Abstract
Key to successful retina regeneration in zebrafish are Müller glia (MG) that respond to retinal injury by dedifferentiating into a cycling population of retinal progenitors. Although recent studies have identified several genes involved in retina regeneration, the signaling mechanisms underlying injury-dependent MG proliferation have remained elusive. Here we report that canonical Wnt signaling controls the proliferation of MG-derived retinal progenitors. We found that injury-dependent induction of Ascl1a suppressed expression of the Wnt signaling inhibitor, Dkk, and induced expression of the Wnt ligand, Wnt4a. Genetic and pharmacological inhibition of Wnt signaling suppressed injury-dependent proliferation of MG-derived progenitors. Remarkably, in the uninjured retina, glycogen synthase kinase-3β (GSK-3β) inhibition was sufficient to stimulate MG dedifferentiation and the formation of multipotent retinal progenitors that were capable of differentiating into all major retinal cell types. Importantly, Ascl1a expression was found to contribute to the multipotential character of these progenitors. Our data suggest that Wnt signaling and GSK-3β inhibition, in particular, are crucial for successful retina regeneration.
Keywords: pyrvinium, XAV939, transgenic zebrafish, heat shock, frizzled
Vision loss is among the top disabilities afflicting the human population. Strategies for repairing the damaged or diseased human retina have remained elusive. Unlike mammals, teleost fish such as zebrafish are able to regenerate a damaged retina that restores structure and function (1–3). Key to successful regeneration are Müller glia (MG) that respond to retinal injury by generating multipotent progenitors that can regenerate all major retinal cell types (4–8). Attempts to stimulate MG dedifferentiation and retina regeneration in mammals have met with little success. In general, retinal injury stimulates a gliotic response where MG undergo morphological, biochemical, and physiological changes (9), but rarely do these cells regenerate new neurons and glia, even when their proliferation is stimulated (10–14).
Mechanisms underlying retina regeneration in zebrafish are just beginning to emerge, and it is anticipated that these mechanisms may suggest novel strategies for stimulating retina regeneration in mammals. After retinal injury in zebrafish, genes associated with the formation of induced pluripotent stem cells are activated in dedifferentiating MG (15). One of these genes, lin-28, participates in an Ascl1a/Lin-28/let-7 microRNA signaling pathway that contributes to MG dedifferentiation (15). Ascl1a may also regulate the proliferation of dedifferentiated MG (16). In addition, injury-dependent induction of Pax6 appears to control the expansion of MG-derived progenitors, but not their initial entry into the cell cycle (17). Although injury-dependent induction of Ascl1a and Pax6 are necessary for proliferation of MG-derived progenitors, it is not clear how they are activated or what signaling pathways underlie their effects.
Here we report that Ascl1a controls proliferation of dedifferentiated MG in the injured zebrafish retina via regulation of a Wnt signaling pathway. We found that Wnt signaling was necessary for proliferation of dedifferentiated MG in the injured retina and that glycogen synthase kinase-3β (GSK-3β) inhibition was sufficient to stimulate MG dedifferentiation into a population of cycling multipotent progenitors in the uninjured retina. Interestingly, Ascl1a knockdown limited the production of neurons by progenitors in the GSK-3β inhibitor-treated retina.
Results
Ascl1a-Dependent Suppression of dkk Gene Expression in Injured Retina.
