Abstract
Astrocytes in the hypothalamus release prostaglandin E2 (PGE2) in response to cell–cell signaling initiated by neurons and glial cells. Upon release, PGE2 stimulates the secretion of gonadotropin-releasing hormone (GnRH), the neuropeptide that controls reproduction, from hypothalamic neuroendocrine neurons. Whether this effect on GnRH secretion is accompanied by changes in the firing behavior of these neurons is unknown. Using patch-clamp recording we demonstrate that PGE2 exerts a dose-dependent postsynaptic excitatory effect on GnRH neurons. These effects are mimicked by an EP2 receptor agonist and attenuated by protein kinase A (PKA) inhibitors. The acute blockade of prostaglandin synthesis by indomethacin (INDO) or the selective inhibition of astrocyte metabolism by fluoroacetate (FA) suppresses the spontaneous firing activity of GnRH neurons in brain slices. Similarly, GnRH neuronal activity is reduced in mice with impaired astrocytic PGE2 release due to defective erbB signaling in astrocytes. These results indicate that astrocyte-to-neuron communication in the hypothalamus is essential for the activity of GnRH neurons and suggest that PGE2 acts as a gliotransmitter within the GnRH neurosecretory system.
Keywords: cyclooxygenase, glia-to-neuron signaling, luteinizing hormone-releasing hormone, preoptic region, fertility
It is increasingly clear that astrocytes play an important role in maintaining central nervous system function (1–3) and controlling key bodily processes, such as breathing (4), sleep (5), and reproduction (6). Because of their perivascular and interneuronal localization, astrocytes are well positioned to sense afferent neuronal and blood-borne signals and ideally suited for the temporal and spatial propagation of these signals (7–9). The activation of astrocytes leads to the release of gliotransmitters (8, 10) that trigger rapid responses in neighboring cells and thus contribute to the region-specific homeostatic regulation of neuronal function.
In the hypothalamus, astrocytes regulate the secretory activity of neuroendocrine neurons (11–14). A subset of such neurons secretes the decapeptide gonadotropin-releasing hormone (GnRH), which controls both the initiation of puberty and adult reproductive function. In rodents, GnRH neurons are mostly located in the preoptic region of the ventral forebrain. They project to the median eminence of the hypothalamus, where GnRH is released into the pituitary portal blood for delivery to the anterior pituitary. In the pituitary, GnRH elicits the secretion of luteinizing hormone and follicle-stimulating hormone, which stimulate gametogenesis and gonadal hormone secretion and thus support reproductive function. It is now clear that the secretory activity of GnRH neurons is controlled by both neuronal and glial input (12, 13, 15, 16). Whereas glutamate is a key neurotransmitter involved in the transsynaptic activation of GnRH neurons (17, 18), prostaglandin E2 (PGE2) mediates cell–cell communication between astrocytes and GnRH neurons (6, 19, 20). A functional connection between the two systems is demonstrated by the ability of glutamate to elicit PGE2 release from astrocytes (21–23). In the hypothalamus, the glutamate-dependent activation of PGE2 release involves astroglial erbB signaling (22), a crucial component of the cell–cell communication process used by astrocytes to facilitate neuroendocrine reproductive development and adult function (6, 24). Whether PGE2 affects the electrical activity of GnRH neurons is, however, unknown. Here we report a remarkably potent postsynaptic excitatory effect of PGE2 on GnRH neurons that requires the activation of the EP2 subclass of PGE2 receptors and of cAMP/protein kinase A (PKA)-mediated downstream signaling pathways.
Results
PGE2 Activates Adult GnRH Neurons in a Potent and Reversible Manner.
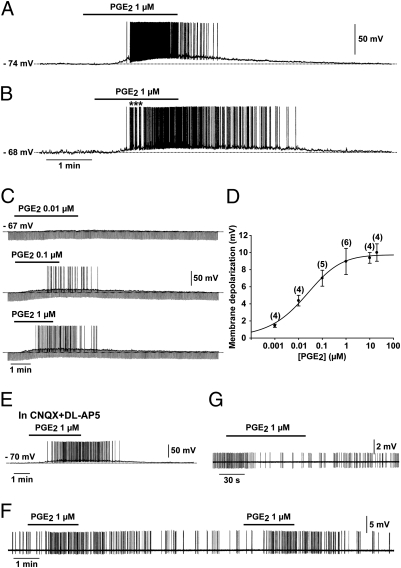
We used whole-cell patch-clamp recording to study the electrical activity of 193 GnRH neurons in brain slices containing the preoptic region from 175 adult transgenic mice (61 males and 114 females) expressing green fluorescent protein (GFP) under the control of the GnRH promoter (25). Pipette solution 1 (ps1) resulted in an average resting potential of −75.54 ± 0.38 mV (n = 151) and an input resistance of 1417.76 ± 43.07 MΩ (n = 97). In this configuration, 87% of the neurons remained silent at the resting potential (SI Results and Fig. S1). Bath application of PGE2 resulted in a striking, dose-dependent depolarizing effect on GnRH neurons (Fig. 1 A–C), with an EC50 of 0.018 μM (Fig. 1 C and D). Among all of the GnRH neurons treated with 1 μM PGE2, 78% (91 out of 116) depolarized rapidly (within 10–190 s; mean 41.00 ± 3.21 s) (Fig. 1 A–C). PGE2-induced (1 μM) depolarization had a mean amplitude of 8.95 ± 0.32 mV (n = 91) and was accompanied by the sustained generation of action potentials (Fig. 1 A–C) and a decrease in membrane resistance (Fig. 1C). Interestingly, 33 cells out of 91 responded to PGE2 with bursting activity (Fig. 1B) similar to that which occurred spontaneously in some neurons (Fig. S1 B and C) and which is thought to be essential for neuropeptide secretion from neuroendocrine cells (26). The PGE2 excitatory effect was fully reversible in 73 neurons (Fig.1 A–C), whereas 18 neurons did not recover their basal membrane potential after cessation of the PGE2 treatment. No difference in the response of GnRH neurons to PGE2 was detected between animals of different sex or stages of the estrous cycle (Table S1). Because PGE2 has previously been shown to stimulate glutamate release from astrocytes (21), we next examined the effects of PGE2 on GnRH-GFP neurons in the presence of 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 100 μM DL-2-amino-5-phosphonopentanoic acid (DL-AP5) (Materials and Methods), to block AMPA/kainate and NMDA receptors, respectively. Under these conditions, the membrane depolarization induced by 1 μM PGE2 was not altered (10.62 ± 1.20 mV, n = 4; Fig. 1E), indicating that glutamate is not an intermediary in the excitatory effects of PGE2 on GnRH neuronal activity.
