Abstract
The cerebellum has a medial, cortico-nuclear zone consisting of the cerebellar vermis and the fastigial nucleus. Functionally, this zone is concerned with whole-body posture and locomotion. The vermis classically is thought to be included within the “spinocerebellum” and to receive somatic sensory input from ascending spinal pathways. In contrast, the lateral zone of the cerebellum is included in the “cerebro-cerebellum” because it is densely interconnected with the cerebral cortex. Here we report the surprising result that a portion of the vermis receives dense input from the cerebral cortex. We injected rabies virus into lobules VB–VIIIB of the vermis and used retrograde transneuronal transport of the virus to define disynaptic inputs to it. We found that large numbers of neurons in the primary motor cortex and in several motor areas on the medial wall of the hemisphere project to the vermis. Thus, our results challenge the classical view of the vermis and indicate that it no longer should be considered as entirely isolated from the cerebral cortex. Instead, lobules VB–VIIIB represent a site where the cortical motor areas can influence descending control systems involved in the regulation of whole-body posture and locomotion. We argue that the projection from the cerebral cortex to the vermis is part of the neural substrate for anticipatory postural adjustments and speculate that dysfunction of this system may underlie some forms of dystonia.
Keywords: motor system, virus tracing
Classically, the cerebellum is divided anatomically into three cortico-nuclear zones: a medial zone consisting of the cerebellar vermis and fastigial nucleus, an intermediate zone consisting of paravermal cortex and interposed nuclei, and a lateral zone consisting of the most lateral cerebellar cortex and the dentate nucleus (1, 2). Each of these zones is considered to be functionally distinct. For example, lesions of the vermis result in deficits in whole-body posture and locomotion, whereas lesions of the lateral zone result in deficits in limb movements (2, 3). Current texts include the vermis in the “spinocerebellum” and emphasize that it receives somatic sensory inputs related to the head and proximal parts of the body from ascending spinal pathways. In contrast, the lateral zone is included in the “cerebro-cerebellum” because it is densely interconnected with the cerebral cortex (4).
We have begun to examine the inputs to the cerebellar cortex using retrograde transneuronal transport of rabies virus (RV) (5). In the course of these experiments we made the surprising observation that the primary motor cortex (M1) and several of the cortical motor areas on the medial wall of the hemisphere provide a major source of input to lobules VB–VIIIB of the vermis. Furthermore, a substantial portion of this input comes from cortical regions involved in the control of distal as well as proximal limb movements. Thus, our results challenge the classical view of the vermis and indicate that it no longer should be considered as entirely isolated from the cerebral cortex. Instead, lobules VB–VIIIB represent a site where the cortical motor areas can influence descending control systems involved in the regulation of whole-body posture and locomotion.
Results
We injected a combination of RV and the β subunit of cholera toxin (CTb) into multiple lobules of the cerebellar vermis (Figs. 1 and 2) in seven monkeys. In four of these animals, the injections were confined largely to lobules VB–VIIIB of the vermis. Our results are based largely on the data from these animals. In the remaining three animals, the injections primarily involved more posterior lobules of the vermis but also minimally involved portions of lobules VB–VIIIB. The data from these three animals are included in the composite maps presented later in the results. All tracer injections were made on or to the right of the cerebellar midline. This report focuses on cortical neurons labeled in the left hemisphere.
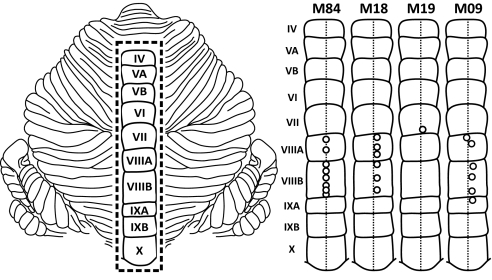
Fig. 1.
Injection sites in posterior vermis. (Left) Flattened map of the cerebellum with the posterior vermis outlined (dashed rectangle). Roman numerals indicate lobules IV–X (adapted from ref. 6). (Right) Injection sites in the posterior vermis of four animals. Open circles indicate the sites of entry for an injection needle. In animal M19, a single injection site was used for needle penetrations at different angles. All injections were made at or near the midline of the vermis (vertical dashed line).
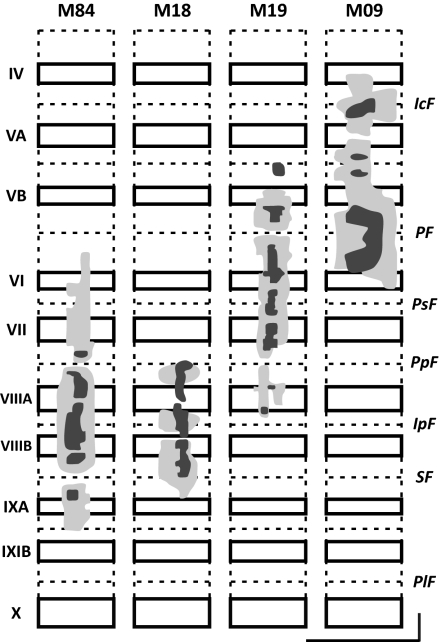
Fig. 2.