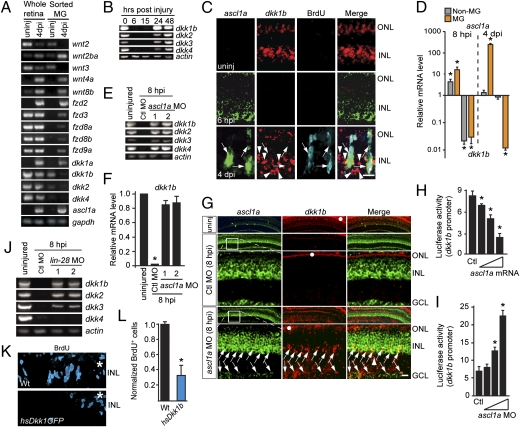
Wnt signaling is a conserved pathway that affects many fundamental developmental processes (18). Deregulated Wnt signaling often underlies cancer cell proliferation (19), and Wnt signaling may also participate in repair of the adult nervous system (20). Here we investigated whether Wnt signaling was necessary for retina regeneration in zebrafish. We first asked whether any Wnt signaling components were regulated during retina regeneration (Fig. 1A and Fig. S1B). For this analysis, RNA was purified from uninjured and injured retinas or from FACS-purified MG and MG-derived progenitors (Fig. S1A) at 4 d post-retinal injury (dpi) as described (15). Interestingly, a number of genes encoding Wnt ligands (wnt2ba, wnt2bb, wnt4a, and wnt8b), Wnt receptors (fzd2, fzd3, fzd8a, fzd8b, and fzd9a), and a Wnt signaling antagonist (dkk1a) were induced in MG-derived progenitors, whereas others (wnt2, wnt3, dkk1b, and dkk2) were suppressed. The expression and injury-dependent regulation of Wnt signaling components in MG may suggest that they signal to each other after retinal injury. However, because components of the Wnt signaling pathway are also expressed by retinal neurons, they, too, may participate in the injury response.
Fig. 1.
Ascl1a inhibits the expression of dkk genes during retina regeneration. (A and B) Injury-dependent regulation of Wnt signaling component mRNAs. (C) Double in situ hybridization shows mutually exclusive ascl1a and dkk1b gene expression. (D) ascl1a and dkk1b expression in FACS-purified MG and non-MG from injured retinas. Values are relative to uninjured retina. *P < 0.009. (E and F) Ascl1a knockdown prevents injury-dependent dkk gene suppression. (F) Quantification of E by qPCR. Values are relative to uninjured retina. *P < 0.0001. (G) In situ hybridization showing Ascl1a knockdown relieves injury-dependent dkk suppression. Boxed region in low-magnification image is shown in higher magnification in the row below. Arrows point to ascl1a+/dkk1b+ cells. White dots identify autofluorescence in ONL. (H and I) Injection of zebrafish embryos with dkk1b:gfp-luciferase reporter and increasing amounts of ascl1a mRNA (H) or ascl1a-targeting MO (I). *P < 0.005. (J) Lin-28 knockdown differentially affects injury-dependent dkk gene suppression. (K and L) Dkk1b overexpression inhibits cell proliferation at 4 dpi. *P < 0.003. (Scale bars, 10 μm.) ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
While analyzing the temporal expression pattern of Wnt component genes, we observed a striking transient decline in dkk expression throughout the retina from 6 to 15 h post-retinal injury (hpi) (Fig. 1 B and C). This pan-retinal decline was unusual in a model of focal retinal injury where all previously studied injury-responsive genes were confined to MG residing close to the injury site (4, 15, 16). Interestingly, Ascl1 expression is associated with DKK repression in human lung cancer (21), and in the injured retina ascl1a induction is correlated with dkk suppression (Figs. 1B and 2A). Therefore, we investigated whether the expression of these two genes was mutually exclusive. Indeed, in the uninjured retina, in situ hybridization showed that ascl1a was undetectable and dkk1b was readily apparent, whereas at 6 hpi the opposite was observed (Fig. 1C and Fig. S2A). At 4 dpi, dkk1b was lacking from ascl1a+ progenitors but restored in neighboring cells (Fig. 1C and Fig. S2A). To further test the idea that ascl1a and dkk1b exhibit a mutually exclusive expression pattern, we used FACS to isolate GFP+ MG and GFP− retinal neurons (non-MG) from gfap:gfp transgenic fish retinas at 0 and 8 hpi and GFP+ dedifferentiated MG from 1016 tuba1a:gfp transgenic fish retinas at 4 dpi (15). Quantitative PCR (qPCR) showed that ascl1a was induced approximately sevenfold in non-MG at 8 hpi, whereas dkk1b was suppressed (>90%) in this cell population (Fig. 1D). Furthermore, at 4 dpi, ascl1a expression was suppressed in non-MG, but increased ∼170-fold in GFP+ MG-derived progenitors, whereas dkk1b was essentially eliminated from these cells (Fig. 1D). These data indicate a mutually exclusive pattern of ascl1a and dkk gene expression.
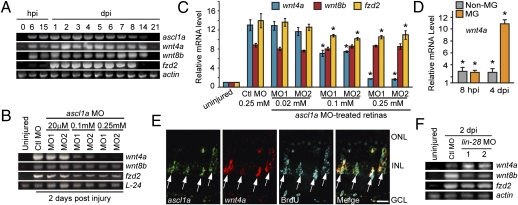
Fig. 2.
