Abstract
A truncated and constitutively active form of the EGF receptor, variant III (EGFRvIII), is a major determinant of tumor growth and progression in glioblastoma multiforme (GBM). Extensive bidirectional crosstalk occurs in the cell-signaling pathways downstream of the EGFR and the urokinase-type plasminogen activator receptor (uPAR); however, crosstalk between EGFRvIII and uPAR has not been examined. Here, we show that uPAR does not regulate ERK activation in EGFRvIII-expressing GBM cells; however, in GBM cells isolated from four separate xenografts in which EGFRvIII expression was down-regulated in vivo, uPAR assumed a major role in sustaining ERK activation. Phosphorylation of Tyr-845 in the EGFR, which is mediated by Src family kinases, depended on uPAR in EGFRvIII-expressing GBM cells. Activation of the mitogenic and prosurvival transcription factor, STAT5b, downstream of EGFRvIII, also required uPAR. The EGFR-selective tyrosine kinase inhibitors, erlotinib and gefitinib, blocked not only EGFRvIII signaling to ERK but also uPAR-dependent STAT5b activation. uPAR gene silencing in EGFRvIII-expressing GBM cells and in cells from tumors that escaped dependency on EGFRvIII decreased cell survival and proliferation. Xenografts of EGFRvIII-expressing cancer cell lines and a human GBM, which was propagated as a xenograft, were robustly immunopositive for uPAR and phospho–Tyr-845 by immunohistochemistry. A human GBM in which the EGFR gene was amplified without truncation was immunonegative for both uPAR and phospho–Tyr-845. These studies identify distinct cell-signaling activities for uPAR in GBM cells that express EGFRvIII and in cells released from dormancy when EGFRvIII is neutralized. uPAR and its crosstalk pathways with EGFRvIII emerge as logical targets for therapeutics development in GBM.
Keywords: c-Src, STAT3, cell death, RNA interference
Glioblastoma multiforme (GBM) is an aggressive astrocytic tumor, rarely cured by surgical resection because of extensive local invasion and widespread neuraxis dissemination (1, 2). The gene for the EGF receptor (EGFR) is amplified in ∼50% of all GBMs; however, EGFR overexpression occurs in the vast majority of GBMs, even in the absence of gene amplification (3–5). EGFR gene amplification is commonly accompanied by an in-frame deletion of exons 2–7, which encode part of the extracellular ligand-binding site of the receptor (6). The resulting truncated receptor, EGFR variant III (EGFRvIII), is constitutively active, contributing to an aggressive phenotype in the GBM cells (7–9). Concurrent EGFRvIII expression and deletion of tumor suppressor genes generates gliomas in mice, suggesting that EGFRvIII may be involved in GBM development as well as progression (10).
The efficacy of EGFR-targeting therapeutics, such as gefitinib and erlotinib, has been evaluated in clinical trials (11, 12). Although these agents are active in some patients, tumors typically escape control, reestablishing aggressive growth and invasion. Failure of EGFR-targeting therapeutics to induce sustained remission may reflect mutations in gene products downstream of the EGFR, such as the tumor suppressor PTEN, or activation of alternative receptors with overlapping cell-signaling activity (10, 12–14). Mukasa et al. (15) developed a derivative of the U373MG GBM cell line in which EGFRvIII is expressed under the control of a doxycycline (Dox)-repressible promoter system. These cells establish xenografts in mice only if EGFRvIII is expressed. When the mice are treated with Dox to suppress EGFRvIII expression, the tumors enter a state of dormancy; however, many tumors eventually reestablish aggressive growth. Tumors that escape dependency on EGFRvIII in mice may represent a model for phenotypes that emerge in patients with GBM who are treated with EGFR-targeting therapeutics (15).
Like the EGFR, the urokinase-type plasminogen activator (uPA) receptor (uPAR) is frequently expressed at high levels in GBMs (16). uPAR promotes cancer invasion by binding uPA and facilitating activation of extracellular proteases (17). uPAR also forms a multiprotein receptor complex with potent cell-signaling activity (17, 18). uPAR-initiated cell signaling may be triggered by uPA or occur independently of uPA (17–21). Crosstalk between uPAR and the EGFR is extensive. uPA binding to uPAR results in EGFR transactivation, which may be important for ERK activation (22, 23). uPAR-initiated cell signaling also leads to phosphorylation of Tyr-845 in the EGFR, which is necessary for activation of the transcription factor STAT5b and for cell proliferation in response to EGF (24).