Fig. 1.
PGE2 powerfully activates GnRH neurons. (A and B) Whole-cell current-clamp recordings showing the effect of bath application of PGE2 on two GnRH neurons. Note that in these two silent GnRH neurons, which had a resting membrane potential of −74 mV (A) and −68 mV (B), respectively, PGE2 induced a reversible membrane depolarization that led to the initiation of spike firing. The effect was short-lived in A and long-lasting in B. Note that in B, the spike firing started with a bursting pattern (*). (C) Whole-cell current-clamp recording of a single GnRH neuron showing that PGE2 (0.01–1 μM) depolarized the membrane in a dose-dependent manner. Note that in this cell, 0.01 μM of PGE2 did not trigger spike firing. In this and the following figures, the downward deflections correspond to voltage responses to 300 ms hyperpolarizing current pulses used to test the membrane input resistance. (D) Dose–response curve of the PGE2-induced membrane depolarization. The numbers of neurons tested at each dose is given in parentheses. Error bars indicate SEM. The EC50 for the PGE2-induced membrane depolarization was 0.018 μM, based on a logistic equation fitted to the data points. (E) The excitatory effect of PGE2 on GnRH neurons persisted in the presence of the AMPA/kainate and NMDA receptor antagonists CNQX (20 μM) and DL-AP5 (100 μM), respectively. (F) Loose patch-clamp recording showing the excitatory effect of PGE2 on a GnRH neuron, characterized by a reversible acceleration of firing. Note that the effect was reproduced by a second application of PGE2 to the same neuron. (G) Loose patch-clamp recording showing the inhibitory effect of PGE2 on a non-GnRH neuron located in the vicinity of GnRH-GFP neurons. The effect was characterized by a reversible slowing down of firing.
PGE2 maintained its potent stimulatory effect on the firing behavior of GnRH neurons recorded using the loose patch-clamp configuration (Fig. 1F). This indicates that the dilution of the intracellular compartment by the patch electrode medium in the whole-cell configuration does not alter the response of GnRH neurons to PGE2. In the loose patch-clamp configuration, GnRH neurons were spontaneously active and all responded to the addition of 1 μM PGE2 to the perfusion medium by a transient acceleration of the action potential discharge (0.51 ± 0.15 Hz before treatment vs. 1.35 ± 0.12 Hz with bath application of PGE2, t test; P < 0.05; n = 5; Fig. 1F). To investigate whether PGE2 had similarly consistent excitatory effects on the electrical activity of non-GnRH neurons in the preoptic region, 14 non-GFP–labeled neurons located near GnRH-GFP neurons were recorded. Whereas 10 neurons responded to the bath application of 1 μM PGE2 with either an acceleration (n = 6) or an inhibition (n = 4, Fig. 1G) of their firing pattern, 4 were insensitive to the prostaglandin.
PGE2 Elicits Membrane Depolarization of GnRH Neurons via a Postsynaptic Effect Involving Activation of an Inward Current.
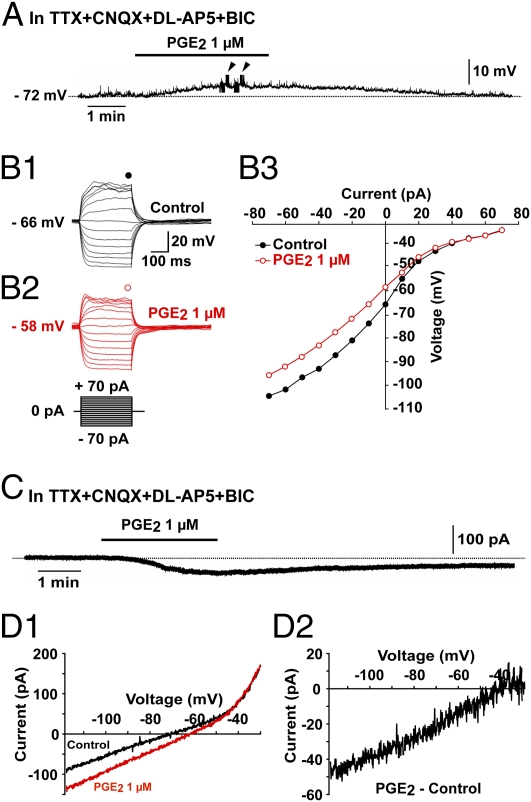
The effect of PGE2 was further investigated in the presence of tetrodotoxin (TTX; 0.5–1 μM) to block action potential-dependent synaptic transmission, in addition to the glutamate and GABAA receptor antagonists DL-AP5 (100 μM), CNQX (20 μM), and bicuculline (BIC) (20 μM) to block ionotropic receptor-mediated presynaptic inputs. Under these conditions, PGE2 (1 μM) consistently depolarized the membrane potential of GnRH neurons (Fig. 2A) with an average depolarization of 7.72 ± 0.48 mV (n = 10). The current–voltage relationship before and after applying PGE2 (1 μM) was obtained by injecting a series of square wave currents from −70 to +70 pA (Fig. 2 B1 and B2). The input resistance was calculated on the basis of the linear part of the current–voltage curve (Fig. 2 B3). PGE2 (1 μM) decreased the input resistance by 27.21 ± 2.38% (n = 10), indicating an increase in conductance. These results suggest that PGE2 triggers firing in GnRH neurons via a direct postsynaptic mechanism.