Tracer spread in lobules IV–IXA of the vermis. For each lobule, solid rectangles indicate cerebellar cortex on the exposed surface, and dashed lines indicate cerebellar cortex buried in fissures. The injection sites include a dense core (dark shading) and a lighter surround (light shading). All injections were confined to the vermis. (Vertical and horizontal scale bars: 10 mm.) IcF, intraculminate fissure; IpF, intrapyramidal fissure; PF, primary fissure; PlF, posterolateral fissure; PpF, prepyramidal fissure; PSF, posterior superior fissure; SF, secondary fissure.
We set the survival time following the virus injections at 42 h to allow for two stages of retrograde transneuronal transport (7). In the first stage of transport, virus was taken up by axon terminals of neurons at the injection site in the vermis and transported in the retrograde direction to label first-order neurons in the pons. The location of the pontine neurons labeled by retrograde transport of CTb to these first-order neurons was consistent with the results of prior studies of pontocerebellar input to the vermis (8, 9). Then, in a second stage, virus was transported transsynaptically in the retrograde direction from first-order neurons in the pons to label second-order neurons in the cerebral cortex. In fact, we found that all the cortical neurons that were labeled after virus injections into the vermis were located in layer V. The laminar location of these neurons is the same as the location of cortico-pontine neurons labeled by retrograde transport of conventional tracers from the pons (10). We did not find any labeled neurons in cortical layers above or below layer V. This observation confirms that the survival time was long enough to allow transneuronal transport of virus from the vermis to second- but not third-order neurons in the cerebral cortex.
Cerebral Cortex Projects to the Vermis.
Our major observation is that retrograde transneuronal transport of virus from lobules IV–IXA of the vermis labeled large numbers of neurons in the cerebral cortex (Figs. 3–5). The total number of neurons labeled in the cerebral cortex after retrograde transneuronal transport of virus from the vermis averaged 10,384 neurons (measured on every fourth section; range, 4,982–16,350, n = 4). In comparison, the total number of neurons labeled in the cerebral cortex after retrograde transneuronal transport of virus from comparable-sized injections into the cerebellar hemisphere averaged 14,472 neurons (range, 4,597–29,775, n = 4) (data from ref. 5). The difference in these averages is not statistically significant (unpaired student's t test). The density of labeled neurons in areas of the cerebral cortex following transneuronal transport of virus from the vermis also was comparable to that following transneuronal transport from the hemisphere. It has long been recognized that the cerebral cortex provides a major input to the hemisphere. Thus, our observations suggest that the cerebral cortex is a major source of input to the vermis.
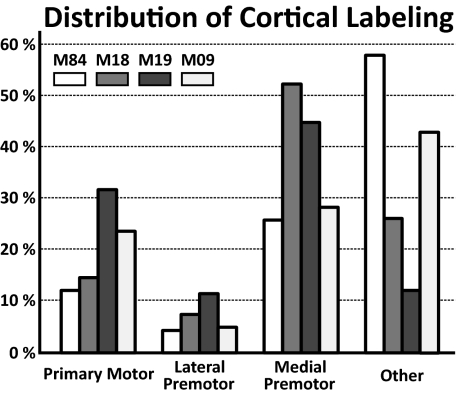
Fig. 3.
Labeled neurons in cerebral cortex. The numbers on the ordinate indicate the percentage of the total number of neurons labeled in different regions of the cerebral cortex after transport of virus from the cerebellar vermis. The shading indicates the specific experimental animal.
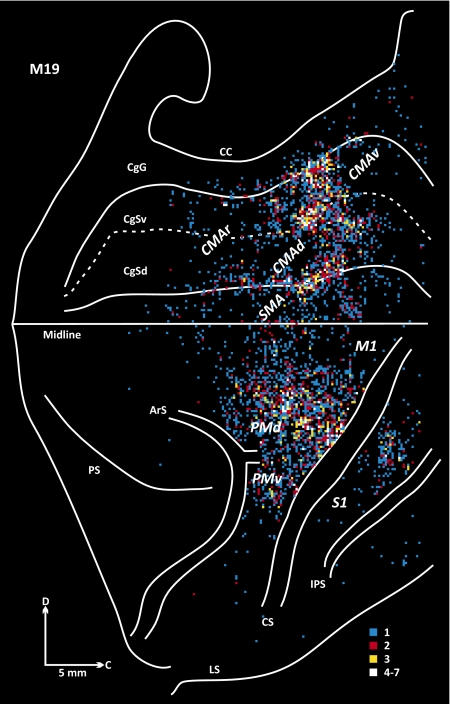
Fig. 5.
Origin of projections to lobule VI of the vermis (M19). See Fig. 4 for conventions.
Cortical Motor Areas Are a Source of Input to Lobules VB–VIIIB.
