Abstract
Recent studies have demonstrated a simple, potentially universal strategy to enhance vaccine potency, via intralymph node (i.LN) injection. To date, intranodal immunization studies have focused on the delivery of unadjuvanted vaccines (e.g., naked DNA, peptide, or protein). We hypothesized that combining i.LN vaccination with controlled release biomaterials permitting sustained dosing of molecular adjuvants to the local tissue microenvironment would further enhance this promising vaccination strategy. To test this idea, we encapsulated the Toll-like receptor-3 ligand poly(inosinic:cytidylic acid) (polyIC) in biodegradable poly(lactide-co-glycolide) microparticles (MPs) designed to remain extracellular and release polyIC in the LN over several days. Intranodal injection of MPs increased persistence of polyIC in LNs compared to the same dose of soluble polyIC or polyIC formulated in nanoparticles, leading to increased accumulation of Toll-like receptor agonist in LN-resident antigen presenting cells and more enduring dendritic cell activation. Intralymph node injection of ovalbumin mixed with polyIC-releasing MPs enhanced the humoral response and expanded ovalbumin-specific T cells to frequencies as high as 18% among all CD8+ cells following a single injection (8.2-fold greater than the same vaccine given i.m.), a response that could not be matched by antigen mixed with polyIC-loaded nanoparticles or a 10-fold greater dose of soluble polyIC. Thus, i.LN immunization with slow release-formulated adjuvants may be a broadly applicable strategy to enhance therapeutic or prophylactic vaccines.
Keywords: drug delivery, immunology, vaccine adjuvants, inflammation
Compared to live attenuated pathogens, vaccines based on nonreplicating viral/bacterial vectors or subunit antigens offer enhanced safety but elicit weaker, less durable immune responses. Thus, enhancing the potency of these vector/subunit vaccines without sacrificing their excellent safety profile is a central focus of vaccine development. One strategy to enhance vaccine potency is to improve the delivery of antigen and adjuvant molecules to critical antigen presenting cells (APCs) in secondary lymphoid organs. In the “geographic” view of immunity, antigen which does not reach these sites for induction of primary immune responses is ignored by the immune system (1). Following traditional vaccine injection in peripheral tissues, soluble proteins or small particles (< 50 nm) drain directly to lymph nodes (LNs), while cell-associated antigen or larger antigen particles access LNs by APC uptake and trafficking (2–4). Recently, intralymph node (i.LN) vaccination—injection of antigens/adjuvants directly into LNs—has shown great promise for vaccine delivery, improving the potency of DNA, RNA, peptide, protein, and dendritic cell-based vaccines (2). Studies have demonstrated as much as 106-fold reductions in antigen dose (5, 6), 100-fold reductions in adjuvant dose (7), and enhanced protection with reduced side effects relative to traditional parenteral immunizations (2). Lymph node injection in humans is readily performed using ultrasound guidance, and the promise of i.LN vaccination has been demonstrated in recent clinical trials (2, 8, 9).
Intranodal injection concentrates vaccine components at the tissue site where naïve T and B lymphocytes are primed, but this beneficial colocalization is short-lived due to rapid flushing of LNs by afferent lymph (10). Too-rapid clearance of the vaccine may limit the quality and duration of immune memory generated by vaccines, as sustained antigen/inflammation over several days appears to maximize adaptive immune responses (11–13). Flushing of extracellular vaccine components from the LN may not limit the exposure of antigen-specific lymphocytes to antigen because nucleic acid vaccines transfect cells on injection (leading to sustained local antigen production) and subunit antigens are internalized by APCs and B cells for subsequent processing/presentation over several days (14, 15). However, vaccine clearance might particularly limit the effectiveness of molecular adjuvants because inflammatory signals [such as Toll-like receptor (TLR) a] are most effective when provided continuously for several days (13, 16, 17).
We hypothesized that combining i.LN vaccination with controlled release biomaterials for adjuvant delivery could allow the inflammatory milieu of LNs to be tailored over defined periods. To test this idea, we employed nonsurgical LN injection of antigen mixed with biodegradable poly(lactide-co-glycolide) microparticles (MPs) or nanoparticles (NPs) encapsulating the TLR3 agonist poly(inosinic:cytidylic acid) (polyIC), a potent molecular adjuvant known to promote CD4+ T-cell responses and drive cross-presentation of soluble antigen to CD8+ T cells (18). Intranodally injected polyIC-loaded MPs (MP-polyIC) mediated sustained polyIC persistence in LNs compared with soluble adjuvant or polyIC-loaded NPs (NP-polyIC), releasing polyIC over several days, increasing adjuvant uptake by LN APCs and prolonging DC activation. When coinjected with the model antigen ovalbumin (OVA), i.LN polyIC-MP elicited serum anti-OVA IgG titers and robust CD8+ T-cell priming following a single immunization that were greatly elevated relative to the same vaccine given parenterally, and unmatched by i.LN injection of NP-polyIC, or by soluble antigen/TLRa even with 10-fold greater doses of polyIC. The increased T-cell frequency observed with MP-polyIC was also long-lasting and conferred an enhanced antibody response after boosting with peripheral injections of soluble antigen/adjuvant. Because LN vaccination is clinically feasible in humans, this approach could provide a broadly applicable route for functionally enhancing vaccination, offering increased potency and reduced systemic exposure to potent adjuvant molecules compared with i.LN injection of soluble vaccines or slow-release vaccine formulations injected peripherally.