Ascl1a regulates wnt4a expression via a Lin-28 independent pathway. (A) Time course of injury-dependent gene expression. (B and C) MO-mediated Ascl1a knockdown suppresses wnt4a gene induction at 2 dpi. *P < 0.0005. (D) wnt4a expression in MG and non-MG after retinal injury relative to uninjured control retina. *P < 0.0007. (E) ascl1a and wnt4a mRNAs are coexpressed in BrdU+ MG-derived progenitors at 4 dpi. (Scale bar, 10 μm.) (F) Lin-28 knockdown does not suppress injury-dependent wnt4a or fzd2 induction. Abbreviations are as in Fig. 1.
The above data suggest that Ascl1a suppresses dkk gene expression. To test this idea, we knocked down Ascl1a with previously validated ascl1a-targeting morpholino-modified antisense oligonucleotides (MOs) (15, 16) and assayed dkk expression at 8 hpi (Fig. 1 E and F). Indeed, Ascl1a knockdown relieved injury-dependent dkk suppression. In situ hybridization assays also showed that dkk1b gene expression returned after Ascl1a knockdown (Fig. 1G). To further substantiate that Ascl1a can repress dkk1b gene expression, we coinjected zebrafish embryos with a dkk1b:gfp–luciferase reporter and various amounts of either in vitro-transcribed ascl1a mRNA or ascl1a-targeting MO (Fig. 1 H and I). In these experiments, Ascl1a overexpression decreased, whereas Ascl1a knockdown increased dkk1b promoter activity. Because Ascl1a is a transcriptional activator, we suggest that it mediates dkk1b suppression via activation of an unidentified transcriptional repressor.
We previously showed that Ascl1a regulates lin-28 expression in the injured retina (15). Therefore, we tested whether Lin-28 mediated the effects of Ascl1a on dkk repression in the injured retina (Fig. 1J). Interestingly, Lin-28 knockdown completely restored dkk1b and dkk2 expression, partially restored dkk3 expression, and had no effect on dkk4 repression. Therefore, Ascl1a uses both Lin-28-dependent and -independent mechanisms to regulate dkk gene expression.
Because Dkk can antagonize Wnt signaling, its early decline may be necessary to initiate Wnt signaling and retina regeneration. To directly test this idea, we took advantage of hs:Dkk1GFP transgenic fish that harbor the zebrafish dkk1b–gfp fusion under control of the hsp70-4 promoter (22). These fish were used previously to demonstrate that canonical Wnt signaling mediates fin regeneration and that transgene activation in developing embryos phenocopies the effects of wnt8 loss of function (22). In addition, Dkk1GFP transgene overexpression blocked activation of the Wnt signaling reporter TOPdGFP (23). Therefore, the hs:Dkk1GFP transgenic line is an excellent tool for studying the function of Wnt signaling. We confirmed that Dkk1b–GFP overexpression blocked fin regeneration and also verified heat shock-dependent Dkk1b–GFP expression in the retina (Fig. S3 A and B). Importantly, Dkk1b overexpression in the injured retina inhibited cell proliferation (Fig. 1 K and L and Fig. S3B). The retention of some progenitors beneath the injury site in Dkk1b-overexpressing fish may indicate a gradient of Wnt signaling emanating from the injury site. In this case, Dkk1b levels may be insufficient to completely block Wnt signaling at the injury site.
Ascl1a-Dependent Induction of Wnt4a in Injured Retina.
We next investigated the temporal expression pattern of Wnt signaling components wnt4a, wnt8b, and fzd2 after retinal injury and compared them to ascl1a expression. We focused on these genes because their expression is undetectable in the uninjured retina but highly induced in MG-derived progenitors at 4 dpi (Fig. 1A). Like ascl1a, wnt4a and wnt8b were induced within 6 hpi, whereas fzd2 was largely induced at ∼24 hpi (Fig. 2A). However, only wnt4a appeared to be completely dependent on Ascl1a expression (Fig. 2 B and C), which is consistent with their coexpression in MG and retinal neurons at 8 hpi (Figs. 1D and 2D) and their coenrichment in MG-derived progenitors at ∼4 dpi (Figs. 1D and 2 D and E and Fig. S2B). Unlike injury-dependent dkk1b repression, wnt4a induction was independent of Lin-28 expression (Fig. 2F).
