On a bone-cold morning in February 2000, hours after their plane touched down at Chicago's O'Hare Airport, Venkatraman “Venki” Ramakrishnan, a structural biologist at the Laboratory of Molecular Biology in Cambridge, England, and three of his colleagues hurried to Argonne National Laboratory, the US Department of Energy's sprawling research center southwest of Chicago. The men had 48 hours to complete their task: obtain high-resolution data on a portion of bacterial cells’ protein synthesizing machinery—the ribosome—by shining X-rays on their crystals to help reveal their structure. The trip was a gamble that Ramakrishnan had planned for months, one that could lead to a signal moment in structural biology or fizzle as a failed experiment. With hard-won time at Argonne's Advanced Photon Source X-ray generator, Ramakrishnan hoped to beat his competitors in assembling a map of the ribosome's small subunit that would pinpoint the location of its atoms in 3D space. Fighting off jet lag, Ramakrishnan and his colleagues collected data on their crystals. As the results trickled in, he realized that the experiment had worked; the researchers were on their way to building the first map of the ribosome's so-called “30S” subunit. Perhaps no length of prose can capture Ramakrishnan's excitement over the experiment's outcome, but he summed up the moment with surprising clairvoyance. “We're going to be famous,” he declared to his team. Nearly a decade later, Ramakrishnan, a member of the National Academy of Sciences, shared the Nobel Prize in chemistry with two other researchers for uncovering the structure of the ribosome. The structure helped pave the way for the advent of novel antibiotics that could someday help people fight infections.

Venkatraman Ramakrishnan. Image courtesy of Medical Research Council Laboratory of Molecular Biology.
Home to a Hindu deity worshipped as part of a holy trinity, Chidambaram is a sun-drenched temple town in southern India, south of the booming metropolis of Chennai. Ramakrishnan was born and lived there until the age of 3, when his parents, both researchers, moved north to Vadodara, where his father was called to lead the university's biochemistry department. There, Ramakrishnan watched his father cobble together a biology laboratory to perform experiments that snagged him a pair of Nature papers early in his tenure despite a dearth of resources. “My childhood was filled with visiting scientists from India and abroad, and that made me realize that science was an international enterprise,” he says. Although he grew up in a household where science held sway, Ramakrishnan developed interests in science and the humanities alike. But as often happens to Indian high-school graduates, thanks to the country's middle-class struggle for social mobility, Ramakrishnan's choice to pursue science in college seemed a foregone conclusion.
After a year-long preparatory course in science at Vadodara's Maharaja Sayajirao University, Ramakrishnan was again at a professional crossroads. This time, however, the choice was between medicine, engineering, and basic science. With his mother's support, he chose basic science, having won a national science talent scholarship similar to the storied Intel Science Talent Search, which scours the United States for young minds of scientific promise. In 1968, Ramakrishnan enrolled at Vadodara for a bachelor's degree in physics, absorbing a then-new curriculum based on a University of California, Berkeley physics course, replete with the now-reputed Feynman Lectures on Physics. After graduation, Ramakrishnan set off for more challenging pastures, arriving in the United States in 1971, a letter of acceptance to Ohio University's physics graduate program in hand.
At Ohio University, Ramakrishnan studied with the physicist Tomoyasu Tanaka, unraveling fine details of ferroelectricity—a property of some materials that allows researchers to apply electric fields to reverse their electrical polarity—in a chemical called monopotassium phosphate, now used in fertilizers, fungicides, and buffering solutions for chemical reactions. Although he soldiered through his Ph.D. in physics, Ramakrishnan had misgivings about his choice of physics as a career, not least because of a growing sense that biologists leapfrogged through scientific advances while physicists inched along. An undercurrent of scientific restlessness combined with an awe of breathless reports of biologists’ advances in Scientific American precipitated something of a career change, close on the heels of his graduation in 1976, a year that marks a turning point in Ramakrishnan's now-luminous career.