Results
Direct i.LN Injection Permits Targeted Delivery of Particulate Biomaterials to LNs.
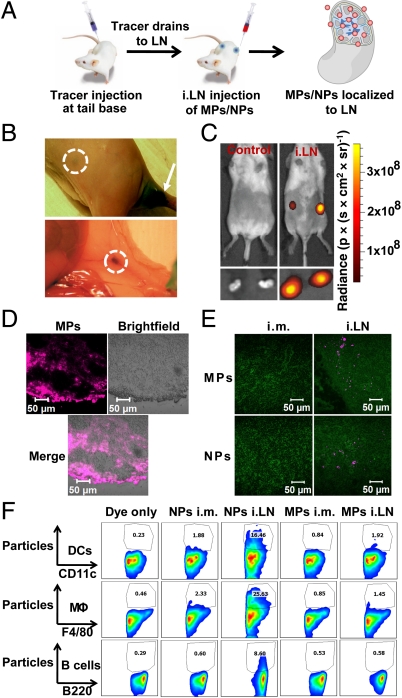
To facilitate studies in small-animal models, we developed a nonsurgical approach for intra-LN injection based on marking LNs with the nontoxic tracer dye Evans blue (Fig. S1A), often employed for mapping lymphatic drainage patterns (19). Optimized doses of this dye injected at the tail base or caudal thigh muscle resulted in drainage to inguinal LNs (Fig. S1B) and permitted a means of visualizing “target” LN sites directly through the skin of shaved mice for subsequent injection of vaccines (Fig. S1 A and B). For adjuvant delivery, we prepared MPs (5.4 ± 0.5-μm diameter) and NPs (300 ± 21-nm diameter) with a biodegradable poly(dl-lactide-co-glycolide (PLGA) core and phospholipid surface coating (Fig. S2A), using an emulsion/solvent evaporation process we previously described (20). To confirm that dye-guided injection permitted accurate i.LN deposition of particles, we administered fluorescently labeled MPs to inguinal LNs of mice. Following these injections, whole-animal fluorescence imaging revealed intense, localized fluorescent signal in the inguinal LN regions (Fig. 1C, Upper). Direct imaging of excised LNs (Fig. 1C, Lower) and histological analysis of frozen sections from these LNs (Fig. 1D) confirmed MPs were localized within LNs. We next compared the efficiency of i.LN injections for localizing MPs or NPs to LNs compared to equivalent particle doses administered via the common vaccination route of i.m. injection. Twenty-four hours after injection, draining LNs were excised and analyzed by immunohistochemistry (Fig. 1E). Intramuscular injections of MPs or NPs did not result in detectable levels of particles in the draining inguinal LNs during histological analysis, confirming inefficient transport of particles from this parenteral site. In contrast, i.LN injections of MPs or NPs both resulted in clear localization of particles within LNs (Fig. 1E, pink signal).
Fig. 1.
Intranodal injection of polymer particles. (A) Intralymph node injection of adjuvant-loaded particles following s.c. injection of tracer to mark draining LNs. (B) Tracer drainage to inguinal LNs 2 h after injection of 10 μg dye. Dashed lines indicate exterior (Upper) and internal (Lower) views of LNs after tracer convection from the injection site (arrow, Upper). (C) Whole-animal imaging of an untreated mouse and a mouse injected i.LN with fluorescent MPs. Shown are live animals (Upper) or excised LNs (Lower) 24 h after injection. (D) Histological analysis of LNs from C showing MPs (pink channel) dispersed within the LN parenchyma. (Scale bar: 50 μm.) (E) Histology of LNs 24 h after i.m. or i.LN injection of fluorescent MPs or NPs, showing particle fluorescence (pink) overlaid with T cells (CD3, green). (Scale bar: 50 μm.) (F) LNs excised 24 h after i.m. or i.LN particle injection were analyzed by flow cytometry to characterize uptake by LN APC populations.
We hypothesized that particle size could be used to control whether adjuvant-carrying particles are taken up by LN-resident APCs or remain as extracellular depots within LNs. To test this idea, we injected MPs or NPs i.m. or i.LN; after 24 h, LNs were digested and analyzed by flow cytometry to quantify particle uptake by LN-resident APCs. Intralymph node injection of NPs increased particle uptake by dendritic cells (DCs), macrophages (MΦ), and B cells by 8-, 10-, and 13-fold, respectively, compared with i.m. delivery (Fig. 1F). In contrast, MPs were too large for efficient internalization, exhibiting low levels of cell-associated particles despite high levels of LN deposition after i.LN injection.