β-Catenin Stabilization Is Necessary for Proliferation of Dedifferentiated MG.
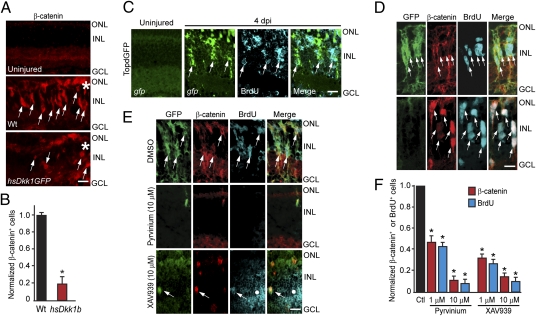
Together, the above studies suggest a role for Wnt signaling in the injured retina. Because β-catenin stabilization is a hallmark of canonical Wnt signaling, we assayed its expression in uninjured and injured retinas. We found that epitope retrieval revealed nuclear β-catenin, whereas standard immunohistochemical protocols showed cytoplasmic β-catenin (compare Fig. 3D Upper, which used standard protocol, with Fig. 3D Lower, which used epitope retrieval protocol). Because epitope retrieval hindered GFP detection, we used standard protocols when anti-GFP and anti-β-catenin antibodies were used on the same section. Retinal injury stimulated nuclear β-catenin expression in MG near the injury site, and this induction was suppressed by Dkk1b overexpression in hs:Dkk1GFP transgenic fish (Fig. 3 A and B and Fig. S4A). Retinal injury in Wnt/β-catenin reporter TOPdGFP transgenic fish (23) stimulated GFP expression in BrdU+/β-catenin+ progenitors located at the injury site (Fig. 3C and Fig. S4 F and G). In 1016 tuba1a:gfp transgenic fish (4), β-catenin was restricted to GFP+ progenitors (Fig. 3D) and coincided with the initiation of cell proliferation at ∼2 dpi (4) (Fig. S4 B and C). Quantification showed that 97% of the GFP+ and 95% of the BrdU+ cells colabeled with β-catenin at 4 dpi. We did not detect large changes in β-catenin (ctnnb1 or ctnnb2) mRNA after injury (Fig. S4E).
Fig. 3.
β-Catenin accumulation in MG-derived progenitors is necessary for retina regeneration. (A and B) Injury-induced β-catenin expression at 4 dpi is blocked by Dkk1b overexpression in hs:Dkk1GFP fish. (C) Injury-dependent induction of GFP expression in the β-catenin reporter fish, TOPdGFP. (D) Accumulation of β-catenin in GFP+ MG-derived proliferating progenitors of 1016 tuba1a:gfp fish at 4 dpi. (Upper) Standard immunohistochemical protocol. (Lower) Epitope retrieval protocol. (E and F) Pyrvinium and XAV939 inhibit the generation of GFP+, β-catenin+, and BrdU+ MG-derived progenitors at 4 dpi in 1016 tuba1a:gfp fish. White dot identifies an area of autofluorescence that results from long exposures to detect fluorescent signals. *P < 0.001. (Scale bars, 10 μm.) Abbreviations are as in Fig. 1.
To determine whether injury-induced β-catenin stabilization is necessary for retina regeneration, we stimulated β-catenin degradation in the injured retina of 1016 tuba1a:gfp fish by injecting eyes with pyrvinium, a casein kinase 1-α activator (24), or XAV939, a tankyrase inhibitor (25), along with a 3-h pulse of BrdU at 4 dpi. Pyrvinium and XAV939 dramatically reduced injury-dependent β-catenin accumulation, GFP transgene expression, and proliferation of MG-derived progenitors (Fig. 3 E and F and Fig. S5). These data are consistent with the idea that a canonical Wnt signaling pathway regulates proliferation of MG-derived progenitors in the injured retina.
β-Catenin Stabilization Stimulates MG Dedifferentiation and Proliferation.