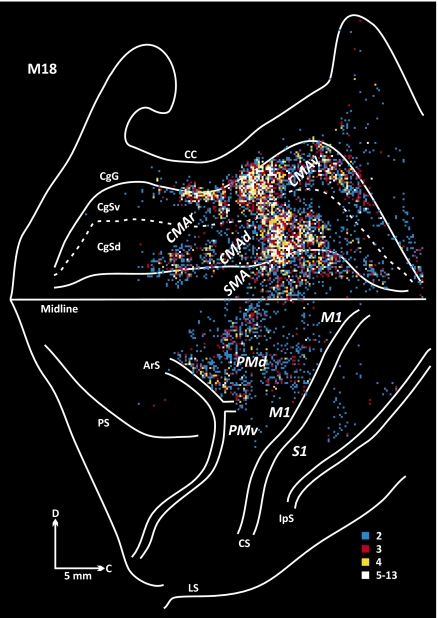
In two animals (M18 and M19), the virus injection sites involved lobules VB–VIIIB (animal M18: lobule VIIIA to VIIIB; animal M19: lobules VB–VIIIA). In these animals we found that the majority (74–88%) of the labeled neurons were located in the cortical motor areas in the frontal lobe (Figs. 3–5). The cortical motor areas include M1 and the six premotor areas that project to it (11, 12). Two of the premotor areas, the dorsal premotor area (PMd) and the ventral premotor area (PMv) lie on the lateral surface of the hemisphere. Four of the premotor areas are located on the medial wall of the hemisphere. One of these areas is the supplementary motor area (SMA) on the superior frontal gyrus. The remaining three premotor areas are located in the cingulate sulcus, either rostrally (the rostral cingulate motor area, CMAr) or more caudally on the dorsal and ventral banks of the sulcus [the dorsal cingulate motor area (CMAd) and the ventral cingulate motor area (CMAv), respectively].
In two other animals the injection site extended either rostrally (animal M09) to involve portions of lobules IV and the intraculminate fissure (between lobules IV and V) or caudally (animal M84) to involve portions of lobule IXA (Fig. 2). The cortical motor areas also contained labeled neurons in these animals. However, approximately half (42–58%) of the labeled neurons were located outside the motor areas (Fig. 3). These observations imply that the cortical input to lobules VB–VIIIB of the vermis originates mainly from the cortical motor areas.
Clearly, some of the cortical motor areas project more densely to lobules VB–VIIIB than others. Approximately half the cortical neurons labeled following transport from lobules VB–VIIIB were located in the premotor areas on the medial wall, especially in the SMA, CMAd, and CMAv (Fig. 3). In contrast, the density of labeled neurons in the two premotor areas on the lateral surface, the PMd and PMv, was relatively sparse. These two cortical areas combined contained 12% or less of the total number of labeled neurons. Thus, the three premotor areas on the medial wall appear to have a more prominent input to the vermis than the two premotor areas on the lateral surface.
Overall, the distribution of labeled neurons in the cortical motor areas varied with location of the injection site within lobules VB–VIIIB. The density of labeling in the SMA, CMAd, and CMAv was greatest in M18, the animal with the more caudally located injection site (Figs. 3–5). In contrast, the density of labeling in M1 was greatest in M19, the animal with the more rostrally located injection site. Labeled neurons were relatively dense just caudal to the superior limb of the arcuate sulcus (ArS) in animal M18 (Fig. 4), whereas labeled neurons were sparse in this region in animal M19 (Fig. 5). These observations suggest that there is a degree of topography in the organization of projections from the cortical motor areas to different portions of lobules VB–VIIIB.
Fig. 4.
Origin of projections to lobule VIII of the vermis (M18). The frontal lobe is displayed with the medial wall of the hemisphere reflected upwards. The map begins at the frontal pole and continues caudally to the intraparietal sulcus (IpS). The cingulate sulcus is unfolded to display the location of labeled neurons on its dorsal (CgSd) and ventral (CgSv) banks. Colored bins indicate the number of labeled neurons in 200-μm bins throughout the cerebral cortex. The number of cells per bin was divided into five levels that are color-coded: white, top 5% of bins (95–100%); yellow, 90–95%; red, 80–90%; and blue, 40–80%. Bins containing 0–40% of cells are omitted. ArS, arcuate sulcus; C, caudal; CC, corpus callosum; CgG, cingulate gyrus; CgSd, cingulate sulcus, dorsal bank; CgSv, cingulate sulcus, ventral bank; CMAd, dorsal cingulate motor area; CMAr, rostral cingulate motor area; CMAv, ventral cingulate motor area; CS, central sulcus; D, dorsal; IpS, intraparietal sulcus; LS, lateral sulcus; M1, primary motor cortex; PMd, dorsal premotor area, PMv, ventral premotor area; S1, primary somatosensory cortex; SMA, supplementary motor area.
Differential Origin from “Proximal” Versus “Distal” M1.
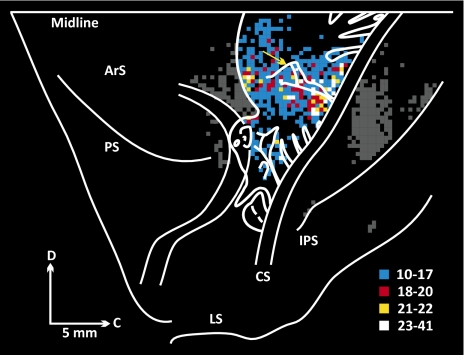
Although the density of the projection from M1 to the cerebellar vermis varied among animals, there was a consistent pattern of projections from different regions of body representation in M1. To illustrate this observation, we have overlapped the maps of labeled neurons from the seven animals with virus injections that involved lobules VB–VIIIB of the vermis (Fig. 6). Compared with the map of Woolsey et al. (13) and more modern maps (14), input to lobules VB–VIIIB of the vermis originates from regions of proximal as well as distal representation in M1. However, the density of labeled neurons was greatest in regions of proximal representation and lowest in regions of distal hand and foot representation (Fig. 6). The peak density of labeled neurons was located in the trunk representation (between the arm and leg representations) and extended into the axial body representation of M1 according to Woolsey's map (lateral to the yellow arrow in Fig. 6). This region includes a portion of the arm representation of the PMd in more current maps (11, 14) and a laterally placed region recently shown to influence muscles involved in eyelid closure (15). Overall, ∼85% of the labeled neurons were located in regions of proximal/axial representation. The proximal/axial emphasis in the origin of inputs from M1 also is apparent in the maps from individual animals (Figs. 4 and 5). These findings imply that the M1 projection to the cerebellar vermis is concerned more with the control of proximal and axial body movements than with the control of distal limb movements.