Previous studies have shown that, in GBM cells, uPAR promotes activation of extracellular proteases and inhibits apoptosis by activating PI3K (25–27). Crosstalk between uPAR and EGFRvIII has not been examined. The goal of the present study was to characterize the function of uPAR in GBM cells that express EGFRvIII and in cells that escape dependency on EGFRvIII in vivo. We find that uPAR plays a central role in sustaining ERK activation in GBM cells isolated from xenografts that escape from dormancy after EGFRvIII is down-regulated in vivo. uPAR-dependent ERK activation is known to promote cancer cell survival, proliferation, and release from states of dormancy (28, 29). In GBM cells that express EGFRvIII, uPAR did not regulate ERK but was necessary for activation of STAT5b, which is a recently identified downstream effector of EGFRvIII (30). Src family kinases (SFKs) play a central role in EGFR-dependent STAT5b activation by phosphorylating Tyr-845 in the EGFR (31, 32). uPAR emerges as an major cell-signaling receptor in GBM because of crosstalk with EGFRvIII and its ability to sustain ERK activation when EGFRvIII is absent.
Results
EGFRvIII-Regulated Cell Signaling in GBM Cells.
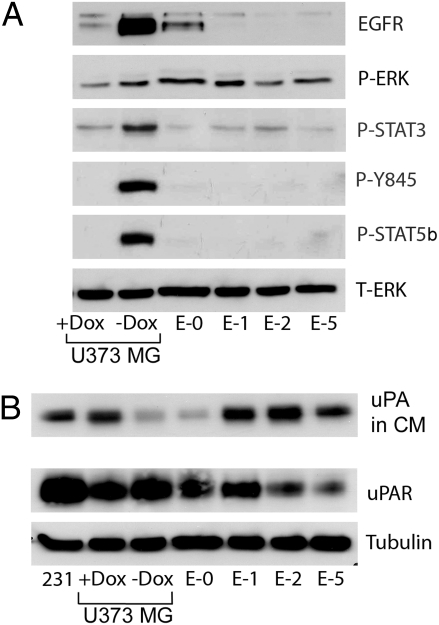
U373MG cells were treated with Dox for 5 d in vitro. Fig. 1A shows that Dox blocked EGFRvIII expression, as anticipated (15). U373MG cells expressed uPAR, which was not significantly altered by Dox treatment (Fig. 1B). Thus, we examined the effects of Dox on the activity of cell-signaling factors known to be regulated by both EGFRvIII and uPAR. In Dox-treated U373MG cells, phospho-ERK (P-ERK) was slightly decreased and P-STAT3 was more substantially decreased, as anticipated (15).
Fig. 1.
Tyr-845 and STAT5b are phosphorylated in EGFRvIII-expressing U373MG cells. (A) U373MG cells were treated with Dox (1 μg/mL) or vehicle for 5 d and maintained in SFM for the last 2 d. Escaper tumor cells (E-0, E-1, E-2, and E-5) were maintained in Dox and treated similarly. Cell extracts were subjected to immunoblot analysis to detect P-ERK, P-STAT3, P–Tyr-845 in the EGFR, P-STAT5b and total ERK (T-ERK). (B) Conditioned SFM (CM) was collected from cultures at equivalent confluency and concentrated 10 times. Immunoblot analysis was performed to detect uPA. The control lane labeled “231” shows CM from MDA-MB-231 cells, which express high levels of uPA (29). Cell extracts were subjected to immunoblot analysis to detect uPAR and tubulin as a loading control.
SFKs and the EGFR are synergistic in promoting cancer progression, which is explained at least in part by the ability of SFKs to phosphorylate Tyr-845 in the EGFR (31). P–Tyr-845 plays a critical role in activation of STAT5b (32), a mitogenic transcription factor implicated in GBM progression (30). In EGFRvIII-expressing U373MG cells, P–Tyr-845 was readily detected. P–Tyr-845 was absent in Dox-treated cells, as anticipated. Similarly, P-STAT5b was abundant in EGFRvIII-expressing cells and undetected in Dox-treated cells. These results demonstrate the importance of the EGFR in STAT5b activation in GBM cells.