We next investigated whether pharmacological stabilization of β-catenin would enhance the regenerative response of MG after retinal injury. For this analysis, we injected either LiCl, GSK-3β inhibitor I, or vehicle (DMSO) into retinas of 1016 tuba1a:gfp transgenic fish at the time of injury, and at 4 dpi fish received a 3-h pulse of BrdU. GSK-3β inhibition resulted in a large expansion in the number of GFP+/BrdU+ retinal progenitors throughout the inner nuclear layer (Fig. S6A). Inspired by this finding, we introduced either the GSK-3β inhibitor or DMSO into the vitreous of an uninjured 1016 tuba1a:gfp fish retina by injection through the front of the eye. Remarkably, the GSK-3β inhibitor stimulated GFP transgene expression, β-catenin accumulation, and BrdU incorporation in MG throughout the retina (Fig. 4A and Fig. S6B). TUNEL showed that the GSK-3β inhibitor did not stimulate cell death (Fig. S6C).
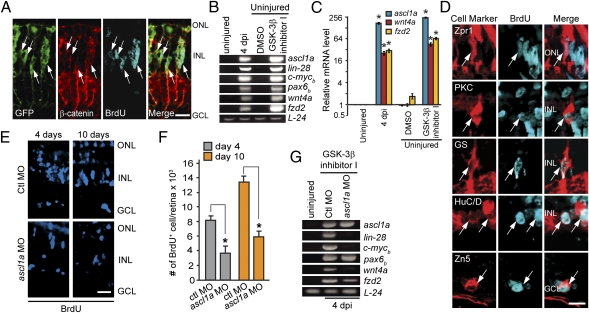
Fig. 4.
β-Catenin stabilization stimulates MG dedifferentiation and the generation of multiple retinal cell types in the uninjured retina. (A) Intravitreal injection of GSK-3β inhibitor induces GFP, β-catenin, and cell proliferation in the uninjured retina of 1016 tuba1a:gfp transgenic fish. (B and C) Expression of regeneration-associated genes in uninjured retinas treated with the GSK-3β inhibitor. *P < 0.0001. (D) GSK-3β inhibitor-induced retinal progenitors proliferate and generate all major retinal cell types. (E and F) Effect of Ascl1a knockdown on MG proliferation in the GSK-3β inhibitor-treated retina. *P < 0.002. (G) Effect of Ascl1a knockdown on genes associated with MG dedifferentiation in the GSK-3β inhibitor-treated retina. [Scale bars, 10 μm (A and D); 20 μm (E).] Abbreviations are as in Fig. 1.
To investigate whether the GSK-3β inhibitor actually stimulated MG dedifferentiation similar to retinal injury or simply forced MG into the cell cycle, we assayed for regeneration-associated genes such as ascl1a, lin-28, pax6b, c-mycb, wnt4a, and fzd2 (15–17). Indeed, the GSK-3β inhibitor stimulated a pattern of gene expression in the uninjured retina that was very similar to that after retinal injury (Fig. 4 B and C), and, at least for ascl1a, this expression was confined to proliferating MG (Fig. S6D). Thus, Wnt signaling feeds back to further activate genes associated with MG dedifferentiation and proliferation.
Dedifferentiated MG in GSK-3β Inhibited Uninjured Retina Are Multipotent.
The above data suggest that GSK-3β inhibition in the uninjured retina stimulates MG dedifferentiation and proliferation. To investigate whether these cells are multipotent, we pulse-labeled them with BrdU 4 d post-GSK-3β inhibition and examined their fate 10–18 d later using retinal cell type-specific antibodies. Remarkably, Zpr1+ cone photoreceptors, PKC+ bipolar cells, GS+ MG, HuC/D+ amacrine, and Zn5+ differentiating ganglion cells were generated from these progenitors (Fig. 4D).
Our data suggest that Ascl1a activates a Wnt signaling pathway that results in β-catenin stabilization and feeds back to further stimulate the expression of genes associated with MG dedifferentiation like ascl1a. Because Ascl1a was previously shown to be necessary for MG dedifferentiation (15), we asked whether its induction in the GSK-3β–inhibited retina was necessary for the generation of multipotent progenitors. To test this idea, we intravitreally delivered GSK-3β inhibitor with a control or ascl1a-targeting MO (Fig. S6E) and pulse labeled with BrdU at 4 dpi. Retinas were harvested 3 h and 6 d post-BrdU injection, and immunofluorescence was used to quantify progenitor-derived (BrdU+) retinal neurons and glia. Although Ascl1a knockdown almost completely suppressed MG proliferation in the injured retina (15), it only reduced MG proliferation by ∼50% in the GSK-3β–inhibited retina (Fig. 4 E and F), suggesting that Ascl1a may contribute to MG proliferation via β-catenin–dependent and –independent mechanisms.