Bend in the Road
It was the developmental biologist Sydney Brenner, a Nobelist and mentor to dozens of molecular biologists, who said, “I'm a strong believer that ignorance is important in science. If you know too much, you start seeing reasons why things won't work. That's why it's important to change your field to collect more ignorance” (1). Those words befit Ramakrishnan's foray into biology, a move partly prompted by his mounting impatience to strike out in a field where he was convinced his efforts would pay off relatively quickly. Moreover, the year of his graduation marked the birth of his son with his wife Vera Rosenberry, a writer of children's books, whom he married at the age of 23. Determined to gain a fundamental understanding of biology, he enrolled for another Ph.D. at the University of California, San Diego. But after 2 fruitful years, he accepted a postdoctoral position in the laboratory of Yale University biophysicist Peter Moore, a long-time maven of ribosomes, the cellular assembly line on which proteins are minted. At Yale, Moore and his coworkers, including the biochemist Don Engelman, put Ramakrishnan through his paces, as he learned to isolate, purify, reconstitute, and assay ribosomes from cells. “The specialized methods I learned in Peter's lab were invaluable to me 20 years later when I started tackling the structure of the 30S subunit that led to the Nobel Prize,” Ramakrishnan writes in his autobiography (2).
Long known to biologists as a dizzying constellation of atoms whose three-dimensional arrangement holds a key to the secret of life, ribosomes help orchestrate protein synthesis. But their mechanism of action was largely shrouded in mystery, partly because their structure, which can be likened to an asymmetrical macaroon of two subunits through which a messenger RNA molecule is threaded, remained elusive. Moore and Engelman hoped to unveil the structure of the bacterial ribosome's small subunit, dubbed 30S for the way it sediments in laboratory experiments, by using a biophysical technique called small angle neutron scattering at Brookhaven National Laboratory in Long Island, New York. The technique exploits differences in the patterns of scattering of neutrons bombarding either regular ribosomes or ribosomes where specific hydrogen atoms are labeled with the isotope deuterium. “Deuterium atoms scatter neutrons very differently from hydrogen atoms,” Ramakrishnan explains, adding that the differences in the scattering signals could yield information about the relative distances between proteins that compose the ribosomal subunit. Signal by painstaking signal, researchers then build a rough map of the subunit, positioning its proteins in 3D space.
Moore and Engelman's map, which Ramakrishnan helped assemble, was a useful guide to navigating the murky world of the 30S subunit. But a closer look at the ribosome still eluded researchers; the technique lacked the sensitivity to explore the subunit's atomic structure. Years later, Ramakrishnan surmounted the problem using an array of strategies in work that garnered the coveted attention of a prize committee in Stockholm.
Despite his contributions to ribosomal structure at Yale, Ramakrishnan's entry into academia was decidedly hard-won. After applying for a number of faculty positions, he accepted an appointment in the biology division at Oak Ridge National Laboratory in Tennessee, where his charge was to help biologists carry out collaborative experiments in neutron scattering. While at Oak Ridge, he struck up partnerships with structural biologists Gerard Bunick and Ed Uberbacher, his sight narrowed on chromatin, the architectural arrangement of DNA and proteins that makes up chromosomes. Eager for bigger challenges, Ramakrishnan approached his erstwhile collaborator Benno Schoenborn at Brookhaven National Laboratory, where he was eventually hired as a tenure-track researcher.
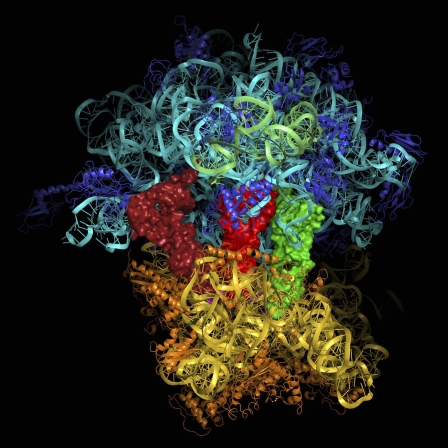
70S ribosomal subunit. Image courtesy of Venkatraman Ramakrishnan.
At Brookhaven, Ramakrishnan began by refining Moore and Engelman's neutron scattering map to help settle a long-standing debate over the relative position of the proteins and RNA that compose the ribosomal small subunit. “I did an experiment where I enhanced the contrast between protein and RNA and showed that the ribosomal proteins were not quite covering the RNA but that there was an asymmetry in their locations,” he says. Those findings led to his first solo publication in Science (3). But neutron maps with their limited ability to reveal atomic details of the ribosome no longer held Ramakrishnan's focus. He wished to unravel the X-ray crystal structure of the ribosomal subunit, a technical feat that could pinpoint the precise locations of most of the subunit's atoms. To that end, he teamed up with Brookhaven biologist Steve White, who tried to purify ribosomal proteins from bacteria that thrive in hot springs. But the meager yield of ribosomal proteins from the bacteria meant that Ramakrishnan needed more powerful techniques to isolate enough protein for analysis. Before long, he learned to produce quantities of the proteins by cloning their genes into Escherichia coli bacteria, a genetic engineering workhorse. By then, the stage had been set for Ramakrishnan's decades-long venture into the world of ribosomal structure. Armed with practical know-how in protein crystallography, thanks to a Cold Spring Harbor Laboratory course, Ramakrishnan handily crystallized and collected preliminary data on two proteins that piqued his interest, the ribosomal subunit protein S5 and the chromosome-forming protein H5, a testament to his continued interest in chromatin. When it came time to further analyze the data in hopes of solving the proteins’ crystal structures, he realized a transatlantic trip was in order.