Fig. 6.
Origin of M1 projection to the posterior vermis. Composite map of labeled neurons in M1 of the seven animals. See Fig. 4 for conventions. Bin size = 400 μm. Labeled neurons in regions outside M1 have been darkened. Woolsey's simunculus is overlaid (adapted from ref. 13). The yellow arrow marks the mediolateral location of the superior precentral sulcus.
Origin from Regions of the SMA, CMAd, and CMAv.
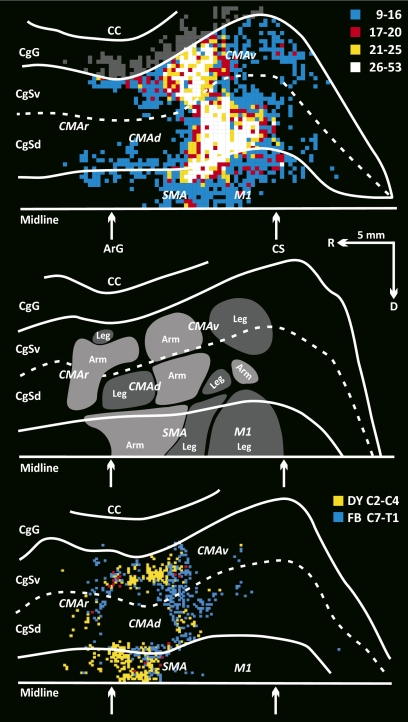
To examine the overall pattern of projections from the SMA, CMAd, and CMAv, we overlapped the maps of labeled neurons on the medial wall of the hemisphere from the seven animals with virus injections that involved lobules VB–VIIIB (Fig. 7 Top). We compared the distribution of the labeled neurons in these animals with maps of arm and leg representation in the SMA, CMAd, and CMAv (Fig. 7 Middle) (see also ref. 16). Based on maps of body representation on the medial wall, the projection from the SMA, CMAd, and CMAv to the vermis originates from regions of arm and leg representation in each cortical area (Fig. 7 Top and Middle).
Fig. 7.
Origin of CMAd, CMAv, and SMA projections to the posterior vermis. (Top) Composite map of the labeled neurons in the SMA, CMAd, and CMAv of the seven animals. See Figs. 4 and 6 for conventions. The region of densely labeled neurons (white, yellow, and red bins) overlaps portions of the arm and leg representations within the CMAd, CMAv, and SMA. (Middle) Locations of the arm and leg representations in the motor areas on the medial wall of the hemisphere (adapted from fig. 8 in ref. 12). (Bottom) Location of corticospinal neurons in the motor areas on the medial wall of the hemisphere. Bins indicate the location of neurons labeled by retrograde transport of conventional tracers from upper (yellow) and lower (blue) cervical segments of the spinal cord (adapted from fig. 13 in ref. 12).
We also compared the location of neurons on the medial wall labeled by virus transport from the vermis (Fig. 7 Top) with the location of corticospinal neurons on the medial wall labeled in our prior studies by transport of conventional tracers from upper and lower segments of the cervical spinal cord (Fig. 7 Bottom) (12). Regions containing large numbers of corticospinal neurons that project to upper segments mark the location of proximal forelimb representation, whereas regions containing large numbers of neurons that project to lower cervical segments mark the location of more distal representation (12). Based on this comparison, the vermis receives equally dense projections from regions of proximal and distal representation in the CMAd and CMAv (Fig. 7 Top and Bottom). In contrast, the vermis receives a denser projection from the caudal and ventral region of the SMA, where the distal forelimb is represented, than from the more rostral and dorsal region of the SMA, where the proximal forelimb is represented. Overall, it is clear that the premotor areas on the medial wall of the hemisphere provide the vermis with a major source of input about the control of distal as well as proximal musculature.
Discussion
We would like to emphasize three major findings from the present study. First, we provide evidence that a portion of the cerebellar vermis is a major target of projections from the cerebral cortex. Second, projections to lobules VB–VIIIB of the vermis originate mainly from motor areas in the frontal lobe and especially from M1, SMA, CMAd, and CMAv. Third, these projections arise from regions of cerebral cortex that represent distal as well as proximal body parts. These results have significant implications for the involvement of the vermis in the normal control of movement as well as its contribution to movement disorders. Before discussing these implications, we need to raise a technical concern.