Intralymph Node Injection of PolyIC-Loaded MPs/NPs Increases Adjuvant Persistence in LNs and LN-Resident APCs.
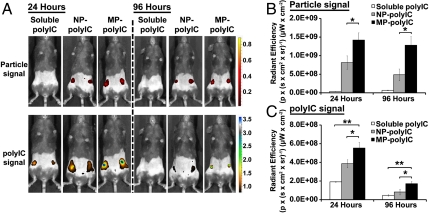
Reasoning that particles continuously releasing adjuvant molecules over a period of days could mimic prolonged stimulation elicited by live replicating vectors and boost the immune response, we explored the impact of adjuvant delivery with intranodal MP or NP injection. MPs and NPs were synthesized that encapsulated polyIC at different loading levels, and we used this parameter to tune adjuvant release rate from 25% release after 7 d, to near complete release over 7 d. (Fig. S2 B–D). For immunization studies, MP-polyIC and NP-polyIC encapsulated approximately 20 μg polyIC/mg of particles (Fig. S2C). Groups of mice received i.LN injections of soluble fluorescently labeled polyIC, NP-polyIC, or MP-polyIC, and the presence of both TLRa and particles in the tissue was analyzed after 1 or 4 d by whole-animal fluorescence imaging. The rate of clearance for MPs was slower than for NPs, and MPs were present at 2.6-fold higher levels in the LNs at 4 d after injection (p < 0.05, Fig. 2 A and B). At 24 h after i.LN injection, polyIC levels detected in LNs increased in the order soluble polyIC < NP-polyIC < MP-polyIC, and this hierarchy was maintained at day 4 (Fig. 2 A–C). MPs mediated 2.9- and 3.6-fold increases in polyIC levels retained in LNs at days 1 and 4, respectively, compared to mice receiving soluble injections of polyIC (p < 0.01, Fig. 2 B and C), and 1.4- and 2.1-fold greater polyIC levels than observed with NP-polyIC injections (p < 0.05).
Fig. 2.
Particle encapsulation increases the persistence of intranodally injected polyIC. (A) In vivo imaging 24 and 96 h after i.LN injection of 50 μg polyIC (soluble, NP-polyIC, or MP-polyIC). Particles (red color scale) and polyIC (blue color scale) were labeled with distinct fluorophores. (B and C) Quantification of whole-animal NP/MP fluorescence (B) and polyIC fluorescence (C) 24 and 96 h after i.LN injection. Shown is mean radiant efficiency ± SEM (n = 4) determined from equivalent regions of interest (*, p < 0.05; **, p < 0.001). Data are from one representative of three independent experiments.
Because soluble polyIC was flushed from the LN most rapidly and MP-polyIC provided the greatest persistence of polyIC intranodally, we used these formulations to explore the distribution of polyIC in LNs. Twenty-four hours after injection of either soluble or MP-encapsulated polyIC, histological analysis revealed TLR agonist dispersed throughout LN sections (Fig. 3A). In some areas with lower particle density, polyIC was observed with a more diffuse pattern than MPs themselves, suggesting significant adjuvant release in vivo by this time (Fig. 3A). In contrast, 96 h after injection, polyIC was only detectable in tissue sections of mice that received MP-polyIC (Fig. 3A). We observed dramatic lymphadenopathy 24 and 96 h after i.LN injection, with soluble polyIC enlarging LNs 2.8- and 3.7-fold (p < 0.05 and 0.01), and MP-polyIC enlarging LNs 3.7- and 5.0-fold (p < 0.01) at these respective times (compared with untreated mice) (Fig. S3).
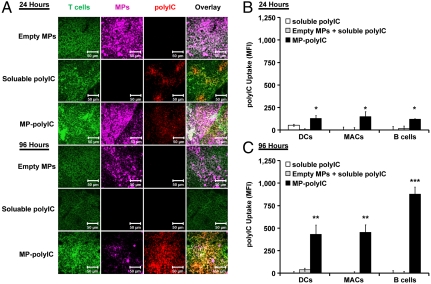
Fig. 3.
Intranodal MP-polyIC mediates sustained polyIC exposure and uptake by LN-resident APCs. Mice received i.LN injections of 50 μg soluble polyIC, MP-polyIC, or empty MPs. (A) Histological images of LNs 24 or 96 h after injection showing T cells (CD3, green), polyIC (red), and MPs (pink). (Scale bar: 50 μm.) (B and C) Flow cytometry analysis of fluorescent polyIC uptake by LN-resident APCs 24 (B) or 96 h (C) after injection. Uptake levels are reported as polyIC mean fluorescent intensity (MFI) ± SEM among dendritic cells, CD11c+; macrophages (MACs), F4/80+; and B cells, B220+. (*, p < 0.05; **, p < 0.01; and ***, p < 0.001 vs. soluble polyIC). Data (n = 4) are from one representative of two independent experiments.