In Dox-treated U373MG cells, uPA accumulated to an increased level in conditioned medium (CM) compared with EGFRvIII-expressing cells (Fig. 1B). Endogenously produced uPA is known to activate uPAR-dependent cell signaling in an autocrine circuit (29). Next, we examined four GBM cell lines (E-0, E-1, E-2, and E-5) generated from different xenografts in which EGFRvIII expression was down-regulated in vivo (“escaper” tumors). Residual EGFRvIII expression was detected in only the E-0 cells. In the three other escaper tumor cell lines, uPA accumulation in CM was increased, similar to the U373MG cells that were treated with Dox in vitro. P-ERK levels were largely unchanged in the escaper tumor cells, compared with U373MG cells that express EGFRvIII. P-STAT3 was decreased (15). P–Tyr-845 and P-STAT5b were not detected in the E-1, E-2, and E-5 cells. In E-0 cells, which retained some EGFRvIII, low levels of P–Tyr-845 and P-STAT5b were observed when we overexposed the immunoblots.
Effects of uPAR on GBM Survival and Proliferation.
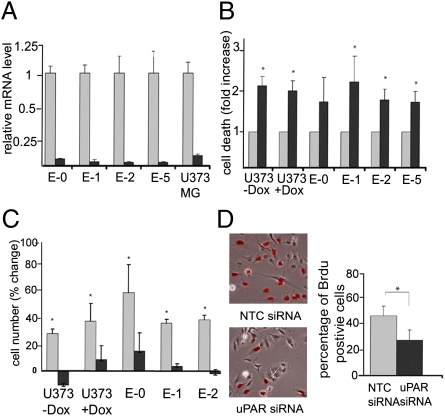
We hypothesized that uPAR-dependent cell signaling may promote GBM cell survival and proliferation when EGFRvIII is absent. To test this hypothesis, uPAR was silenced in U373MG cells as well as in E-0, E-1, E-2, and E-5 cells. Control cells were transfected with nontargeting control (NTC) siRNA. Fig. 2A shows that uPAR gene silencing was at least 95% effective at the mRNA level in all of the cell lines. Fig. 2B shows that uPAR gene silencing significantly increased cell death (P < 0.05) in cultures of Dox-treated U373MG cells and in escaper tumor cells that lacked EGFRvIII (E-1, E-2, and E-5). E-0 cells, which retained a low level of EGFRvIII, demonstrated increased cell death when uPAR was silenced; however, the increase was not statistically significant at the P < 0.05 level. uPAR gene silencing also significantly increased cell death in EGFRvIII-expressing U373MG cells (no Dox treatment), which was an unanticipated result.
Fig. 2.
Effects of uPAR on growth and survival of EGFRvIII-positive and -negative GBM cells. (A) U373MG and escaper tumor cells were transfected with NTC (gray bars) or uPAR-specific (black bars) siRNA. uPAR mRNA levels were determined by qPCR and standardized against the levels present in cells treated with NTC siRNA (mean ± SEM, n = 3). (B) GBM cells were transfected with uPAR-specific (black bars) or NTC (gray bars) siRNA. After culturing in SFM for 48 h, cell death was determined with the Cell Death Detection ELISA Kit (mean ± SEM, n = 3). (C) Cells transfected with uPAR-specific (black bars) or NTC (gray bars) siRNA were cultured for 48 h in SFM. The viable cell number was determined by MTT assay before (0 h) and after the 48-h incubation. The change in cell number is plotted (mean ± SEM, n = 3). (D) U373MG cells were transfected with uPAR-specific or NTC siRNA. BrdU (100 μM) was added for 6 h in SFM. BrdU-positive cells (red) were detected by immunofluorescence microscopy (representative fields are shown). Cell proliferation was determined as the percentage of BrdU-positive cells (mean ± SEM, n = 3). *Values are significantly different at the P < 0.05 level.
As a second approach to study the effects of uPAR gene silencing in GBM cells, we performed 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, measuring the overall change in cell number during a 48-h incubation in serum-free medium (SFM). All of the cells that were transfected with NTC siRNA demonstrated cell growth in SFM. Cell growth was decreased (P < 0.05) by uPAR gene silencing (Fig. 2C). The number of EGFRvIII-expressing U373MG cells actually decreased during the 48-h incubation when uPAR was silenced.