The cortex of the cerebellum is extensively foliated. As a consequence, large portions of cerebellar lobules are buried within deep fissures that are not directly accessible from the surface. This configuration makes it difficult to achieve uniform and complete injections of individual lobules. It is possible that more extensive and more complete injections of lobules VB–VIIIB would reveal additional cortical inputs to this region of the cerebellum. For example, portions of lobules VI and VII have been included within the “oculomotor vermis” (17). Although it is likely that our injection sites included some of this region, we found only limited numbers of labeled neurons in either the frontal eye field or the supplementary eye field. This result may reflect limited involvement of the oculomotor vermis in our injection sites and/or limited projection from the eye fields to this region of the cerebellum. In any event, we wish to emphasize that this report is not intended as a comprehensive description of all of the inputs to the vermis. Rather our intention is to present the striking finding that lobules VB–VIIIB in the vermis receive dense, disynaptic inputs from motor areas in the frontal lobe.
Our observations clearly challenge longstanding concepts about the functional organization of the cerebellum. As noted above, the cerebellum classically has been divided into three cortico-nuclear zones: (i) a medial zone consisting of the cerebellar vermis and the fastigial nucleus; (ii) an intermediate zone consisting of paravermal cortex and the interposed nuclei; and (iii) a lateral zone consisting of the most lateral cerebellar cortex and the dentate nucleus. Current texts consider the vermis to be part of the “spino-cerebellum” and emphasize that ascending spinal pathways provide the vermis with somatic sensory information about the head and proximal parts of the body. In contrast, the lateral zone is included in the “cerebro-cerebellum” because it is densely interconnected with the cerebral cortex (4). Our results show that one portion of the vermis is the target of dense output from the cerebral cortex. In fact, the density of projections from the cerebral cortex to lobules VB–VIIIB in the vermis is as great as the density of projections to the lateral zone in the cerebellar hemisphere. The input to this portion of the vermis originates from regions of arm, leg, and proximal body representation in multiple cortical areas. These results suggest that for the control of movement the disynaptic cortical pathway to the vermis may be as important as the disynaptic cortical pathway to the hemisphere.
In general, the results from prior experiments that used conventional tracers to study cortico-pontine and ponto-cerebellar connections are consistent with our conclusions about a disynaptic connection from M1 and the SMA to lobules in the posterior vermis (8, 9, 18, 19). However, because the individual experiments with conventional tracers were performed in different animals, and the planes of sections from the different studies may not match, it is difficult to say with complete confidence that the pontine terminations seen in one experiment overlap with the pontine projection neurons found in another experiment. This limitation of studies using conventional tracers clearly illustrates the benefits of using transneuronal transport to define multisynaptic circuits. Our results also provide evidence on inputs to the vermis from the cingulate motor areas.
What is the function of the projection from the cortical motor areas to the vermis? Neurons in the motor cortex are likely to send the vermis signals related to force, speed, and direction of motor output at the time of movement execution (20). Neurons in the SMA, CMAd, and CMAv are likely to send the vermis signals related not only to the kinematics of motor output but also to earlier stages in movement processing (21–24). The signals from the SMA, CMAd, and CMAv will arrive at the vermis during the period of motor preparation, before movement onset, as well as during movement execution. Thus the outputs from the cortical motor areas are likely to provide the vermis with information about the motor commands associated with the preparation for and performance of limb and body movements.
The output of lobules VB–VIIIB is directed largely at the fastigial nucleus. A component of the output from the fastigial nucleus is directed at regions of the thalamus that may influence the same cortical areas that provide input to the vermis (25, 26). However, the major output of the fastigial nucleus is to brainstem nuclei that are the origin of descending pathways to the spinal cord (27). Multiple lines of evidence suggest that the descending component of fastigial output is involved in the control of whole-body posture and locomotion (2–4).
We believe that the input–output organization of the projection from the cortical motor areas to the vermis is precisely what would be required to trigger and modify adaptively the anticipatory postural adjustments that are associated with limb movements (28). In essence, the control of posture is not limited to responding to external disturbances but also occurs during voluntary movement. The forces generated by normal movements are large enough to cause perturbations in posture. To prevent such perturbations and to provide a stable base of support, anticipatory postural adjustments precede and accompany movement. These adjustments result from internal commands, are adaptable, and are strongly influenced by task demands (29). Signals from the SMA, CMAd, and CMAv during the period of motor preparation could provide the vermis with information about upcoming movements. This information would be essential for the generation of the anticipatory component of postural responses. The timing of anticipatory postural adjustments could be linked to early signals from the cortical motor areas at the time of movement execution. The specific role of the vermis could be to provide the adaptive plasticity necessary to modify anticipatory adjustments for the full range of motor outputs. This speculation is supported by the finding that lesions of the cortical motor areas disrupt anticipatory postural adjustments (30), and cerebellar lesions abolish their plasticity (31).
If our speculation about the function of the input to lobules VB–VIIIB of the vermis is correct, it may provide some insight into the basis of a common disorder of movement, dystonia, which recently has been associated with dysfunction of the vermis. Dystonia is a heterogeneous, involuntary movement disorder that is characterized by excessive activation of muscle groups. The overactivation leads to the development of unusual, twisting movements and abnormal postures that generally begin and/or worsen with attempted movement (32). Some have argued that dystonia is an expression of abnormal postural control mechanisms (33). Although dystonia generally is considered to be caused by dysfunction of the basal ganglia, there is increasing evidence that the cortical motor areas and the vermis of the cerebellum contribute to the genesis and maintenance of some forms of this disorder (34). Based on our findings, an abnormal signal from the cortical motor areas, an abnormal vermal response to this input, or a combination of the two could lead to a form of “action” dystonia in which attempted movement would be accompanied by excessive anticipatory postural responses. This mechanism would explain the “overflow” seen when attempted movement results in dystonic postures in body regions apparently unrelated to the intended movement (32).