Fig. 2.
The PGE2-induced activation of GnRH neurons is direct and involves an inward current. (A) Whole-cell current-clamp recording showing that PGE2 depolarized GnRH neurons in the presence of TTX (0.5 μM), CNQX (20 μM), DL-AP5 (100 μM), and bicuculline (BIC, 20 μM). Arrowheads indicate the time of application of hyperpolarizing and depolarizing current pulses to trace current–voltage relationships. (B1–B3) Responses of a GnRH neuron to current injection from −70 pA to +70 pA before (B1, control) and during application of PGE2 (B2). The current–voltage relationship (B3) for the corresponding neuron obtained before (control) and during the application of PGE2 indicate that PGE2 decreased the slope of the linear part of the curve, indicating a decrease in the membrane input resistance. Note that the two curves converged at −40 mV in this example. (C) Whole-cell voltage-clamp recording showing that PGE2 evoked an inward current in GnRH neurons in the presence of TTX (0.5 μM), CNQX (20 μM), DL-AP5 (100 μM), and BIC (20 μM). The inward current was recorded at a holding potential of −70 mV. (D1 and D2) Current traces evoked by voltage ramps (duration, 12.5 s) from −120 mV to −30 mV at a holding potential of −70 mV in GnRH neurons. (D1) Traces showing the current responses to the voltage ramp in GnRH neurons before (control) and during application of PGE2. (D2) PGE2-induced current obtained after subtracting the control from the PGE2 curve. Note that the PGE2-induced current is suppressed at −40 mV.
To identify the conductance involved in the PGE2-induced membrane depolarization, we performed voltage-clamp recordings at a holding potential of −70 mV and observed that 1 μM PGE2 elicited an inward current of 22.92 ± 5.57 pA (n = 8) in the presence of TTX (0.5 μM), DL-AP5 (100 μM), CNQX (20 μM), and bicuculline (20 μM). This current appeared 40.00 ± 13.89 s (10–130 s, n = 8) after the initiation of PGE2 treatment and ended 193.75 ± 32.07 s (n = 8) after the removal of PGE2 from the bath solution (Fig. 2C). We then investigated the current evoked by PGE2 using a ramp voltage-clamp protocol from −120 mV to −30 mV and a holding potential of −70 mV, in the presence of TTX (0.5 μM), DL-AP5 (100 μM), CNQX (20 μM), and bicuculline (20 μM; Fig. 2 D1). PGE2 (1 μM) increased the current evoked by this protocol (Fig. 2 D1). After subtracting control values from the current recorded after applying PGE2, a PGE2-induced inward current was obtained (Fig. 2 D2). This inward current had a linear voltage dependence from −120 to −40 mV and was suppressed at a mean value of −41.12 ± 3.54 mV (n = 4; Fig. 2 D2), suggesting the activation of a nonselective cation conductance (27, 28). This value was also close to the membrane potential measured in current-clamp mode (−45.66 ± 1.68 mV, t test; P > 0.05; n = 7; Fig. 2 B3), for which the two current–voltage curves obtained in the presence and absence of PGE2 converged.
EP2 Receptors Are Expressed in GnRH Neurons and Their Activation Mimics the Effects of PGE2.
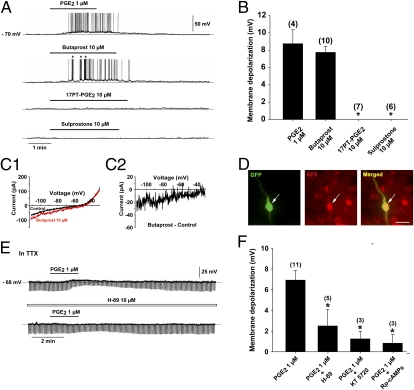
To gain insight into the nature of the receptors that mediate the potent postsynaptic excitatory action of PGE2 on GnRH neurons, we tested the effects of 17-phenyl trinor PGE2 (17PT-PGE2), sulprostone, and butaprost, which are EP1, EP1/EP3, and EP2 receptor agonists (29), respectively, on GnRH neuronal activity. The bath application of 17PT-PGE2 or sulprostone at 10 μM, a concentration previously shown to promote GnRH release in GnRH-secreting cell lines (20), had no effect on GnRH neuronal activity (Fig. 3 A and B). However, 10 μM butaprost resulted in membrane depolarization (Fig. 3 A and B). Analysis of the voltage-clamp ramps in the presence or absence of butaprost (Fig. 3 C1) revealed that this EP2 receptor agonist activated an inward current that was suppressed at a mean value of −48.33 ± 6.23 mV (n = 3, Fig. 3 C2), close to the value obtained with PGE2 (Fig. 2 D2; t test, P > 0.05). Perfusion of the slices with 30 μM AH 23848, an antagonist of the EP4 receptor (29), did not modify the response of GnRH neurons to PGE2 treatment (n = 3). Taken together, these observations strongly suggest that the excitatory effects of PGE2 on GnRH neuronal activity are mediated by EP2 receptor activation. To confirm the presence of EP2 receptors in GnRH neurons, we used immunohistochemistry. The EP2 receptor was abundantly expressed in the preoptic region; among the 128 GnRH-GFP neurons analyzed, 72 (56%) displayed EP2 receptor immunostaining (n = 4 animals) (Fig. 3D).
