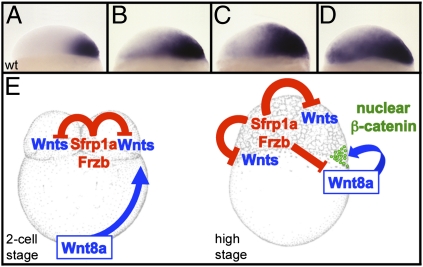
Taken together, our study demonstrates that the canonical Wnt/β-catenin signaling pathway is activated during cleavage and blastula stages by Wnt8a, which is translated from mRNAs initially located at the vegetal pole and transported, in a microtubule-dependent manner, toward what will become the future dorsal marginal blastomeres. The presence of multiple Wnts and all components of the Wnt signaling pathway in the blastomeres during the early cleavage stages does not result in Wnt pathway activity because of the negative regulation by the Wnt inhibitors Sfrp1a and Frzb. Gradual attenuation of this Wnt antagonistic activity results in a progressive extension of chordin expression domain from the dorsal margin toward the ventral animal territory (Fig. 4 A–D), showing the existence of a gradient of Wnt activity, maximal at the dorsal margin, which extends across the entire blastula. This clearly establishes that Sfrp1a and Frzb are essential for the spatial regulation of the Wnt signaling pathway, restricting the nuclear accumulation of β-catenin to the dorsalmost cells where the overall stimulation by Wnt ligands (translated both from blastomeres and from mRNAs that were transported from the vegetal pole) is maximal (Fig. 4E).
Fig. 4.
Sfrp1a and Frzb regulate the activation of the maternal Wnt/β-catenin signaling pathway. Expression pattern of chordin are shown in wild type (A) or in embryos injected with 4 ng (B), 5.5 ng (C), and 7 ng (D) of Frzb morpholinos. (E) At the two-cell stage Sfrp1a and Frzb prevent activation of the maternal Wnt pathway in the blastomeres, whereas the dorsal determinant, Wnt8a, is transported from the vegetal pole toward the blastomeres in a microtubule-dependent manner. At the high stage (immediately after the midblastula transition), Wnt8a translated from these transported mRNAs stimulates the canonical β-catenin pathway. Sfrp1a and Frzb are required to limit this activation to the cells receiving the highest level of stimulation.
Although the molecular mechanism responsible for the initial radial symmetry breaking (an mRNA encoding a Wnt ligand, asymmetrically transported along microtubules, stimulating the canonical pathway) is conserved between amphibians and fish, different regulatory factors are used for these two classes of vertebrates. In frog, the dorsal determinants are Wnt11 and Wnt5a negatively regulated by Dkk-1. In fish, the dorsal determinant is Wnt8a (a known activator of the canonical Wnt signaling pathway) negatively regulated by Sfrp1a and Frzb. Considering how essential this initial break of radial symmetry is for the establishment of the vertebrate body plan, we find these differences very surprising. Such a fundamental process would have been expected to exhibit a high degree of conservation of both the mechanisms and the specific determinants involved. This important finding requires a reconsideration of the evolutionary history of the process of dorsal specification in vertebrates.
Materials and Methods
In Situ Hybridization and Immunofluorescence.
Whole mount in situ hybridization was performed as previously described (29). Immunolocalization of β-catenin was performed as previously described (30). Confocal fluorescence images were taken using a Leica TCS-LSI confocal macroscope.
Nocodazole Treatment.
Nocodazole treatment was performed as previously described (8) by incubating embryos, dechorionated with pronase, for 2 min in a solution of 4 μg/mL nocodazole (Sigma) prepared from a stock solution of 5 mg/mL in DMSO diluted in 0.3× Danieau buffer [1× Danieau buffer: 58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, and 5.0 mM Hepes (pH 7.6), with sterile water].
mRNA and Morpholino Injections.
All injected mRNAs were transcribed from a pCS2+ plasmid digested with NotI and synthesized with the SP6 RNA polymerase kit (Ambion). Wnt8a (13) and Wnt11 (31) mRNA were injected together with GFP mRNA (100 pg) or with RFP mRNA (50 pg) into one blastomere at the 64-cell stage. As a control for the activity of Wnt11 mRNA, 400 pg was injected at the 1-cell stage. This overexpression results in cyclopia phenotypes typical for overexpression of Wnt ligands activating the noncanonical Wnt pathway (32) (Fig. S6 E and F). ORFs for Wnt2, Wnt3a, Wnt4a, Wnt4b, Wnt5a, Wnt5b, Wnt9b, Wnt10b, and Wnt11r were isolated by RT-PCR and subcloned into the pCS2+ vector. After sequence verification, mRNA for all Wnt genes was synthesized and their activities verified by overexpression through mRNA injection (injection of 10–200 pg) into embryos at the 1-cell stage.