In summary, our results indicate that the vermis no longer should be considered as entirely isolated from the cerebral cortex. Instead, lobules VB–VIIIB, at least, represent a site where the cortical motor areas can influence descending control systems involved in the regulation of whole-body posture and locomotion. Whether additional areas of the cerebral cortex, including nonmotor areas in the frontal lobe, influence the function of other regions of the vermis remains to be determined.
Materials and Methods
This report is based on observations from seven macaques (Macaque mulatta and fascicularis, 2.3–9.6 kg, three males and four females). In each monkey, we injected a mixture of RV and a conventional tracer (CTb) into selected lobules of the cerebellar vermis. We coinjected CTb with RV to facilitate identification of the injection site. The protocol for these experiments was approved by appropriate Institutional Animal Care and Use and Biosafety Committees. Biosafety practices conformed to Biosafety Level 2 regulations outlined in Biosafety in Microbiological and Biomedical Laboratories (US Department of Health and Human Services publication No. 93–8395). Details of the procedures for handling virus and virus-infected animals have been published previously (35).
Surgical Preparation.
All surgical procedures were performed under deep general anesthesia (1.5–2.5% isoflurane) using aseptic conditions. The night before surgery, the monkeys were administered dexamethasone (0.5 mg/kg, i.m.). Respiratory rate, blood oxygen level, body temperature, venous blood gas, serum electrolytes, glucose, and sensitivity to noxious stimuli were monitored at regular intervals during the procedure.
Cerebellar Vermis Injections.
Each monkey's head was restrained in a Kopf stereotactic frame (Kopf Instruments). We made a craniotomy to expose the posterior vermis and the paramedian lobules of the cerebellum. Prior structural MRIs of each cerebellum enabled define the angle of approach and the depth of injections into the vermis. We used a Hamilton syringe with a 30-gauge needle to inject a mixture of RV (N2c strain, 4.5 × 109 pfu/mL; provided by M. Schnell, Thomas Jefferson University, Philadelphia, PA) and CTb (0.02%; List Biological Laboratories). Small amounts of the mixture (0.2 μL) were injected at 0.5-mm intervals along needle penetrations into the cerebellum. The cerebellar fissures in the posterior vermis can extend more than 9 mm below the surface. As a consequence, substantial portions of the vermis are buried within these fissures. To reach the full depths of vermal folia with angled penetrations, we injected up to 15 mm below the cerebellar surface. In six of the seven animals, we injected tracer at multiple locations in the posterior vermis by inserting the injection needle at six to eight sites. In one animal, we injected tracer at multiple locations in the posterior vermis by varying the angle of each needle penetration made at a single entry site. When we completed the injections, we covered the cerebellum with artificial dura, closed the incision in anatomical layers, and gave an analgesic (buprenorphine) and an antibiotic (ceftriaxone) at the appropriate intervals.
Prior studies have demonstrated that RV is transported exclusively in the retrograde direction in a time-dependent fashion. The time to infect first-, second-, and third-order neurons depends on the strain of RV and its concentration. Based on prior results (5, 7), we set the survival time following the cerebellar injections to 42 h. This interval is sufficient to allow transport of the N2c strain to second-, but not third-order neurons.
Histological Procedures.
At the end of the survival time, each animal was deeply anesthetized (ketamine, 20–25 mg/kg, i.m. followed by sodium pentobarbital, 40 mg/kg, i.p.) and was perfused transcardially with a three-step procedure. The perfusates included (i) 0.1 M phosphate buffer; (ii) 10% buffered formalin; and (iii) 10% buffered formalin with 10% glycerol added. We separated the brain into a cerebellar and a cerebral cortical block and stored the blocks at 4 °C in buffered 10% formalin with 20% glycerol for 5–7 d. The cerebral cortical block extended from the frontal pole to at least 2 mm beyond the posterior extent of the central sulcus. We cut serial 50-μm frozen sections in the sagittal plane of the cerebellar block and in the coronal plane of the cerebral cortical block. Every tenth section was stained with cresyl violet to reveal cytoarchitecture. To identify virus-infected neurons, we processed at least every fourth section of the cerebral cortex as free-floating tissue according to the avidin-biotin peroxidase method (Vectastain; Vector Laboratories). Rabies antigen was detected using a monoclonal antibody directed against the rabies virus phosphoprotein (M957, diluted 1:300) (supplied by A. Wandeler, Animal Diseases Research Institute, Ottawa, ON, Canada). To identify the injection sites, we processed every fourth section of the cerebellum, as described above, to detect rabies antigen. We also processed another set of free-floating sections (every fourth section) using the avidin-biotin peroxidase method and goat anti-choleragenoid (diluted 1:10,000; List Biological Laboratories) to detect CTb. Reacted tissue sections were mounted on gelatin-coated glass slides, air dried, and coverslipped.
Analytic Procedures.
We used an Olympus BX51 microscope, (40, 100, and 200× magnification) and bright-field illumination to determine the spread of tracers at injection sites in the vermis and the location of virus-infected neurons in the cerebral cortex. We plotted the results of this analysis using a computer-based charting system (MD2; AccuStage). The system is based on linear optical encoders that are coupled to X–Y movements of the microscope stage.