Fig. 3.
The PGE2-induced activation of GnRH neurons is mediated by the EP2 receptor and requires the cAMP/PKA pathway. (A) In a single GnRH neuron recorded with a whole-cell current clamp, the EP2 receptor agonist, butaprost, evoked a membrane depolarization similar to that induced by PGE2, whereas the EP1 receptor agonist 17-phenyl trinor PGE2 (17PT-PGE2) and the EP1–3 receptor agonist, sulprostone, had no effect. Note that the discharge elicited by the butaprost-induced membrane depolarization led to bursts of action potentials (*). (B) Bar graph illustrating the membrane depolarization in GnRH neurons induced by PGE2, butaprost, 17PT-PGE2, and sulprostone (*P < 0.05 compared with the membrane depolarization induced by PGE2, one-way ANOVA; n = 4–10 neurons). Error bars indicate SEM. (C1 and C2) Current traces evoked by voltage ramps (duration, 12.5 s) from −120 mV to −30 mV at a holding potential of −70 mV in GnRH neurons. (C1) Traces showing the current response to the voltage ramp in GnRH neurons before (control) and during application of butaprost, a selective EP2 receptor agonist. (C2) Butaprost-induced current obtained after subtracting the control from the butraprost curve. Note that the butraprost-induced current was suppressed at −35 mV in this example. (D) EP2 receptor immunoreactivity (red) was detected in the cell body of GnRH-GFP neurons (green, arrow). (Scale bar, 20 μm.) (E) Whole-cell current-clamp recording of a single GnRH neuron in the presence of TTX (0.5 μM) showing the effect of PGE2 in the absence (Upper) and presence (Lower) of the membrane-permeable PKA inhibitor H89. Note that H89 attenuated the membrane depolarization and the decrease in membrane input resistance (downward deflections) induced by PGE2. (F) Bar graph illustrating the membrane depolarization induced by PGE2 alone or in the presence of H89 and two other membrane-permeable compounds, the more selective PKA inhibitor KT 5720 and the cAMP antagonist Rp-cAMPs (*P < 0.05 compared with the membrane depolarization induced by PGE2 alone, one-way ANOVA; n = 3–11 neurons). Error bars indicate SEM.
PGE2-Mediated Membrane Depolarization in GnRH Neurons Requires Protein Kinase A Activation.
Because EP2 receptors are linked to the Gs-cAMP/PKA pathway (29, 30), we used PKA inhibitors to determine whether the excitatory effect of PGE2 on GnRH neurons could be inhibited or attenuated. In the presence of 0.5 μM TTX, the bath application of the PKA inhibitors H89 (10 μM, n = 5) and KT 5720 (10 μM, n = 3) or the competitive PKA antagonist Rp-cAMP (20 μM, n = 3) for 30 min significantly attenuated the stimulatory effect of PGE2 on membrane depolarization in GnRH neurons (Fig. 3 E and F). The activation of EP1 and EP3 receptors is coupled to the mobilization of intracellular calcium stores (29), but did not cause membrane depolarization in GnRH neurons (Fig. 3 A and B). Consistent with this observation, the depletion of intracellular calcium stores with thapsigargin (2 μM) did not block the depolarizing effects of PGE2 on GnRH neurons (n = 2). Taken together, our data suggest that the excitatory effects of PGE2 on GnRH neuronal activity are exerted via an EP2-Gs-cAMP/PKA signaling pathway.
Blockade of Endogenous Cyclooxygenase Activity Inhibits Spontaneous Firing of GnRH Neurons.
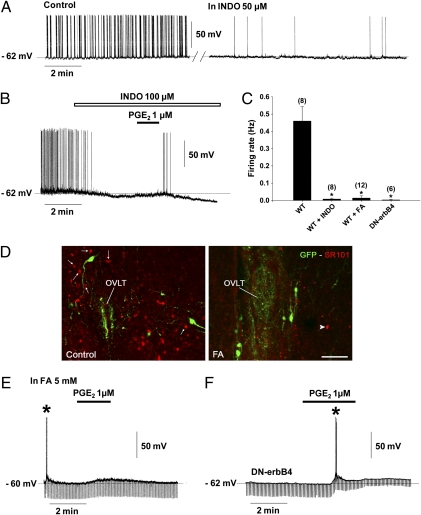
To monitor spontaneous GnRH neuronal activity, whole-cell patch-clamp recordings were performed using a pipette solution 2 (ps2) that conferred the cells with an average resting potential of −61.50 ± 0.62 mV (n = 10) and an input resistance of 1384.27 ± 73.71 MΩ (n = 10), as shown previously (31). At this resting potential, all neurons exhibited spontaneous activity with a mean discharge of 0.45 ± 0.07 Hz (n = 10). To explore the contribution of PGE2 to this spontaneous activity, we bath applied indomethacin (INDO), an inhibitor of cyclooxygenase, the rate-limiting enzyme in prostaglandin synthesis, to slices of the preoptic region during recording from GnRH neurons (Fig. 4). Bath application of this inhibitor at 50–100 μM either greatly reduced (by 95%; n = 3; Fig. 4A) or fully suppressed (n = 5; Fig. 4B) the spontaneous discharge of GnRH neurons (Fig. 4C). These effects were either reversible (n = 2) or irreversible (n = 6). Lower concentrations of indomethacin (5–10 μM) had no effect on GnRH neuronal activity, except in the case of one cell that exhibited a reversible reduction of its basal firing rate by 57%. At 100 μM indomethacin, the suppression of firing was accompanied by a membrane hyperpolarization (4.5 ± 0.6 mV, n = 6) (Fig. 4B). However, the bath application of PGE2 (1 μM) remained capable of triggering membrane depolarization (8.12 ± 2.39 mV) and action potentials (n = 4) (Fig. 4B). These experiments suggest that the endogenous production of PGE2 contributes to the maintenance of the activity of GnRH neurons in the preoptic region.