Because we did not anticipate that uPAR gene silencing would decrease cell growth and survival in EGFRvIII-expressing U373MG cells, we further studied the effects of uPAR gene silencing by examining BrdU incorporation. The BrdU pulse-exposure time was 6 h. Fig. 2D shows that uPAR gene silencing decreased BrdU incorporation by 43%.
uPAR-Dependent Cell Signaling in GBM Cells.
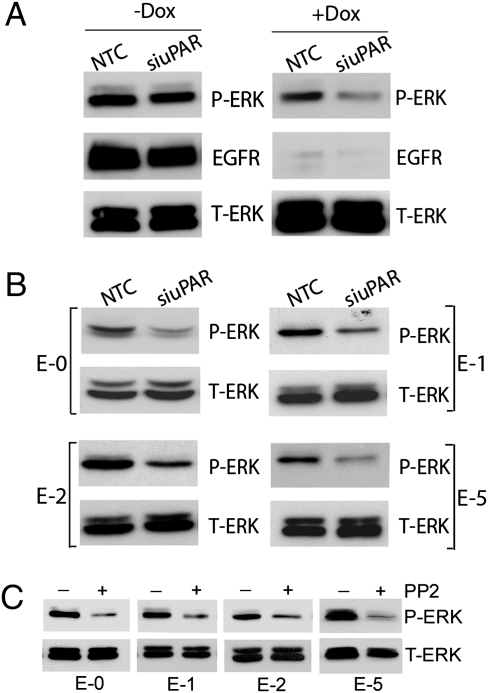
Next, we assessed the effects of uPAR gene silencing on cell signaling. In EGFRvIII-expressing U373MG cells transfected with NTC siRNA (no Dox treatment), ERK was highly phosphorylated. uPAR gene silencing had no effect on P-ERK (Fig. 3A), most likely reflecting the dominant activity of EGFRvIII. By contrast, in U373MG cells that were treated with Dox, uPAR gene silencing decreased P-ERK. Similarly, in cells derived from all four escaper tumors, uPAR gene silencing decreased P-ERK (Fig. 3B). Thus, in GBM cells that lose EGFR activity, uPAR provides a back-up pathway for sustaining ERK activation independently of the EGFR.
Fig. 3.
uPAR regulates ERK activation in EGFRvIII-negative GBM cells. (A) EGFRvIII-positive and -negative U373MG GBM cells were transfected with uPAR-specific or NTC siRNA, allowed to recover, and then cultured in SFM for 24 h. Cell extracts were prepared and subjected to immunoblot analysis to detect P-ERK, EGFR, and total ERK (T-ERK). (B) Extracts were prepared from escaper tumor cells that were transfected with uPAR-specific or NTC siRNA and subjected to immunoblot analysis. (C) Escaper tumor cells were transferred to SFM and treated with PP2 (1.0 μM) or vehicle for 12 h. Immunoblot analysis was performed.
In many cell types, SFKs function as essential mediators of uPAR-initiated cell signaling, upstream of diverse uPAR-activated factors, such as ERK and Rac1 (33, 34). Fig. 3C shows that the SFK-selective inhibitor 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine (PP2) inhibited ERK activation in all of the escaper tumor cells.
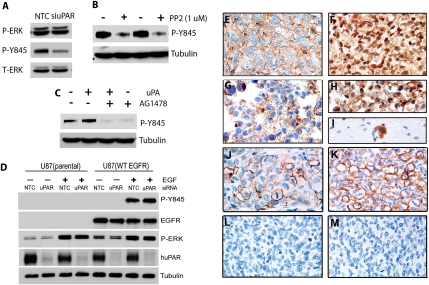
uPAR is one of many receptors capable of activating SFKs, which induce phosphorylation of Tyr-845 in the EGFR and activation of STAT5b (24, 35). Because uPAR gene silencing did not decrease P-ERK in EGFRvIII-expressing U373MG cells, we examined the effects of uPAR gene silencing on P–Tyr-845. Fig. 4A shows that uPAR gene silencing almost entirely eliminated P–Tyr-845 (a weak signal was apparent at high exposure). Similarly, phosphorylation of STAT5b was essentially blocked by uPAR gene silencing. As a control, we examined phosphorylation of Tyr-1173 in the EGFR, which is not an SFK substrate. uPAR gene silencing had no effect on P–Tyr-1173. These results show that, in EGFRvIII-expressing U373MG GBM cells, uPAR is essential for phosphorylation of Tyr-845 in EGFR and STAT5b activation.