Interestingly, GSK-3β inhibitor-induced progenitors (BrdU+), with and without Ascl1a knockdown, were not equivalent. First, progenitors with Ascl1a knockdown generated fewer neurons (40% less photoreceptor, 67% less bipolar, 43% less amacrine, and 100% less ganglion cells) and retained more MG characteristics (38% increase in BrdU+/GS+ MG) than those with Ascl1a expression. Second, Ascl1a knockdown suppressed expression of a subset of genes associated with MG dedifferentiation, such as lin-28, mycb, and wnt4a (Fig. 4G). These results suggest that injury-dependent or GSK-3β inhibitor-dependent Ascl1a induction may not only stimulate MG dedifferentiation and proliferation but also influence their differentiation into neurons and glia.
Discussion
MG dedifferentiation and proliferation are key to retina regeneration in zebrafish. We report here that activation of a canonical Wnt signaling pathway is necessary for proliferation of dedifferentiated MG. This conclusion is supported by the observations that: (i) Stabilized β-catenin is first detected in MG at ∼2 dpi when they begin proliferating; (ii) Dkk overexpression or β-catenin destabilization suppressed injury-dependent MG proliferation; and (iii) β-catenin stabilization induced MG proliferation in the uninjured retina.
While investigating the temporal pattern of expression of Wnt signaling components to a focal retinal injury, we uncovered a surprisingly robust pan-retinal suppression of dkk gene expression that appeared to be dependent on Ascl1a induction. These data suggest that signals reporting retinal injury are not confined to MG immediately surrounding the injury site, as previously thought, but, rather, may rapidly diffuse throughout the retina. Although the nature of these signals remains unknown, reactive oxygen species are potential candidates because they are rapidly induced by injury and can diffuse in tissues and cross-membranes and regulate transcription factor activity (26). Interestingly, a gradient of hydrogen peroxide was recently shown to mediate wound detection in zebrafish (27). One consequence of this retinal wide response to injury is the induction of ascl1a gene expression, which leads to repression of dkk gene expression. Interestingly, ascl1a induction and dkk repression is transient in retinal neurons, but maintained in MG-derived progenitors. Although the mechanisms underlying this regulation are not completely clear, it is likely that Ascl1a mediates dkk repression indirectly via the activation of a dkk transcriptional repressor.
Although dkk suppression and wnt4a and 8b induction were observed at ∼6 hpi, β-catenin accumulation was not detected until 2 dpi when MG-derived progenitors began proliferating. This lag may reflect the need for induction of other Wnt signaling components, such as Fzd2, that are induced at later times during retina regeneration. Alternatively, low levels of Wnt signaling mediated by undetectable levels of β-catenin may participate in MG dedifferentiation at early times after retinal injury, whereas at later times, higher levels of Wnt signaling may regulate events such as cell proliferation and differentiation.
We were most intrigued by the observation that GSK-3β inhibition in the uninjured retina was sufficient to stimulate MG dedifferentiation into a cycling population of multipotent retinal progenitors. A similar result has been reported in rat retinal explants treated with GSK-3β inhibitors (11), suggesting a common mechanism underlying MG proliferation in zebrafish and mammals. Interestingly, ciliary neurotrophic factor, FGF/insulin, and glutamate have also been reported to stimulate MG proliferation in fish, birds, and mammals, respectively (14, 28, 29). Whether these factors stimulate a genetic program similar to that found in zebrafish MG-derived progenitors is not known; also unknown is whether Wnt signaling is necessary for these factors to stimulate MG proliferation.
Wnt signaling in the adult regenerating zebrafish retina appears to recapitulate development where Wnt signaling also regulates retinal progenitor proliferation (30). Interestingly, Wnt signaling components are enriched in adult mouse MG (31, 32), and this pathway appears to be involved in several mouse models of retinal degeneration (33, 34). These studies suggest that, although mammalian MG appear capable of activating the Wnt signaling pathway, this pathway may need to collaborate with other signal transduction cascades to stimulate MG dedifferentiation into multipotent progenitors.