Fig. 4.
Astrocytic prostaglandin production sustains the electrical activity of GnRH neurons. Recordings of GnRH neurons were performed under whole-cell current-clamp using pipette solution 2 (ps2) (Materials and Methods) to obtain a background of spontaneous activity from GnRH neurons. (A) GnRH neurons showed spontaneous activity (control), which was reduced after 10 min of perfusion with indomethacin (INDO; 50 μM), an inhibitor of the cyclooxygenases, enzymes responsible for prostaglandin production. (B) INDO at a higher concentration (100 μM) strongly attenuated the spontaneous activity of GnRH neurons, accompanied by membrane hyperpolarization. Note that PGE2 reversed the inhibitory effect of INDO. (C) Bar graph illustrating the firing rate of GnRH neurons recorded in brain slices from wild-type mice (WT) exposed to INDO or fluoroacetate (FA) and from DN-erbB4 mice. (*P < 0.05 compared with the firing rate of GnRH neurons recorded in brain slices from WT mice, one-way ANOVA; n = 6–12 neurons). Error bars indicate SEM. (D) Pretreatment of brain slices with fluoroacetate (FA, 5 mM, 60–120 min), a glial toxin, impaired the astrocytic uptake of sulforhodamine101 (SR101, red). Arrows show cells that took up SR101 in the vicinity of GnRH neurons (GFP, green) under control conditions (Left). After FA treatment, very few cells were labeled with SR101 (Right, arrowhead). Images were acquired at the level of the organum vasculosum of the lamina terminalis (OVLT). (Scale bar, 100 μm.) (E) FA strongly reduced the firing rate of GnRH neurons. In this example, the slice was pretreated with FA for 60 min and action potentials could be driven by a brief injection of a depolarizing current (*). Note that PGE2 retained its depolarizing effect. (F) In DN-erbB4 mice, in which astrocytic PGE2 release is diminished, most GnRH neurons were silent. In this recording, action potentials could be driven by a brief injection of depolarizing current (*). Note that PGE2 retained its depolarizing effect and reduced the membrane input resistance (downward deflections).
Astrocytic PGE2 Regulates GnRH Neuronal Activity in Situ.
Hypothalamic astrocytes are a source of PGE2 (19), which is required for the normal release of GnRH (6, 24). To determine whether astrocytes contribute to the regulation of GnRH neuronal firing, we pretreated hypothalamic slices with the glial toxin fluoroacetate (5 mM; 60–120 min) (2, 32–34). Fluoroacetate significantly impaired astrocyte function as assessed by the inability of astrocytes to take up sulforhodamine 101 (SR101) (Fig. 4D), a fluorescent dye that is selectively taken up by astrocytes both in vivo (35) and in living brain slices (36). The number of astrocytes capable of taking up the dye in the vicinity of GnRH neurons was reduced by 78 ± 1% compared with untreated slices (n = 4; t test, P < 0.01). The spontaneous firing of GnRH neurons (detected using ps2 conditions) was strikingly reduced in fluoroacetate-treated slices (9 out of 12) (Fig. 4 C and E). The remaining neurons (3 out of 12) either exhibited a sporadic pattern of action potential discharge (0.01 Hz, n = 2) or fired in repetitive bursts (0.15 Hz, n = 1). In contrast, fluoroacetate treatment did not affect the basal membrane properties of GnRH neurons (resting potential of −74.29 ± 1.44 mV and input resistance of 1123.50 ± 130.18 MΩ in ps1, n = 7; t test for treated vs. control slices; P > 0.05). Importantly, in most GnRH neurons, fluoroacetate treatment did not affect the membrane depolarizing effect of PGE2 (1 μM; 6 out of 9 neurons; 7.92 ± 0.42 mV; Fig. 4E). The treatment also failed to affect the latency of the membrane depolarization upon initiation of PGE2 treatment (50.00 ± 13.36 s in artificial cerebrospinal fluid (ACSF) vs. 31.25 ± 6.10 s in FA, n = 8 for each treatment, t test, P > 0.05), the duration of the effect (234.28 ± 36.70 s in ACSF vs. 377.14 ± 74.08 s in FA, n = 7 for each treatment, t test, P > 0.05) or the ability of the prostaglandin to trigger action potentials (5 out of 6 neurons, Fig. S2).
We next sought to investigate the firing activity of GnRH neurons in mice with deficient astrocytic PGE2 production. We used transgenic mice expressing a dominant-negative form of the erbB4 receptor (DN-erbB4) specifically targeted to astrocytes by means of the human GFAP promoter (6). This mutated receptor blocks the ligand-dependent activation of erbB2 and erbB4 receptors without affecting signaling through other receptors, such as erbB1 or Notch1 (6, 37). GnRH secretion is deficient in GFAP–DN-erbB4 mice due to the inability of astrocytes to respond to erbB4 activation by producing PGE2 (6). To visualize GnRH neurons in these animals we crossed GFAP–DN-erbB4 mice with GnRH-GFP animals. GnRH neurons recorded from four double-transgenic mice under ps2 conditions were either silent (n = 5; Fig. 4F) or displayed a sporadic low-frequency (0.02 Hz) pattern of action potential discharge (n = 1), in contrast to GnRH-GFP neurons recorded from wild-type controls (Fig. 4C). These results suggest that the excitability of GnRH neurons is reduced in GFAP–DN-erbB4 mice due to the failure of PGE2 production by erbB4-deficient astrocytes. We tested this assumption by determining whether exogenous PGE2 could restore GnRH membrane depolarization in GFAP–DN-erbB4 mice. The bath application of PGE2 (1 μM) elicited membrane depolarization equally strongly in hypothalamic slices from wild-type and GFAP–DN-erbB4 mice (8.67 ± 1.32 mV in DN-erbB4 mutant mice vs. 8.72 ± 067 mV in wild-type controls, n = 6 and 10, respectively, t test, P > 0.05) (Fig. 4F).