For animal pole injections, mRNA coding for the different Wnt ligands was coinjected together with 100 pg of GFP or 50 pg of RFP mRNAs. Only embryos displaying a fluorescent clone of cells at their animal pole at the sphere stage were analyzed for the activation of the maternal β-catenin signaling pathway.
Dominant-negative forms of the zebrafish Wnt8a ORF1 and ORF2 were generated as described for the Xenopus dominant-negative X-Wnt8 (33) and 500 pg of mRNA coding for DN-Wnt8a ORF2 was injected at the one-cell stage to probe the requirement of maternal Wnt8a in the formation of the initial dorsal center (Fig. 2C and Fig. S2). Use of mRNA for the DN-Wnt8a ORF1 gives similar results but requires injection of a higher quantity (1.5 ng). Antisense morpholino oligonucleotides (GeneTools) designed for zebrafish and interfering with translation of the genes Sfrp1a (GGACAAAGATGCAAGGGACTTCATT and TGCAGTCAAAGCAACCCCTGAAAAC) and Frzb (GAGTTGATAGAAGAATGAC ATGCGG and TGTTCTGGAGACTCTAGCGCGTAAA) were injected (8 ng each) into one-cell-stage embryos during the first 20 min after laying. Control experiments demonstrating the specificity of these morpholinos (inhibition of translation of a Sfrp1a-GFP and Frzb-GFP fusion proteins) are presented in Figs. S8 and S9. Antisense morpholino oligonucleotides used to knock down Wnt8a (Fig. S2) are those antagonizing translation of ORF1 and ORF2 of Wnt8a (24). Five nanograms of each Wnt8a morpholino was injected.
For all experimental conditions (gain and loss of function) at least 50 embryos were analyzed and the typical result presented in Fig. 1 B and C, Fig. 2 B and C, Fig. 3, Fig. 4 A–D, Figs. S2–S4, Fig. S8, and Fig. S9 was observed in at least 75% of embryos.
RT-PCR Analysis.
The sequences of the oligonucleotides used in RT-PCR analysis are provided in Tables S1 and S2. Gene names are those established by the Zebrafish Nomenclature Committee (ZNC) and in use by the Zebrafish Model Organism Database (www.zfin.org).
Supplementary Material
Acknowledgments
We thank R. Bloodgood and B. Gumbiner for careful reading of the manuscript, Sandy Snyder for technical assistance, and Susan Vecchio for taking care of the fish. This work was supported by funds from the University of Virginia.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106801108/-/DCSupplemental.
References
- 1.Schneider S, Steinbeisser H, Warga RM, Hausen P. β-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- 2.Dougan ST, Warga RM, Kane DA, Schier AF, Talbot WS. The role of the zebrafish nodal-related genes squint and cyclops in patterning of mesendoderm. Development. 2003;130:1837–1851. doi: 10.1242/dev.00400. [DOI] [PubMed] [Google Scholar]
- 3.Bellipanni G, et al. Essential and opposing roles of zebrafish β-catenins in the formation of dorsal axial structures and neurectoderm. Development. 2006;133:1299–1309. doi: 10.1242/dev.02295. [DOI] [PubMed] [Google Scholar]
- 4.Kelly C, Chin AJ, Leatherman JL, Kozlowski DJ, Weinberg ES. Maternally controlled (β)-catenin-mediated signaling is required for organizer formation in the zebrafish. Development. 2000;127:3899–3911. doi: 10.1242/dev.127.18.3899. [DOI] [PubMed] [Google Scholar]
- 5.Ober EA, Schulte-Merker S. Signals from the yolk cell induce mesoderm, neuroectoderm, the trunk organizer, and the notochord in zebrafish. Dev Biol. 1999;215:167–181. doi: 10.1006/dbio.1999.9455. [DOI] [PubMed] [Google Scholar]
- 6.Jesuthasan S, Stähle U. Dynamic microtubules and specification of the zebrafish embryonic axis. Curr Biol. 1997;7:31–42. doi: 10.1016/s0960-9822(06)00025-x. [DOI] [PubMed] [Google Scholar]
- 7.Nojima H, et al. Genetic evidence for involvement of maternally derived Wnt canonical signaling in dorsal determination in zebrafish. Mech Dev. 2004;121:371–386. doi: 10.1016/j.mod.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Nojima H, et al. Syntabulin, a motor protein linker, controls dorsal determination. Development. 2010;137:923–933. doi: 10.1242/dev.046425. [DOI] [PubMed] [Google Scholar]
- 9.Weaver C, Kimelman D. Move it or lose it: Axis specification in Xenopus. Development. 2004;131:3491–3499. doi: 10.1242/dev.01284. [DOI] [PubMed] [Google Scholar]