Fig. 4.
uPAR regulates P–Tyr-845 and STAT5b in EGFRvIII-expressing U373MG cells. (A) EGFRvIII-positive U373MG GBM cells were transfected with uPAR-specific or NTC siRNA and transferred to SFM for 48 h. Immunoblot analysis was performed. (B) U373MG cells were transfected with NTC (gray bar) or uPA-specific (black bar) siRNA. uPA mRNA levels were determined by qPCR (mean ± SEM, n = 3). Extracts were prepared from cells in which uPA was silenced and control cells and subjected to immunoblot analysis. (C) U373MG cells were transferred to SFM and treated with PP2 (1 μM) or vehicle for 12 h. Immunoblot analysis was performed. (D) U373MG cells were transfected with uPAR-specific or NTC siRNA, transferred to SFM, and treated with erlotinib (100 nM), gefitinib (100 nM), or vehicle for 12 h. Cell extracts were subjected to immunoblot analysis.
uPAR-initiated cell signaling occurs in response to uPA binding and, under some conditions, in the absence of uPA (17–20, 29). To test whether endogenously produced uPA is involved in the pathway by which uPAR regulates STAT5b, we silenced uPA gene expression in U373MG cells. Fig. 4B shows that uPA gene silencing was greater than 90% effective at the mRNA level. uPA gene silencing substantially inhibited but did not entirely block Tyr-845 phosphorylation. Similarly, P-STAT5b was substantially decreased in uPA gene-silenced cells.
The SFK-selective tyrosine kinase inhibitor PP2 decreased P–Tyr-845 and P-STAT5b in U373MG cells (Fig. 4C), consistent with the known role of SFKs in mediating Tyr-845 phosphorylation and EGFR-dependent STAT5b activation (31, 32). These results support a model in which endogenously produced uPA activates uPAR-dependent cell signaling in EGFRvIII-expressing U373MG GBM cells, activating SFKs and promoting STAT5b activation.
EGFR-Selective Tyrosine Kinase Inhibitors Block uPAR-Dependent STAT5b Activation in GBM Cells.
EGFRvIII-expressing U373MG cells were transfected with uPAR-specific siRNA or with NTC siRNA. The cells were then treated for 24 h with erlotinib, gefitinib, or vehicle. In the vehicle-treated cells, uPAR gene silencing did not affect P-ERK, as anticipated (Fig. 4D). The two EGFR-selective tyrosine kinase inhibitors decreased P-ERK comparably in uPAR gene-silenced and control cells. Thus, uPAR-initiated cell signaling did not compensate for loss of ERK activation when the EGFR was present but inactivated for 24 h.
In the absence of the EGFR-targeting drugs, uPAR gene silencing substantially decreased P–Tyr-845 (Fig. 4D). Erlotinib and gefitinib reduced P–Tyr-845 to undetectable levels in cells in which uPAR was silenced and in control cells. These results demonstrate that EGFR-selective tyrosine kinase inhibitors block a key step in the pathway by which uPAR activates STAT5b. Mechanistically, the activity of the EGFR tyrosine kinase inhibitors is explained by the fact that the EGFR needs to be activated to induce the conformational change necessary to reveal the otherwise cryptic Tyr-845 (31, 32).
uPAR Activates STAT5b in EGFRvIII-Expressing but Not in EGFR-Expressing U87MG Cells.
As a second model system to test the hypothesis that uPAR plays an essential role in EGFRvIII-dependent STAT5b activation in GBM cells, we studied U87MG GBM cells that express EGFRvIII. These cells were transfected with uPAR-specific or NTC siRNA. uPAR gene silencing was greater than 95% effective in these cells as determined by quantitative PCR (qPCR). Fig. 5A shows that P–Tyr-845 was decreased by uPAR gene silencing. P–Tyr-845 also was inhibited by PP2, confirming the role for SFKs (Fig. 5B). P–Tyr-845 was blocked by the EGFR-selective tyrosine kinase inhibitor, tyrphostin AG1478 (Fig. 5C), as anticipated, because the EGFR must be activated to reveal Tyr-845 for phosphorylation (31).