Discussion
The disruption of astrocytic erbB4 receptor signaling by the overexpression of a dominant-negative erbB4 receptor leads to diminished astrocytic PGE2 release in response to ligand-dependent erbB4 activation, leading in turn to reduced GnRH release, delayed puberty, and disrupted adult reproductive function (6, 24). The present results show that GnRH neuronal activity is decreased in these animals and that this deficiency is mimicked by the bath application of either fluoroacetate, an inhibitor of astrocyte metabolism (2, 32, 34), or the cyclooxygenase blocker indomethacin, to slices of the preoptic region from wild-type animals. Our findings that GnRH neurons respond to PGE2 with an enhancement of the firing rate and that PGE2 rescues GnRH neuronal activity in brain slices with reduced astrocytic PGE2 output indicate that glial PGE2 is an important component of the homeostatic mechanism controlling GnRH neuronal activity.
The presence of EP2 receptors in GnRH neurons (ref. 38 and present study) and the finding that the selective EP2 receptor agonist butaprost mimics the effect of PGE2 suggest that the excitatory effect of PGE2 on GnRH neuronal activity involves the activation of EP2 receptors. This inference is supported by an earlier report showing that the stimulation of GnRH-producing GT1–7 cells with butaprost results in GnRH release (20). In addition, the attenuation of the effect of PGE2 by the bath application of PKA inhibitors or biologically inactive cAMP is consistent with earlier findings demonstrating the coupling of the EP2 receptor to the cAMP/PKA pathway (29) and the involvement of cAMP in the intracellular mechanism underlying the stimulatory effect of PGE2 on GnRH secretion (39). A previous study has reported that native GnRH neurons also express EP1 receptors (20), which are coupled to the mobilization of intracellular calcium stores (29) and to GnRH release (20). Our electrophysiological data show that the EP1 receptor agonists 17PT-PGE2 and sulprostone had no effect on GnRH neuronal activity and that the depletion of intracellular calcium stores with thapsigargin did not alter the depolarizing response of GnRH neurons to PGE2. These results, together with earlier findings demonstrating that PGE2-induced GnRH release from median eminence explants requires the mobilization of intracellular calcium stores (40), suggest that the EP1 receptor-dependent stimulation of GnRH release is exerted at the level of GnRH nerve terminals rather than GnRH cell bodies. Thus, PGE2 may stimulate both GnRH neuron firing and GnRH release by acting at the cell soma and nerve terminal levels, respectively.
An intriguing finding in this study is that the cyclooxygensase (COX) inhibitor indomethacin failed to affect spontaneous GnRH neuronal activity at doses lower than 50 μM, which is >1,700 times the EC50 of COX-1 (0.028 μM), but only ∼25 times the EC50 of COX-2 (1.68 μM) (41). This finding suggests that eicosanoids sustaining spontaneous GnRH neuronal activity are mostly COX-2–derived products, a conclusion consistent with evidence that COX-2 is the most abundant COX form expressed in astrocytes (42, 43). Because endocannabinoids, such as 2-arachidonoyl glycerol (2-AG) and arachidonoyl ethanolamide (AEA or anandamide) were recently shown to be substrates for COX-2 (44) and because endocannabinoids modulate GABAergic excitatory inputs to GnRH neurons (45), we cannot rule out that part of the effects of indomethacin on the spontaneous activity of GnRH neurons may be due to inhibition of endocannabinoid oxygenation by COX-2 (46).
Equally intriguing is the apparent lack of sex and estrous cycle effects of PGE2 on GnRH neuronal activity. Previous studies have shown that GnRH firing activity is modified by estradiol in a diurnal-dependent manner (47), and that COX-2 expression in the preoptic region is greater in males than females (48). Perhaps the lack of changes in GnRH firing activity is related to the fact that all recordings were made at the same time of day and using a saturating dose of PGE2. In addition to its postsynaptic effect on GnRH neurons, PGE2 may also modulate the activity of steroid-sensitive afferent neuronal populations (49), including kisspeptin neurons that ensure coordinated progression of neuroendocrine events sustaining ovulatory cyclicity (50). The recent development of a mouse model that enables identification of kisspeptin neurons using fluorescent reporter genes (51) now renders these neurons amenable to scrutiny.
PGE2 has long been known to play a role in the control of GnRH neuronal function (52, 53). More recent work points to PGE2 as a mediator of astrocyte-to-GnRH-neuron communication initiated by the activation of erbB signaling in astrocytes (6, 19, 24, 54). Astrocytes, which are sophisticated sensors of neuronal activity (7, 8), release PGE2 at active synapses in response to glutamate (21, 23, 55). Interestingly, a ligand-dependent increase in erbB signaling appears to mediate glutamate-stimulated PGE2 release in hypothalamic astrocytes (22). Within the hypothalamus, both glutamate and glial erbB signaling play physiological roles in promoting GnRH release at the onset of puberty (6, 18, 54, 56) and during adult reproductive life (17, 24). The present results identify PGE2 as a potent excitatory regulator of GnRH neuronal activity and indicate that the production of PGE2 by astroglial cells plays a hitherto unappreciated role in the homeostatic regulation of GnRH neuronal excitability.