To test if sustained release of polyIC from MPs promoted accumulation of the TLR agonist in LN-resident APCs, we analyzed LN DCs, macrophages, and B cells for uptake of polyIC. One day after injection, MPs mediated increased polyIC uptake compared with soluble TLR agonist injections (p < 0.05, Fig. 3B). This difference in TLRa uptake was amplified by day 4, with MP-polyIC formulations causing accumulation of polyIC in DCs, macrophages, and B cells compared with near-baseline levels observed in LNs from mice receiving soluble agonist (p < 0.01 and 0.001, Fig. 3C). Mixing polyIC mixed with empty MPs just before injection also showed low uptake of soluble TLRa, suggesting that increased polyIC internalization did not reflect an alteration of the LN APC response to TLRa caused by exposure to PLGA particles per se (Fig. 3 B and C).
MPs Promote Sustained Activation of LN-Resident DCs.
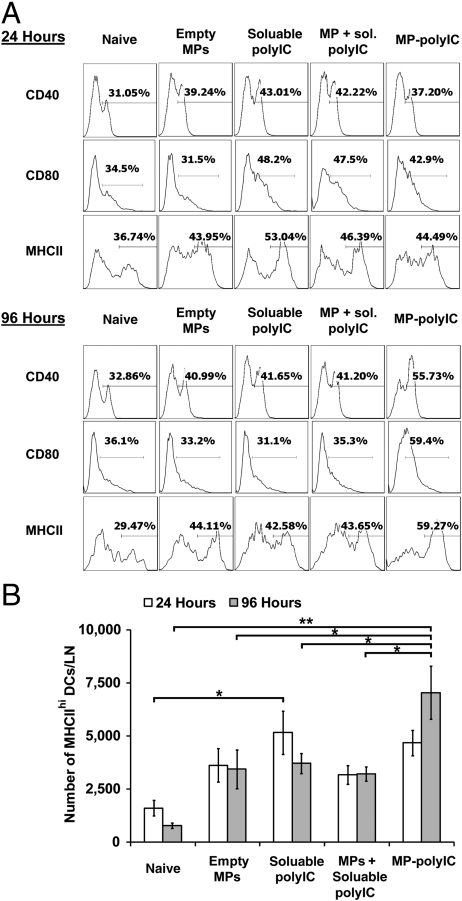
We next investigated whether increased persistence and accumulation of polyIC in APCs following i.LN MP deposition would alter DC activation compared to soluble TLRa. In these studies, we mixed soluble OVA with soluble or MP-encapsulated polyIC as adjuvant. Twenty-four hours after i.LN immunization, comparable increased frequencies of activated lymph node CD11c+ DCs were found in each treatment group relative to naïve mice (Fig. 4A). Empty MPs, though negative for endotoxin, also elicited a modest level of CD40 and MHCII up-regulation intermediate between soluble polyIC and DCs from naïve mice. This up-regulation may reflect internalization of the smallest particles in the MP preparation leading to inflammasome activation (21). At 4 d after injection, DCs with a mature phenotype were decreasing toward naïve levels in the empty MP, soluble polyIC, or soluble polyIC mixed with empty MP groups. In contrast, MP-polyIC generally mediated increasing frequencies of activated DCs compared with other formulations and compared with the same vaccine at day 1 (Fig. 4A). The absolute number of mature-phenotype MHCIIhi DCs in these subpopulations also increased from day 1 to day 4, while the number of mature DCs in the other conditions remained constant or contracted (p < 0.05 and 0.01, Fig. 4B). These results suggest MP-encapsulated polyIC promoted more enduring DC activation, in a manner which required sustained polyIC release and which was not simply the sum of responses triggered by PLGA and polyIC.
Fig. 4.
i.LN injection of MP-polyIC induces enduring DC activation. (A) Flow cytometry analysis of surface markers on CD11c+ DCs from LNs of naïve mice or mice receiving i.LN injections of 50 μg of soluble OVA antigen mixed with either empty MPs, 50 μg soluble polyIC, empty MPs mixed with 50 μg polyIC, or MP-polyIC encapsulating 50 μg polyIC, 24 or 96 h after injection. (B) Quantification of number of MHCIIhi DCs for each immunization condition at 24 or 96 h. Shown are mean frequencies ± SEM measured per LN (*, p < 0.05; **, p < 0.01). Data (n = 5) are from one representative of two independent experiments.
Intranodal Microparticle Injection Enhances the Potency of PolyIC.