- 10.Tao Q, et al. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Cha SW, Tadjuidje E, Tao Q, Wylie C, Heasman J. Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development. 2008;135:3719–3729. doi: 10.1242/dev.029025. [DOI] [PubMed] [Google Scholar]
- 12.Cha SW, et al. Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr Biol. 2009;19:1573–1580. doi: 10.1016/j.cub.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 13.Kelly GM, Greenstein P, Erezyilmaz DF, Moon RT. Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development. 1995;121:1787–1799. doi: 10.1242/dev.121.6.1787. [DOI] [PubMed] [Google Scholar]
- 14.Tendeng C, Houart C. Cloning and embryonic expression of five distinct sfrp genes in the zebrafish Danio rerio. Gene Expr Patterns. 2006;6:761–771. doi: 10.1016/j.modgep.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Agathon A, Thisse C, Thisse B. The molecular nature of the zebrafish tail organizer. Nature. 2003;424:448–452. doi: 10.1038/nature01822. [DOI] [PubMed] [Google Scholar]
- 16.Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–766. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- 18.Molenaar M, et al. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 19.Ku M, Melton DA. Xwnt-11: A maternally expressed Xenopus wnt gene. Development. 1993;119:1161–1173. doi: 10.1242/dev.119.4.1161. [DOI] [PubMed] [Google Scholar]
- 20.Kloc M, Etkin LD. RNA localization mechanisms in oocytes. J Cell Sci. 2005;118:269–282. doi: 10.1242/jcs.01637. [DOI] [PubMed] [Google Scholar]
- 21.Marlow F. Maternal Control of Development in Vertebrates (Colloquium Series in Developmental Biology) San Rafael, CA: Morgan and Claypool Life Sciences; 2010. [PubMed] [Google Scholar]
- 22.Fekany K, et al. The zebrafish bozozok locus encodes Dharma, a homeodomain protein essential for induction of gastrula organizer and dorsoanterior embryonic structures. Development. 1999;126:1427–1438. doi: 10.1242/dev.126.7.1427. [DOI] [PubMed] [Google Scholar]
- 23.Thorpe CJ, Weidinger G, Moon RT. Wnt/β-catenin regulation of the Sp1-related transcription factor sp5l promotes tail development in zebrafish. Development. 2005;132:1763–1772. doi: 10.1242/dev.01733. [DOI] [PubMed] [Google Scholar]
- 24.Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 25.Heisenberg CP, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 26.Matsui T, et al. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev. 2005;19:164–175. doi: 10.1101/gad.1253605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larabell CA, et al. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in β-catenin that are modulated by the Wnt signaling pathway. J Cell Biol. 1997;136:1123–1136. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu RJ, et al. Novel intronic microRNA represses zebrafish myf5 promoter activity through silencing dickkopf-3 gene. Nucleic Acids Res. 2010;38:4384–4393. doi: 10.1093/nar/gkq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 30.Lyman Gingerich J, Westfall TA, Slusarski DC, Pelegri F. hecate, a zebrafish maternal effect gene, affects dorsal organizer induction and intracellular calcium transient frequency. Dev Biol. 2005;286:427–439. doi: 10.1016/j.ydbio.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Makita R, Mizuno T, Koshida S, Kuroiwa A, Takeda H. Zebrafish wnt11: Pattern and regulation of the expression by the yolk cell and No tail activity. Mech Dev. 1998;71:165–176. doi: 10.1016/s0925-4773(98)00013-6. [DOI] [PubMed] [Google Scholar]
- 32.Ungar AR, Kelly GM, Moon RT. Wnt4 affects morphogenesis when misexpressed in the zebrafish embryo. Mech Dev. 1995;52:153–164. doi: 10.1016/0925-4773(95)00386-f. [DOI] [PubMed] [Google Scholar]
- 33.Hoppler S, Brown JD, Moon RT. Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 1996;10:2805–2817. doi: 10.1101/gad.10.21.2805. [DOI] [PubMed] [Google Scholar]
- 34.Kim CH, et al. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houart C, et al. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 2002;35:255–265. doi: 10.1016/s0896-6273(02)00751-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.