Materials and Methods
Animals.
Electrophysiological recordings were performed on adult GnRH-GFP and GFAP–DN-erbB4/GnRH-GFP transgenic mice. The generation of GFAP–DN-erbB4 mice has been described previously (6).
Electrophysiological Experiments.
GnRH-GFP neurons were recorded as described in a previous study (31). Pipette solution 1 (ps1) contained (in mM) K-gluconate, 125; Hepes, 10; CaCl2, 1; MgCl2, 1; ATP-Mg, 2; EGTA, 11; GTP, 0.3; and NaCl, 15 (pH 7.3 with KOH; osmolarity, 270–280 mOsm). Pipette solution 2 (ps2) contained (in mM): K-gluconate, 140; Hepes, 10; ATP-Mg, 2; EGTA, 1; and KCl, 10 (pH 7.3 with KOH; osmolarity, 270–290 mOsm). Drugs were applied to the perfusing system (bath application) to obtain the final concentrations indicated. The drugs used were: PGE2, butaprost, sulprostone, 17-phenyl trinor PGE2 (Cayman Chemical), CNQX, DL-AP5, TTX, thapsigargin (Tocris), sodium fluoroacetate, indomethacin, AH 23848, H-89, KT 5720, Rp-cAMPs, and bicuculline methiodide (Sigma). Detailed methods are provided in SI Materials and Methods.
Fluorescent Staining.
Detection of EP2 receptor (rabbit polyclonal antibody, Cayman Chemical) and SR101 uptake were performed using protocols described previously in refs. 6 and 36, respectively. Detailed methods are provided in SI Materials and Methods.
Data Analysis.
Statistical analysis was performed using Sigma-Stat software (Jandel). Differences between several groups were analyzed by one-way ANOVA, followed by a Student-Newman-Keuls multiple comparison test. The Student t test was used to compare two groups. The data points of the dose–response curve were fitted with a four-parameter logistic curve using Sigma Plot 2001 (SPSS). A P value of <0.05 was considered to indicate a statistically significant difference. Values are reported as the mean ± SEM.
Supplementary Material
Acknowledgments
We thank Julien Devassine (animal facility, Institut Fédératif de Recherche, IFR 114) for his brilliant management of our mouse colony. This research was supported by the Agence National pour la Recherche Grants ANR-07-NEURO-026-03 and ANR-09-BLAN-0267, the Fondation pour la Recherche Médicale (equipe FRM 2005), and the IFR 114 electrophysiological platform (to V.P.), in addition to National Institutes of Health Grants HD25123 and RR000163 for the operation of the Oregon National Primate Research Center (to S.R.O.). J.C. was supported by a doctoral fellowship from the Institut National de la Santé et de la Recherche Médicale and the Région Nord Pas de Calais.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107533108/-/DCSupplemental.
References
- 1.Gordon GR, et al. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64:391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panatier A, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 4.Gourine AV, et al. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halassa MM, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prevot V, et al. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci. 2003;23:230–239. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barres BA. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 9.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 10.Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Brain Res Rev. 2010;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatton GI. Function-related plasticity in hypothalamus. Annu Rev Neurosci. 1997;20:375–397. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- 12.Ojeda SR, et al. Glia-to-neuron signaling and the neuroendocrine control of female puberty. Ann Med. 2003;35:244–255. doi: 10.1080/07853890310005164. [DOI] [PubMed] [Google Scholar]
- 13.Prevot V, et al. Function-related structural plasticity of the GnRH system: A role for neuronal-glial-endothelial interactions. Front Neuroendocrinol. 2010;31:241–258. doi: 10.1016/j.yfrne.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- 15.Herbison AE, Neill JD. Physiology of the gonadotropin-releasing hormone neuronal network. In: Knobil E, Neill JD, editors. Knobil and Neill's Physiology of Reproduction. 3rd Ed. New York: Elsevier; 2006. pp. 1415–1482. [Google Scholar]
- 16.Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 17.Brann DW, Mahesh VB. Excitatory amino acids: evidence for a role in the control of reproduction and anterior pituitary hormone secretion. Endocr Rev. 1997;18:678–700. doi: 10.1210/edrv.18.5.0311. [DOI] [PubMed] [Google Scholar]
- 18.Urbanski HF, Ojeda SR. A role for N-methyl-D-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology. 1990;126:1774–1776. doi: 10.1210/endo-126-3-1774. [DOI] [PubMed] [Google Scholar]
- 19.Ma YJ, Berg-von der Emde K, Rage F, Wetsel WC, Ojeda SR. Hypothalamic astrocytes respond to transforming growth factor-alpha with the secretion of neuroactive substances that stimulate the release of luteinizing hormone-releasing hormone. Endocrinology. 1997;138:19–25. doi: 10.1210/endo.138.1.4863. [DOI] [PubMed] [Google Scholar]
- 20.Rage F, Lee BJ, Ma YJ, Ojeda SR. Estradiol enhances prostaglandin E2 receptor gene expression in luteinizing hormone-releasing hormone (LHRH) neurons and facilitates the LHRH response to PGE2 by activating a glia-to-neuron signaling pathway. J Neurosci. 1997;17:9145–9156. doi: 10.1523/JNEUROSCI.17-23-09145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bezzi P, et al. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 22.Dziedzic B, et al. Neuron-to-glia signaling mediated by excitatory amino acid receptors regulates ErbB receptor function in astroglial cells of the neuroendocrine brain. J Neurosci. 2003;23:915–926. doi: 10.1523/JNEUROSCI.23-03-00915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zonta M, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 24.Prevot V, Lomniczi A, Corfas G, Ojeda SR. erbB-1 and erbB-4 receptors act in concert to facilitate female sexual development and mature reproductive function. Endocrinology. 2005;146:1465–1472. doi: 10.1210/en.2004-1146. [DOI] [PubMed] [Google Scholar]