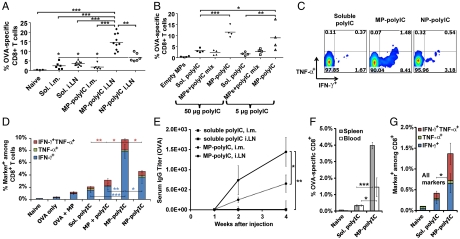
To determine if increased accumulation of polyIC in LN-resident APCs and prolonged DC activation elicited by i.LN injection of MP-polyIC would translate to enhanced efficacy of this TLRa, we measured antibody and CD8+ T-cell responses following immunization with soluble OVA, using polyIC as an adjuvant in soluble, NP, or MP form. Groups of mice were immunized either i.m. or i.LN and antigen-specific CD8+ T-cell frequencies in peripheral blood were enumerated 1 wk after injection. OVA mixed with soluble polyIC elicited similar frequencies of OVA-specific CD8+ T cells when administered i.m. or i.LN, suggesting that intranodal vaccination did not greatly benefit this soluble protein/TLRa immunization (Fig. 5A and Fig. S4). OVA mixed with MP-polyIC administered i.m. also elicited a modest response, producing 1.8 ± 0.6% OVA-specific T cells, indicating that sustained release of polyIC at a peripheral vaccination site did not enhance the immune response triggered by this adjuvant. In contrast, OVA and MP-polyIC administered i.LN expanded up to 21% (mean 15 ± 1.4%) OVA-specific endogenous T cells, a 4.1-fold increase compared with i.LN injections of OVA/soluble polyIC (p < 0.001), and an 8.2-fold increase compared with MP-polyIC administered i.m. (p < 0.001, Fig. 5A and Fig. S4). Interestingly, NP-polyIC, which gave polyIC persistence in LNs intermediate between soluble and MP-polyIC adjuvant delivery, also gave a CD8+ response intermediate between these two other immunization groups (Fig. 5A). Empty MPs were a poor adjuvant for the CD8+ T-cell response, and empty MPs mixed with polyIC just before injection gave responses essentially equivalent to soluble polyIC alone (Fig. 5B), suggesting that polyIC encapsulation and the resulting sustained release played a critical role in the synergistic enhancement seen with MP-polyIC. Further, MP-polyIC administered at a 10-fold lower polyIC dose (5 μg) generated a stronger T-cell response compared with other polyIC formulations at this dose (p < 0.01), or compared with soluble polyIC given at a 10-fold greater dose (p < 0.05), demonstrating the potential for dose-sparing using this approach (Fig. 5B). To assess the functionality of T cells expanded by these vaccines, we analyzed cytokine production from T cells isolated 7 d after immunization. MP-polyIC induced 5.3-fold (p < 0.001) and 3.7-fold (p < 0.01) increases in frequencies of IFN-γ+ CD8+ T cells compared with soluble polyIC or polyIC mixed with empty MPs, respectively (Fig. 5 C and D). We also observed higher frequencies of T cells producing multiple cytokines, with i.LN MP-polyIC eliciting 5.2-fold (p < 0.01) and 1.8-fold (p < 0.05) increases in the frequency of IFN-γ+TNF-α+ CD8+ T cells, compared with soluble TLRa or TLRa mixed with empty MPs (Fig. 5 C and D). Again correlating with adjuvant persistence, NP-polyIC elicited cytokine responses intermediate between soluble polyIC and MP-polyIC vaccinations (Fig. 5 C and D). We also assessed OVA-specific serum IgG levels in immunized mice and found that, following a single injection i.m. or i.LN, MP-polyIC elicited readily detectable OVA-specific titers that developed over several weeks, compared to soluble polyIC immunizations, where no response was detected even following i.LN vaccination (p < 0.05 and 0.01, Fig. 5E). Thus, i.LN immunization with adjuvant-releasing biomaterials significantly enhanced the potency of this TLR agonist, amplifying both primary CD8+ T-cell and humoral responses.
Fig. 5.
Enhanced vaccine responses following i.LN immunization with MP-polyIC. (A) Mice were immunized with 50 μg OVA and 50 μg polyIC (soluble polyIC alone, NP-polyIC, or MP-polyIC), administered i.m. or i.LN. T-cell responses were analyzed by tetramer staining on day 7. Shown are mean frequencies of tetramer+ T cells. Data (n = 6) is representative of one of three independent experiments. (B–D) Mice were immunized i.LN with 50 μg of soluble OVA antigen and either empty MPs, soluble polyIC, empty MPs mixed with polyIC just prior to injection, MP-polyIC, or NP-polyIC. (B) Shown are mean tetramer+ CD8+ T-cell frequencies ± SEM from one (n = 4) representative of three independent experiments. (C and D) Seven days after immunization with 50 μg polyIC, peripheral blood mononuclear cells were restimulated ex vivo with SIINFEKL peptide, fixed, and stained to detect intracellular IFN-γ/TNF-α. Shown are flow cytometry analyses (C) and quantification of frequencies (D) of CD8+ T cells positive for IFN-γ, TNF-α. (E) Mice were immunized with soluble polyIC or MP-polyIC as in A, and OVA-specific IgG serum titers were measured by ELISA. Shown are mean titers ± SEM from onee (n = 4) representative of two independent experiments. (F and G) Mice were immunized i.LN with soluble polyIC or MP-polyIC as in A and, 6 wk after vaccination, antigen-specific CD8+ T-cell frequencies (F) and cytokine production (G) were measured in T cells isolated from blood or spleen. Data are mean ± SEM and are representative of one (n = 4) of two independent experiments. For all panels, *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Importantly, soluble polyIC or particle injections did not induce notable toxicity to key LN-resident APCs relative to untreated mice following i.LN injections (Fig. S5A). However, to determine whether i.LN particle injections might cause tissue damage that could impair the development of immune memory, we assessed T- and B-cell responses over time. Six weeks after i.LN priming, ova-specific T cells were readily detectable in soluble- or MP-polyIC-immunized mice at levels well above the background of naïve mice, suggesting the establishment of persisting memory populations. Mirroring earlier time points, ova-specific T-cell frequencies in spleens and LNs of mice vaccinated with MP-polyIC were 4.3-fold (p < 0.05) and 12-fold (p < 0.001) higher than those immunized with soluble polyIC, respectively (Fig. 5F), and approximately 3-fold more T cells from MP-polyIC-immunized mice produced cytokines on ex vivo restimulation (p < 0.05, Fig. 5G). To assess B-cell memory and determine whether i.LN priming left animals responsive to booster injections, a second set of mice were primed i.LN with OVA mixed with soluble or MP-polyIC, followed 5-wk later by an i.m. boost of 20 μg soluble OVA and 20 μg soluble polyIC. Both soluble- and MP-polyIC-primed animals responded 1 wk after boost with elevated serum titers, but MP-polyIC-primed mice exhibited 9.5-fold greater titers compared to mice receiving a soluble polyIC prime (p < 0.001, Fig. S5B).