In a Crystal, Darkly
In the summer of 1991, supported by a Guggenheim Fellowship, Ramakrishnan moved for a year-long sabbatical to the Laboratory of Molecular Biology in Cambridge, England, hailed as the birthplace of crystallography. At the LMB, whose hallowed halls have hosted the laboratories of researchers like James Watson, Francis Crick, Sydney Brenner, and countless others, he learned how to solve the structure of his protein crystals, publishing his findings in Nature (4, 5).
Crystallographers glean atomic detail from protein crystals by shining X-rays on them; the wavelength of X-rays is attuned to interatomic distances. Protein crystals, which house millions of identical protein molecules arrayed in a lattice, scatter the X-rays, which are then recombined using computer software, not unlike a lens, into concentrated spots that help reconstruct the protein's 3D architecture. But to recombine the scattered rays meaningfully, researchers must first solve a physical parameter called the “phase” of the scattered X-rays. “Phase provides information about where each of the scattered waves is positioned relative to the others. That information helps recombine the rays into an image,” Ramakrishnan explains. To solve the phase problem, Ramakrishnan used a particle accelerator called a synchrotron and a technique known as multiwavelength anomalous scattering (6), which helped produce stunningly realized structural maps of the H5 protein crystals.
Encouraged by the success of his sabbatical, Ramakrishnan returned to Brookhaven, determined to focus on the crystallography of ribosomal proteins together with White. But declining support at Brookhaven for investigator-led research, compared with the LMB's seemingly bottomless freedom and funding, left him yearning for an environment where he could nurture his dream of mapping the ribosomal subunit into reality. Thanks to an acquaintance struck with University of Utah biochemist Wes Sundquist during the sabbatical, Ramakrishnan accepted a faculty position in the biochemistry department at the University of Utah. There, he set to work on the ribosomal subunit, solving the structure of a handful of its proteins. As a picture emerged, he realized that the project would lose steam without sustained funding, technical expertise, and scientific free rein of the sort he enjoyed at the LMB. That is partly why, when then-director Richard Henderson of the LMB offered him a position—one that came with a sizeable pay cut—he accepted.
A Dream Realized
Months before his move to the LMB, Ramakrishnan began work on solving the structure of the entire 30S subunit, a feat attempted by few until then. Among them was Ada Yonath, a crystallographer at the Weizmann Institute of Science in Israel, who went on to share the Nobel Prize with Ramakrishnan. Beginning in the early 1980s, Yonath had carved for herself a niche in ribosome crystallography by successfully coaxing bacteria to yield crystals of the ribosome's larger, 50S subunit that revealed relatively high-resolution structural details. And a Russian group had made the first crystals of the 30S subunit, albeit ones that did not lend themselves well to high-resolution structural study. “By then, I had the idea that if I could get enough crystals of the 30S subunit, I could use the techniques I had used to solve the H5 phase problem to solve the subunit's structure,” Ramakrishnan says.
But to crystallize a behemoth like the 30S subunit was no mean task. Unlike sugar and salt, which readily crystallize thanks to close-knit repeating motifs that make up their lattice like a tightly woven carpet, crystals of large proteins, not to mention molecular machines like ribosomes, contain far-flung building blocks held together by fewer contacts between atoms. Disturb the delicate equilibrium between the building blocks, and bedlam ensues, collapsing the crystal like a house of cards. Also, the uniformity of orientation among the molecules that make up a crystal determines how well the crystal can scatter X-rays, in turn determining the level of detail that researchers can wrest from it.