The observation that early response genes (ascl1a, lin-28, c-mycb, pax6b, and wnt4a) associated with MG dedifferentiation were activated by GSK-3β inhibition in the uninjured retina suggests that stabilized β-catenin contributes to their expression in MG-derived progenitors. These genes may be important for proliferating MG to function as multipotent stem cells and/or contribute to the capacity of these progenitors to differentiate into neurons and glia. Indeed, we found that knocking down Ascl1a expression in the GSK-3β inhibited retina dramatically reduced the production of new neurons from these progenitors.
It is tempting to speculate that the poor regenerative capacity of mammals may be associated with their lack of expression of dedifferentiation-associated genes in proliferating MG. Consistent with this idea, we note that NMDA-mediated retinal damage combined with EGF-induced MG proliferation did not stimulate retina regeneration or Ascl1 induction in the mouse (13). In contrast, the postnatal chick retina does exhibit a limited amount of regeneration that is accompanied by a modest induction of Ascl1 expression (35). In addition, overexpression of basic helix–loop–helix and homeobox genes in the injured rat retina promoted the regeneration of cells with rod photoreceptor, amacrine, and horizontal cell phenotypes (36). Although the full constellation of genes necessary for MG dedifferentiation into a multipotent retinal progenitor is not yet defined, the zebrafish retina provides an ideal system for discovering these factors because of its robust regenerative response.
The fact that Wnt signaling activation was sufficient to stimulate MG proliferation in both rodent and zebrafish retinas suggests that this signaling cascade is a major control point for MG dedifferentiation and proliferation. However, unlike in zebrafish, activation of Wnt signaling in mammals does not appear to be sufficient to stimulate robust retina regeneration. This difference may result from incomplete activation of gene expression programs in mammals that underlie MG dedifferentiation. It will be interesting to determine whether genes associated with retina regeneration in fish such as ascl1a, lin-28, and pax6 remain repressed in the injured mammalian retina exposed to GSK-3β inhibitors and whether overexpression of these genes or signaling pathways that activate these genes improves retina regeneration in mammals.
Methods
Plasmid Construction, RNA Isolation, PCR, and mRNA Synthesis.
All primers used in this study are listed in Table S1. A 4-kb zebrafish dkk1b promoter was cloned to create dkk1b:gfp-luciferase. Total RNA was isolated by using TRIzol (Invitrogen). PCRs were as described (15). Capped mRNAs were synthesized by using the mMESSAGE mMACHINE kit (Ambion) according to manufacturer's instructions. Detailed information is provided in SI Methods.
Animals, Heat Shock, Retinal injury, MO Delivery, and FACS.
gfap:GFP, hs:Dkk1GFP, TOPdGFP, and 1016 tuba1a:gfp fish and retinal lesions were described (4, 22, 23, 37). For heat shock, fish were transferred to preheated 36.5 °C water for 1 h every 12 h beginning 1 d before injury. MO delivery to cells was facilitated by electroporation (16). MG and non-MG were purified by FACS (15). Detailed information is provided in SI Methods.
BrdU Labeling, Immunofluorescence, and in Situ Hybridization.
BrdU labeling, immunofluorescence, and in situ hybridization were performed as described (4, 8, 15, 16). Double in situ hybridizations were performed according to manufacturer's instructions (Perkin-Elmer). Detailed information is provided in SI Methods.
Supplementary Material
Acknowledgments
We thank D. Hyde, R. Moon, and R. Dorsky for sharing transgenic fish; K. Cadigan for XAV939; J. Wan for oubain-treated retinal sections; M. Uhler for expression vectors and reagents; J. Beals for help with confocal microscopy; the University of Michigan Flow Cytometry Core for cell sorting; P. Macpherson for statistics; and R. Karr and T. Melendez for fish care. This work was supported by National Institutes of Health Grant NEI R01 EY018132 (to D.G.), National Institutes of Health National Institute of Child Health and Human Development Grant T32HD007507 (to R.R.), and a University of Michigan Center for Organogenesis grant (to X.-F.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.R.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107220108/-/DCSupplemental.