Discussion
Efficient delivery of vaccine components into LNs is critical for mounting effective immune responses because antigen that fails to reach the lymphoid organs may be effectively ignored by the immune system (1). Recently, it has been shown that subunit vaccine responses can be enhanced relative to soluble antigen/adjuvant or alum formulations by association of vaccine components with NPs small enough (< 50-nm diameter) to directly drain to LNs following parenteral injection (3, 4). However, with these approaches, the majority of injected material still remains at the vaccination site (4), where it may be essentially nonfunctional in promoting the immune response and might promote undesirable granuloma formation or reactogenicity. In contrast, i.LN injection deposits the entire vaccine dose directly into the site where the primary immune response is initiated, maximizing the per-dose response. Indeed, i.LN administration allows potent antibody and T-cell responses to be mounted using doses as low as one-millionth that needed for parenteral immunizations (2, 5, 6). This approach also alters the functional quality of immune response, selectively promoting Th1-biased cytokine and humoral responses (22), and eliciting enhanced protection in viral and tumor challenge models (6).
In addition to its physical location, the timing of the immune system’s exposure to a vaccine must be considered for generation of optimal protective immunity. Recent studies evaluating the impact of the kinetics of antigen and adjuvant availability on T-cell and antibody responses suggest sustained exposure of the immune system over a period of several days to both antigen and adjuvant molecules amplifies vaccine immunogenicity (11–13, 16, 17). However, parenterally injected soluble vaccines are rapidly cleared from the injection site and flush through the draining LNs in a time course of minutes to hours (10, 23), and here we have shown similar rapid clearance of soluble vaccine components by efferent lymph flow following i.LN injection. To sustain exposure of the immune system to vaccines, parenteral immunization with antigen or adjuvants in controlled release formulations has been explored. Polymer matrices releasing antigen over days or weeks following parenteral injection have been shown to robustly promote antibody responses (23, 24) and biodegradable MPs loaded with TLR agonists can promote CD4+ and CD8+ T-cell responses, as well as antibody responses (25–27).
Because controlled release from peripheral tissue “depots” does not necessarily enhance delivery of vaccine components to draining LNs and intranodal injections do not necessarily sustain the exposure of immune cells to vaccine components, we hypothesized there could be substantial synergy in combining these two approaches. Biomaterials suitable for antigen delivery with appropriate release kinetics need to be tailored to individual antigens, and encapsulation of antigens can lead to denaturation (28). In addition, the ability of APCs to rapidly take up antigen should allow antigen processing and presentation to be sustained for several days in the presence of inflammatory signals despite rapid clearance of extracellular free vaccine (14, 15). Thus, we focused on the impact of regulating delivery of molecular adjuvants to provide local danger signals during priming of the immune response. The potent TLR agonist polyIC was formulated in biodegradable MPs or NPs using the Food and Drug Administration-approved polymer poly(lactide-co-glycolide). Consistent with earlier studies suggesting that phagocytosis by macrophages and DCs is efficient for submicron-sized particles but much less effective for particles > 3.0 μm in diameter (29, 30), we found that the fate of particles injected i.LN could be controlled by the simple physical parameter of particle size: NPs were taken up to high levels by LN-resident APCs, whereas MPs remained largely extracellular over several days (Fig. 1 D–F). We hypothesized that an extracellular depot of particles releasing polyIC over several days in LNs would amplify the immune response by allowing DCs and immune cells newly recruited to the LN over time to be continuously stimulated, mimicking the sustained inflammatory signaling achieved by local infection and live attenuated vaccines. Intranodal deposition of PLGA MPs releasing polyIC allowed the dosing of the tissue with the TLRa to be substantially sustained over a period of at least 4 d relative to injections of soluble polyIC. Interestingly, TLRa-loaded NPs exhibited levels of polyIC persistence falling between that of rapidly draining soluble adjuvant and slowly cleared MPs. PLGA microspheres and other particulate materials have been shown to have intrinsic immune modulatory activity via activation of the inflammasome in innate immune cells (21), and, in agreement with these studies, we saw some level of DC activation triggered by empty MPs, intermediate between soluble polyIC and control naïve mice. However, prolonged exposure to TLR3 agonist released from MPs correlated with substantially increased polyIC accumulation in LN-resident APCs and more persistent DC activation over 4 d compared to these control cases or empty MPs mixed with soluble polyIC.