- 25.Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng G, Brown D. The origins and significance of pulsatility in hormone secretion from the pituitary. J Neuroendocrinol. 1997;9:493–513. doi: 10.1046/j.1365-2826.1997.00615.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roland AV, Moenter SM. Glucosensing by GnRH neurons: Inhibition by androgens and involvement of AMP-activated protein kinase. Mol Endocrinol. 2011;25:847–858. doi: 10.1210/me.2010-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: Properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 30.Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clasadonte J, Poulain P, Beauvillain JC, Prevot V. Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: A possible local synchronizing signal. Endocrinology. 2008;149:587–596. doi: 10.1210/en.2007-1260. [DOI] [PubMed] [Google Scholar]
- 32.Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia. 1997;21:106–113. [PubMed] [Google Scholar]
- 33.Okada-Ogawa A, et al. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J Neurosci. 2009;29:11161–11171. doi: 10.1523/JNEUROSCI.3365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsons MP, Hirasawa M. ATP-sensitive potassium channel-mediated lactate effect on orexin neurons: Implications for brain energetics during arousal. J Neurosci. 2010;30:8061–8070. doi: 10.1523/JNEUROSCI.5741-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods. 2004;1:31–37. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- 36.Kafitz KW, Meier SD, Stephan J, Rose CR. Developmental profile and properties of sulforhodamine 101—Labeled glial cells in acute brain slices of rat hippocampus. J Neurosci Methods. 2008;169:84–92. doi: 10.1016/j.jneumeth.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Patten BA, Sardi SP, Koirala S, Nakafuku M, Corfas G. Notch1 signaling regulates radial glia differentiation through multiple transcriptional mechanisms. J Neurosci. 2006;26:3102–3108. doi: 10.1523/JNEUROSCI.4829-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jasoni CL, Todman MG, Han SK, Herbison AE. Expression of mRNAs encoding receptors that mediate stress signals in gonadotropin-releasing hormone neurons of the mouse. Neuroendocrinology. 2005;82:320–328. doi: 10.1159/000093155. [DOI] [PubMed] [Google Scholar]
- 39.Ojeda SR, Urbanski HF, Katz KH, Costa ME. Stimulation of cyclic adenosine 3′,5′-monophosphate production enhances hypothalamic luteinizing hormone-releasing hormone release without increasing prostaglandin E2 synthesis: Studies in prepubertal female rats. Endocrinology. 1985;117:1175–1178. doi: 10.1210/endo-117-3-1175. [DOI] [PubMed] [Google Scholar]
- 40.Ojeda SR, Negro-Vilar A. Prostaglandin E2-induced luteinizing hormone-releasing hormone release involves mobilization of intracellular Ca+2. Endocrinology. 1985;116:1763–1770. doi: 10.1210/endo-116-5-1763. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koyama Y, et al. Endothelins stimulate expression of cyclooxygenase 2 in rat cultured astrocytes. J Neurochem. 1999;73:1004–1011. doi: 10.1046/j.1471-4159.1999.0731004.x. [DOI] [PubMed] [Google Scholar]
- 43.Luo J, Lang JA, Miller MW. Transforming growth factor beta1 regulates the expression of cyclooxygenase in cultured cortical astrocytes and neurons. J Neurochem. 1998;71:526–534. doi: 10.1046/j.1471-4159.1998.71020526.x. [DOI] [PubMed] [Google Scholar]
- 44.Yang H, Chen C. Cyclooxygenase-2 in synaptic signaling. Curr Pharm Des. 2008;14:1443–1451. doi: 10.2174/138161208784480144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farkas I, et al. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:5818–5829. doi: 10.1210/en.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prusakiewicz JJ, Duggan KC, Rouzer CA, Marnett LJ. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochemistry. 2009;48:7353–7355. doi: 10.1021/bi900999z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA. 2005;102:15682–15687. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- 49.Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayer C, et al. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107:22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cravo RM, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ojeda SR, Harms PG, McCann SM. Effect of inhibitors of prostaglandin synthesis on gonadotropin release in the rat. Endocrinology. 1975;97:843–854. doi: 10.1210/endo-97-4-843. [DOI] [PubMed] [Google Scholar]
- 53.Ojeda SR, Aguado LI. Prostaglandins in reproductive neuroendocrinology. In: Steger RW, Johns A, editors. Handbook of Pharmacologic Methodologies for the Study of the Neuroendocrine System. Boca Raton, FL: CRC; 1985. pp. 205–243. [Google Scholar]
- 54.Ma YJ, et al. Neuregulins signaling via a glial erbB-2-erbB-4 receptor complex contribute to the neuroendocrine control of mammalian sexual development. J Neurosci. 1999;19:9913–9927. doi: 10.1523/JNEUROSCI.19-22-09913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zonta M, et al. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J Physiol. 2003;553:407–414. doi: 10.1113/jphysiol.2003.046706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ojeda SR, Urbanski HF, Costa ME, Hill DF, Moholt-Siebert M. Involvement of transforming growth factor alpha in the release of luteinizing hormone-releasing hormone from the developing female hypothalamus. Proc Natl Acad Sci USA. 1990;87:9698–9702. doi: 10.1073/pnas.87.24.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.