Through rigorous biochemistry, Ramakrishnan's LMB team first obtained crystals of the 30S subunit that scattered X-rays at a respectable resolution of 5 Å. By then, however, Yonath had set her sights on the 30S subunit, working furiously to solve its structure through somewhat different methods. In a now-storied race to solve the ribosomal structure, Ramakrishnan's team rushed to collect X-ray data from their improved crystals at Argonne's Advanced Photon Source facility. With further technical refinements, the team published in a 2000 Nature report a higher resolution atomic structure of the 30S subunit from the bacterium Thermus thermophilus, a denizen of hot springs (7). At around the same time, Yonath published her high-resolution structure of the 30S subunit, ending what had been a fiercely competitive race in a resounding tie (8).
Ramakrishnan's structure of the 30S subunit led to his 2009 Nobel Prize in chemistry, which he shared with Yonath and Yale University structural biologist Thomas Steitz, who unraveled the structure of the larger ribosomal subunit. The prize committee summarized years of scientific toil and a nail-biting race to the finish line in a few sentences, “…for having showed what the ribosome looks like and how it functions at the atomic level. All three have used a method called X-ray crystallography to map the position for each and every one of the hundreds of thousands of atoms that make up the ribosome.”
Pursuit in Progress
Ramakrishnan's crystal structure of ribosomes attached to antibiotics, such as tetracycline and streptomycin, revealed how the antibiotics attack bacteria. Because many antibiotics target ribosomes, whose atomic structure differs between people and bacteria, targeting those differences could help researchers develop antibiotics with improved potency and help address the growing problem of antibiotic resistance that plagues the treatment of infectious diseases. That reasoning was a driving force behind Connecticut-based Rib X, a biotechnology firm founded by Steitz that Ramakrishnan advises. “Right now, Rib X is using 50S and whole-bacterial ribosome structures to design better antibiotics. The hope is that they will eventually also use the 30S and 70S structures from our lab,” he says.
His future goals are ambitious: to help uncover the workings of the eukaryotic ribosome, a molecular machine of staggering complexity whose unraveling would no doubt call for the combination of drive and determination that have come to define Ramakrishnan. “I am an avid hiker. Sometimes, you have to force yourself to keep going even though you may be exhausted. In long and challenging structural projects, where the next breakthroughs are not within sight, a hiker's endurance comes in handy,” he says.
Now known everywhere as a part of a standard-bearing triumvirate of authorities on ribosomal structure, Ramakrishnan says he is a member of a larger scientific workforce devoted to solving this structural puzzle. The Nobel Prize, he points out, inevitably lessens the efforts of other researchers who toiled alongside the eventual winners, often contributing to their discoveries. “The Nobel Prize was instituted at a time when the scientific enterprise was very different from what it is today. So, it gives the impression of science as a sporting contest. But science isn't like that, and I have deep misgivings about prizes,” he says. Yet given the constraints, he hastens to suggest, the committee may have picked the trio because the structures and ensuing insights they uncovered made the single biggest difference to the field.
Footnotes
This is a Profile of a recently elected member of the National Academy of Sciences to accompany the member's contributed article on page 15798.
References
- 1.Duncan DE. The Geneticist Who Played Hoops with My DNA. New York: Harper Collins; 2005. p. 192. [Google Scholar]
- 2.Ramakrishnan V. Autobiography. From Chidambaram to Cambridge: A life in science. 2009. Available at http://nobelprize.org/nobel_prizes/chemistry/laureates/2009/ramakrishnan.html. Accessed June 29, 2011.
- 3.Ramakrishnan V. Distribution of protein and RNA in the 30S ribosomal subunit. Science. 1986;231:1562–1564. doi: 10.1126/science.3513310. [DOI] [PubMed] [Google Scholar]
- 4.Ramakrishnan V, White SW. The structure of ribosomal protein S5 reveals sites of interaction with 16S rRNA. Nature. 1992;358:768–771. doi: 10.1038/358768a0. [DOI] [PubMed] [Google Scholar]
- 5.Ramakrishnan V, Finch JT, Graziano V, Lee PL, Sweet RM. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature. 1993;362:219–223. doi: 10.1038/362219a0. [DOI] [PubMed] [Google Scholar]
- 6.Hendrickson WA. Determination of macromolecular structures from anomalous diffraction of synchrotron radiation. Science. 1991;254:51–58. doi: 10.1126/science.1925561. [DOI] [PubMed] [Google Scholar]
- 7.Wimberly BT, et al. Structure of the 30S ribosomal subunit. Nature. 2000;407:327–339. doi: 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- 8.Schluenzen F, et al. Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution. Cell. 2000;102:615–623. doi: 10.1016/s0092-8674(00)00084-2. [DOI] [PubMed] [Google Scholar]