Correlating with the enhanced persistence of polyIC and DC activation, we found that intranodal vaccination and adjuvant-loaded MP depots synergistically enhanced both the antibody and T-cell response to a model soluble antigen. Sustained release of the TLRa in LNs permitted a significant increase in the expansion of antigen-specific CD8+ T cells, which could not be matched by soluble adjuvant injection even using a 10-fold higher dose of polyIC (Fig. 5). This enhanced response required both encapsulation of the polyIC in MPs for slow release and intranodal deposition to concentrate the agonist in the lymphoid tissue, and also translated to increased T-cell cytokine production. NP-polyIC, which gave polyIC persistence in LNs intermediate between soluble and MP formulations, also elicited T-cell responses intermediate between these other groups. Considering that NPs were efficiently internalized by APCs while MPs were not, NP-polyIC likely resulted in a larger but more transient intracellular adjuvant dose, whereas MP-polyIC remained extracellular, delivering a lower but more prolonged polyIC exposure in LNs. Here, the latter delivery dynamic provided the strongest immune response. The ability to elicit potent responses using lower doses of TLR agonist via MP delivery is attractive for avoiding undesirable systemic immune stimulation. The high levels of antigen-specific T cells observed in our experiments were generated rapidly (7 d after injection) and with no additional boosting (though, as shown in Fig. S5B, i.LN-primed mice were not refractory to further boosting). Future studies examining more complex immunization regimens (e.g., additional boost injections, heterologous prime boosting, encapsulation of antigen and adjuvant, or delivery of multiple agents) may offer room for further enhancements of vaccine responses and immunological memory generation. For example, antigen-releasing PLGA particles injected i.LN have recently been reported to elicit stronger antibody responses compared with other injection routes of these particles (31), though comparisons to soluble antigen injections were not made in this study. Intralymph node injection of soluble antigen-encoding RNA has also been reported to significantly enhance vaccination and may serve as a synergistic extension to our current strategy of delivering synthetic RNA adjuvants (i.e., polyIC) (32).
We note that the clinical feasibility of LN injections in humans is well established from a number of clinical trials (2, 8, 9), and safe, injectable biomaterials are available that can encapsulate both small molecule and macromolecular cargos. These factors make the approach described here attractive as a general strategy for engineering the LN microenvironment not only for vaccination but also for therapeutic contexts, such as cancer immunotherapy or immunomodulation for allergy or autoimmune disorders.
Materials and Methods
Detailed methods are provided in the SI Text.
Particle Synthesis.
Lipid-coated PLGA MPs and NPs were synthesized by an emulsion/solvent evaporation process (20). Particle size and charge were measured by laser diffraction and electrophoretic mobility, respectively. PolyIC loading was quantified by UV absorbance following hydrolysis of MPs/NPs.
Animals and Injections.
Mice were immunized i.m. or i.LN with soluble OVA and polyIC in soluble, NP, or MP form. Intralymph node injections were performed by injecting tracer dye s.c. at the tail base, then injecting vaccines i.LN after tracer drainage.
Imaging.
Whole-animal imaging was performed using an in vivo fluorescence imaging system and quantitative region of interest analysis. Histology sections were prepared from LNs, then fixed, stained, and imaged by Cofocal Laser Scanning Microscopy.
Flow Cytometry.
Uptake of fluorescent particles/polyIC and DC activation were measured by digesting LNs after injection and staining with antibodies for phenotypic markers. OVA-specific CD8+ T-cell levels in spleen or blood were determined using antibodies for CD8α and SIINFEKL/H-2Kb peptide-MHC tetramers. Cytokine secretion was measured by staining for IFN-γ and TNF-α after ex vivo restimulation with SIINFEKL peptide.
Statistical Analysis.
Student’s t test was used to compare two groups. One-way ANOVAs with a Bonferroni posttest was used to compare > 2 groups. p values < 0.05 were considered significant.
Supplementary Material
Acknowledgments.