Injection Sites.
We confirmed the sites of entry of the injection needle on the fixed cerebellum (Fig. 1) and on histological sections. Throughout the text we use the nomenclature of Madigan and Carpenter (36) to describe the lobules and fissures of the vermis. To define the spread of tracer, we plotted the location of CTb staining in every fourth section through the vermis. To analyze the full extent of tracer spread, we created unfolded maps of the cerebellar vermis from the charts of single sections using software generated in the laboratory (Fig. 2). This analysis confirmed that all injections were confined to the vermis.
Cortical Labeling.
We plotted the location of RV-labeled neurons in every fourth section through the cerebral cortex. The plots began at the frontal pole and extended to at least 2 mm past the posterior extent of the central sulcus. Then, we used laboratory-generated software to construct maps showing the location and density of labeled neurons in the frontal lobe. The procedures used to create these maps have been described in detail previously (12). Briefly, we marked major morphological features (e.g., surface and depths of sulci) on each charted section and drew the border between the cerebral cortex and white matter. The computer program used this information along with the location of labeled neurons to create flattened maps from regions of the cerebral cortex. To examine the density of labeled neurons, the computer program divided labeled regions into 200- or 400-μm bins, counted the number of labeled neurons in each bin, and color-coded the result for display.
Composite Maps of Cortical Labeling.
We overlapped the flattened maps of labeled neurons from individual animals using the map of animal M18 as the template. We selected animal M18 because (i) sections in this animal included the full rostro-caudal extent of the cingulate sulcus, and (ii) the pattern of sulci in animal M18 was typical of macaques. We aligned each of the maps on the medial edge of the hemisphere and warped each map to that of animal M18. The warping procedure involved an overall scale adjustment followed by a section-by-section adjustment along the medio-lateral axis using landmarks in individual sections to guide the match to sections of the template. The maps of individual animals were rebinned after warping. Then the number of labeled neurons in each of the bins was added to the corresponding bins in a composite map and color-coded for display.
Acknowledgments
We thank Dr. M. Schnell for supplying rabies virus strain N2c and Dr. A. Wandeler for supplying antibodies to rabies. We thank M. Page and M. Semcheski for developing computer programs and M. O'Malley, M. Watach, and D. Sipula for their expert technical assistance. This work was supported in part by funds from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, by National Institutes of Health Grants R01 NS24328 (to P.L.S.), R01 MH56661 (to P.L.S.), P40 RR018604 (to P.L.S.), K12 HD00850 (to K.A.C.), and K12 HD052892 (to K.A.C.), and by a grant from the Pennsylvania Department of Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Jansen J, Brodal A. Experimental studies on the intrinsic fibers of the cerebellum. II. The cortico-nuclear projection. J Comp Neurol. 1940;73:267–321. [PubMed] [Google Scholar]
- 2.Chambers WW, Sprague JM. Functional localization in the cerebellum. I. Organization in longitudinal cortico-nuclear zones and their contribution to the control of posture, both extrapyramidal and pyramidal. J Comp Neurol. 1955a;103:105–129. doi: 10.1002/cne.901030107. [DOI] [PubMed] [Google Scholar]
- 3.Chambers WW, Sprague JM. Functional localization in the cerebellum. II. Somatotopic organization in cortex and nuclei. AMA Arch Neurol Psychiatry. 1955b;74:653–680. doi: 10.1001/archneurpsyc.1955.02330180071008. [DOI] [PubMed] [Google Scholar]
- 4.Ghez C, Thach WT. The cerebellum. In: Kandel ER, Schwartz JH, Jessel TM, editors. Principles of Neural Science. New York: McGraw-Hill; 2000. [Google Scholar]
- 5.Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsell O. In: The Comparative Anatomy and Histology of the Cerebellum from Monotremes Through Apes. Jansen J, editor. Minneapolis: Univ of Minnesota; 1970. [Google Scholar]
- 7.Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 8.Brodal P. The pontocerebellar projection in the rhesus monkey: An experimental study with retrograde axonal transport of horseradish peroxidase. Neuroscience. 1979;4:193–208. doi: 10.1016/0306-4522(79)90082-4. [DOI] [PubMed] [Google Scholar]
- 9.Thielert CD, Thier P. Patterns of projections from the pontine nuclei and the nucleus reticularis tegmenti pontis to the posterior vermis in the rhesus monkey: Astudy using retrograde tracers. J Comp Neurol. 1993;337:113–126. doi: 10.1002/cne.903370108. [DOI] [PubMed] [Google Scholar]
- 10.Glickstein M, May JG, 3rd, Mercier BE. Corticopontine projection in the macaque: The distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol. 1985;235:343–359. doi: 10.1002/cne.902350306. [DOI] [PubMed] [Google Scholar]