References
- 1.Otteson DC, Hitchcock PF. Stem cells in the teleost retina: Persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43:927–936. doi: 10.1016/s0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- 2.Sherpa T, et al. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev Neurobiol. 2008;68:166–181. doi: 10.1002/dneu.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mensinger AF, Powers MK. Visual function in regenerating teleost retina following cytotoxic lesioning. Vis Neurosci. 1999;16:241–251. doi: 10.1017/s0952523899162059. [DOI] [PubMed] [Google Scholar]
- 4.Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thummel R, Kassen SC, Montgomery JE, Enright JM, Hyde DR. Inhibition of Müller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev Neurobiol. 2008;68:392–408. doi: 10.1002/dneu.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandran R, Reifler A, Parent JM, Goldman D. Conditional gene expression and lineage tracing of tuba1a expressing cells during zebrafish development and retina regeneration. J Comp Neurol. 2010;518:4196–4212. doi: 10.1002/cne.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bringmann A, et al. Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009;28:423–451. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Wan J, Zheng H, Xiao HL, She ZJ, Zhou GM. Sonic hedgehog promotes stem-cell potential of Müller glia in the mammalian retina. Biochem Biophys Res Commun. 2007;363:347–354. doi: 10.1016/j.bbrc.2007.08.178. [DOI] [PubMed] [Google Scholar]
- 11.Osakada F, et al. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer AJ, Scott MA, Ritchey ER, Sherwood P. Mitogen-activated protein kinase-signaling regulates the ability of Müller glia to proliferate and protect retinal neurons against excitotoxicity. Glia. 2009;57:1538–1552. doi: 10.1002/glia.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karl MO, et al. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci USA. 2008;105:19508–19513. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeda M, et al. alpha-Aminoadipate induces progenitor cell properties of Müller glia in adult mice. Invest Ophthalmol Vis Sci. 2008;49:1142–1150. doi: 10.1167/iovs.07-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28:1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thummel R, et al. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res. 2010;90:572–582. doi: 10.1016/j.exer.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Yang X, Yang S, Zhang J. The Wnt /β-catenin signaling pathway in the adult neurogenesis. Eur J Neurosci. 2011;33:1–8. doi: 10.1111/j.1460-9568.2010.7483.x. [DOI] [PubMed] [Google Scholar]
- 21.Osada H, et al. Roles of achaete-scute homologue 1 in DKK1 and E-cadherin repression and neuroendocrine differentiation in lung cancer. Cancer Res. 2008;68:1647–1655. doi: 10.1158/0008-5472.CAN-07-5039. [DOI] [PubMed] [Google Scholar]
- 22.Stoick-Cooper CL, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 23.Dorsky RI, Sheldahl LC, Moon RT. A transgenic Lef1/beta-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev Biol. 2002;241:229–237. doi: 10.1006/dbio.2001.0515. [DOI] [PubMed] [Google Scholar]
- 24.Thorne CA, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 26.Oktyabrsky ON, Smirnova GV. Redox regulation of cellular functions. Biochemistry (Mosc) 2007;72:132–145. doi: 10.1134/s0006297907020022. [DOI] [PubMed] [Google Scholar]
- 27.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassen SC, et al. CNTF induces photoreceptor neuroprotection and Müller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp Eye Res. 2009;88:1051–1064. doi: 10.1016/j.exer.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Müller glia of the chicken retina. J Neurosci. 2002;22:9387–9398. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi M, et al. Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development. 2005;132:3027–3043. doi: 10.1242/dev.01881. [DOI] [PubMed] [Google Scholar]
- 31.Jadhav AP, Roesch K, Cepko CL. Development and neurogenic potential of Müller glial cells in the vertebrate retina. Prog Retin Eye Res. 2009;28:249–262. doi: 10.1016/j.preteyeres.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roesch K, et al. The transcriptome of retinal Müller glial cells. J Comp Neurol. 2008;509:225–238. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi H, Nakamura RE, Mohamed O, Dufort D, Hackam AS. Characterization of Wnt signaling during photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2007;48:5733–5741. doi: 10.1167/iovs.07-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu C, Nathans J. An essential role for frizzled 5 in mammalian ocular development. Development. 2008;135:3567–3576. doi: 10.1242/dev.028076. [DOI] [PubMed] [Google Scholar]
- 35.Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- 36.Ooto S, et al. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci USA. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassen SC, et al. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67:1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.