This work was supported in part by the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard and The Gates Foundation. C.M.J. is a Ragon Institute Postdoctoral Fellow. D.J.I. is an investigator of the Howard Hughes Medical Institute. Core facilities were supported by Koch Institute Cancer Center Support Grant P30-CA14051 (National Cancer Institute).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.A.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105200108/-/DCSupplemental.
References
- 1.Zinkernagel RM, et al. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: A geographical view of immune reactivity. Immunol Rev. 1997;156:199–209. doi: 10.1111/j.1600-065x.1997.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 2.Senti G, Johansen P, Kundig TM. Intralymphatic immunotherapy. Curr Opin Allergy Clin Immunol. 2009;9:537–543. doi: 10.1097/ACI.0b013e3283310ff7. [DOI] [PubMed] [Google Scholar]
- 3.Reddy ST, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 4.Manolova V, et al. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 5.Johansen P, et al. Direct intralymphatic injection of peptide vaccines enhances immunogenicity. Eur J Immunol. 2005;35:568–574. doi: 10.1002/eji.200425599. [DOI] [PubMed] [Google Scholar]
- 6.Maloy KJ, et al. Intralymphatic immunization enhances DNA vaccination. Proc Natl Acad Sci USA. 2001;98:3299–3303. doi: 10.1073/pnas.051630798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Beust BR, et al. Improving the therapeutic index of CpG oligodeoxynucleotides by intralymphatic administration. Eur J Immunol. 2005;35:1869–1876. doi: 10.1002/eji.200526124. [DOI] [PubMed] [Google Scholar]
- 8.Senti G, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: A randomized controlled trial. Proc Natl Acad Sci USA. 2008;105:17908–17912. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber J, et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J Immunother. 2008;31:215–223. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- 10.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann MF, et al. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur J Immunol. 2006;36:842–854. doi: 10.1002/eji.200535730. [DOI] [PubMed] [Google Scholar]
- 12.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen P, et al. Antigen kinetics determines immune reactivity. Proc Natl Acad Sci USA. 2008;105:5189–5194. doi: 10.1073/pnas.0706296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh NJ, Cox M, Schwartz RH. TLR ligands differentially modulate T cell responses to acute and chronic antigen presentation. J Immunol. 2007;179:7999–8008. doi: 10.4049/jimmunol.179.12.7999. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 17.Shaulov A, Murali-Krishna K. CD8 T cell expansion and memory differentiation are facilitated by simultaneous and sustained exposure to antigenic and inflammatory milieu. J Immunol. 2008;180:1131–1138. doi: 10.4049/jimmunol.180.2.1131. [DOI] [PubMed] [Google Scholar]
- 18.Stahl-Hennig C, et al. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 2009;5:e1000373. doi: 10.1371/journal.ppat.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrell MI, Iritani BM, Ruddell A. Lymph node mapping in the mouse. J Immunol Methods. 2008;332:170–174. doi: 10.1016/j.jim.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bershteyn A, et al. Polymer-supported lipid shells, onions, and flowers. Soft Matter. 2008;4:1787–1791. doi: 10.1039/b804933e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp FA, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci USA. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansen P, Mohanan D, Martinez-Gomez JM, Kundig TM, Gander B. Lympho-geographical concepts in vaccine delivery. J Control Release. 2010;148:56–62. doi: 10.1016/j.jconrel.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Gupta RK, Chang AC, Griffin P, Rivera R, Siber GR. In vivo distribution of radioactivity in mice after injection of biodegradable polymer microspheres containing 14C-labeled tetanus toxoid. Vaccine. 1996;14:1412–1416. doi: 10.1016/s0264-410x(96)00073-4. [DOI] [PubMed] [Google Scholar]
- 24.Preis I, Langer RS. A single-step immunization by sustained antigen release. J Immunol Methods. 1979;28:193–197. doi: 10.1016/0022-1759(79)90341-7. [DOI] [PubMed] [Google Scholar]
- 25.Singh M, et al. Cationic microparticles are an effective delivery system for immune stimulatory CpG DNA. Pharm Res. 2001;18:1476–1479. doi: 10.1023/a:1012269226066. [DOI] [PubMed] [Google Scholar]
- 26.Schlosser E, et al. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine. 2008;26:1626–1637. doi: 10.1016/j.vaccine.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Bourquin C, et al. Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. J Immunol. 2008;181:2990–2998. doi: 10.4049/jimmunol.181.5.2990. [DOI] [PubMed] [Google Scholar]
- 28.Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide) Nat Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 29.Tabata Y, Ikada Y. Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials. 1988;9:356–362. doi: 10.1016/0142-9612(88)90033-6. [DOI] [PubMed] [Google Scholar]
- 30.Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohanan D, et al. Administration routes affect the quality of immune responses: A cross-sectional evaluation of particulate antigen-delivery systems. J Control Release. 2010;147:342–349. doi: 10.1016/j.jconrel.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Kreiter S, et al. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 2010;70:9031–9040. doi: 10.1158/0008-5472.CAN-10-0699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.