- 11.Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav. 2002;77:677–682. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- 12.He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: Motor areas on the medial surface of the hemisphere. J Neurosci. 1995;15:3284–3306. doi: 10.1523/JNEUROSCI.15-05-03284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolsey CN, et al. Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis. 1952;30:238–264. [PubMed] [Google Scholar]
- 14.Boudrias MH, McPherson RL, Frost SB, Cheney PD. Output properties and organization of the forelimb representation of motor areas on the lateral aspect of the hemisphere in rhesus macaques. Cereb Cortex. 2010;20:169–186. doi: 10.1093/cercor/bhp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong S, DeCuypere M, Zhao Y, LeDoux MS. Cerebral cortical control of orbicularis oculi motoneurons. Brain Res. 2005;1047:177–193. doi: 10.1016/j.brainres.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 16.Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolatti G. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: An intracortical microstimulation study in the macaque monkey. J Comp Neurol. 1991;311:463–482. doi: 10.1002/cne.903110403. [DOI] [PubMed] [Google Scholar]
- 17.Noda H, Fujikado T. Topography of the oculomotor area of the cerebellar vermis in macaques as determined by microstimulation. J Neurophysiol. 1987;58:359–378. doi: 10.1152/jn.1987.58.2.359. [DOI] [PubMed] [Google Scholar]
- 18.Wiesendanger R, Wiesendanger M. Cerebello-cortical linkage in the monkey as revealed by transcellular labeling with the lectin wheat germ agglutinin conjugated to the marker horseradish peroxidase. Exp Brain Res. 1985;59:105–117. doi: 10.1007/BF00237671. [DOI] [PubMed] [Google Scholar]
- 19.Schmahmann JD, Rosene DL, Pandya DN. Motor projections to the basis pontis in rhesus monkey. J Comp Neurol. 2004;478:248–268. doi: 10.1002/cne.20286. [DOI] [PubMed] [Google Scholar]
- 20.Sergio LE, Hamel-Pâquet C, Kalaska JF. Motor cortex neural correlates of output kinematics and kinetics during isometric-force and arm-reaching tasks. J Neurophysiol. 2005;94:2353–2378. doi: 10.1152/jn.00989.2004. [DOI] [PubMed] [Google Scholar]
- 21.Okano K, Tanji J. Neuronal activities in the primate motor fields of the agranular frontal cortex preceding visually triggered and self-paced movement. Exp Brain Res. 1987;66:155–166. doi: 10.1007/BF00236211. [DOI] [PubMed] [Google Scholar]
- 22.Cadoret G, Smith AM. Input-output properties of hand-related cells in the ventral cingulate cortex in the monkey. J Neurophysiol. 1995;73:2584–2590. doi: 10.1152/jn.1995.73.6.2584. [DOI] [PubMed] [Google Scholar]
- 23.Backus DA, Ye S, Russo GS, Crutcher MD. Neural activity correlated with the preparation and execution of visually guided arm movements in the cingulate motor area of the monkey. Exp Brain Res. 2001;140:182–189. doi: 10.1007/s002210100807. [DOI] [PubMed] [Google Scholar]
- 24.Russo GS, Backus DA, Ye S, Crutcher MD. Neural activity in monkey dorsal and ventral cingulate motor areas: Comparison with the supplementary motor area. J Neurophysiol. 2002;88:2612–2629. doi: 10.1152/jn.00306.2002. [DOI] [PubMed] [Google Scholar]
- 25.Kievit J, Kuypers HGJM. Fastigial cerebellar projections to the ventrolateral nucleus of the thalamus and the organization of the descending pathways. In: Frigyes T, Rinvik E, Yahr MO, editors. Corticothalamic Projections and Sensorimotor Activities. New York: Raven; 1972. pp. 91–114. [Google Scholar]
- 26.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 27.Asanuma C, Thach WT, Jones EG. Brainstem and spinal projections of the deep cerebellar nuclei in the monkey, with observations on the brainstem projections of the dorsal column nuclei. Brain Res. 1983;286:299–322. doi: 10.1016/0165-0173(83)90017-6. [DOI] [PubMed] [Google Scholar]
- 28.Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. J Neurophysiol. 1982;47:287–302. doi: 10.1152/jn.1982.47.2.287. [DOI] [PubMed] [Google Scholar]
- 29.Massion J. Postural changes accompanying voluntary movements. Normal and pathological aspects. Hum Neurobiol. 1984;2:261–267. [PubMed] [Google Scholar]
- 30.Jacobs JV, Lou JS, Kraakevik JA, Horak FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson's disease. Neuroscience. 2009;164:877–885. doi: 10.1016/j.neuroscience.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diedrichsen J, Verstynen T, Lehman SL, Ivry RB. Cerebellar involvement in anticipating the consequences of self-produced actions during bimanual movements. J Neurophysiol. 2005;93:801–812. doi: 10.1152/jn.00662.2004. [DOI] [PubMed] [Google Scholar]
- 32.Berardelli A, et al. The pathophysiology of primary dystonia. Brain. 1998;121:1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- 33.Blood AJ. New hypotheses about postural control support the notion that all dystonias are manifestations of excessive brain postural function. Biosci Hypotheses. 2008;1:14–25. doi: 10.1016/j.bihy.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asanuma K, et al. The metabolic pathology of dopa-responsive dystonia. Ann Neurol. 2005;57:596–600. doi: 10.1002/ana.20442. [DOI] [PubMed] [Google Scholar]
- 35.Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods. 2000;103:63–71. doi: 10.1016/s0165-0270(00)00296-x. [DOI] [PubMed] [Google Scholar]
- 36.Madigan JC, Carpenter MB. Cerebellum of the Rhesus Monkey: Atlas of Lobules, Laminae and Folia, in Sections. Baltimore: University Park Press; 1971. [Google Scholar]