Fig. 5.
uPAR regulates P–Tyr-845 in EGFRvIII-expressing but not EGFR-expressing GBM cells. (A) EGFRvIII-expressing U87MG cells were transfected with uPAR-specific or NTC siRNA. After transfer to SFM for 48 h, immunoblot analysis was performed. (B) EGFRvIII-expressing U87MG cells were transferred to SFM and treated with PP2 or vehicle for 12 h. Immunoblot analysis was performed. (C) EGFRvIII-expressing U87MG cells were transferred to SFM for 12 h and treated with AG1478 (50 nM) or vehicle for 2 h. The cells were then treated with uPA (10 nM) or vehicle for 10 min. Immunoblot analysis was performed. (D) Wild-type U87MG cells (parental) and cells that overexpress full-length EGFR were transfected with uPAR-specific or NTC siRNA. The cells were transferred to SFM and treated with EGF (10 ng/mL) or vehicle for 10 min, as indicated. Immunoblot analysis was performed. (E) IHC was performed to detect uPAR in an orthotopic xenograft of EGFRvIII-expressing U87MG cells. The field shows intense immunoreactivity that is accentuated around cell borders. (F) The equivalent U87MG xenograft shows cytoplasmic and nuclear immunoreactivity for P–Tyr-845. (G) uPAR immunoreactivity is restricted primarily to cell borders in xenografts of BA/F3 cells transfected with EGFRvIII. (H) The equivalent xenograft is intensely immunopositive for P–Tyr-845. (I) IHC for P–Tyr-845 detected individual BA/F3 cells invading normal brain. (J) IHC for uPAR in an EGFRvIII-expressing human GBM. (K) IHC for P–Tyr-845 in the same specimen as in J. (L) uPAR IHC of a human GBM with EGFR gene amplification but without EGFRvIII was negative. (M) IHC for P–Tyr-845 in the same specimen as in L was also negative.
For comparison, we studied control U87MG cells and cells that were transfected to express full-length (wild-type) EGFR. Although the EGFR was not detected in the control cells by immunoblot analysis, low levels of EGFR were apparently present because ERK was activated in response to EGF (Fig. 5D). P–Tyr-845 was detected in the EGFR-overexpressing cells only after treatment with EGF for 10 min. uPAR gene silencing did not decrease P–Tyr-845 in the EGFR-overexpressing cells. Thus, the role of uPAR in supporting SFK-dependent Tyr-845 phosphorylation seems to be unique to EGFRvIII in U87MG cells.
To confirm that uPAR expression and phosphorylation of Tyr-845 are maintained in vivo, we performed immunohistochemistry (IHC) studies, assessing tissue isolated from orthotopic xenografts of EGFRvIII-expressing U87MG cells. Fig. 5E shows that uPAR was expressed by these cells in vivo. Antigen was identified throughout the cell but most was concentrated at the cell surface. Similarly, P–Tyr-845 was robustly present in EGFRvIII-expressing U87MG cells (Fig. 5F). This antigen was identified diffusely throughout the cell, including the nucleus, again with some concentration at the cell surface.
For comparison, we examined intracerebral implants of a mouse tumor cell line (BA/F3) that expresses EGFRvIII. As shown in representative images of the tumor tissue, the cancer cells were immunopositive for uPAR (Fig. 5G) and intensely immunopositive for P–Tyr-845 (Fig. 5H). Compared with EGFRvIII-expressing U87MG cells, uPAR and P–Tyr-845 in the BA/F3 cells were more demarcated to the cell surface. Because of the high intensity of P–Tyr-845 immunoreactivity in EGFRvIII-expressing cells, this epitope was particularly useful for identifying individual tumor cells invading through normal brain. A representative image of an isolated, invading BA/F3 cell is shown in Fig. 5I.
Finally, we examined two previously characterized human GBMs that were propagated as xenografts and shown to retain molecular and histological characteristics of the parent tumors (36). One tumor, which was EGFRvIII-positive, demonstrated strong uPAR immunopositivity in a plasma membrane distribution (Fig. 5J). The same tumor was robustly immunopositive for P–Tyr-845 (Fig. 5K). The second tumor demonstrated EGFR gene amplification without EGFRvIII and was PTEN-null. This tumor was immunonegative for both uPAR (Fig. 5L) and P–Tyr-845 (Fig. 5M).
Discussion
The transient nature of the clinical response of most GBMs to EGFR-targeting therapeutics suggests that alternative receptors and cell-signaling pathways are activated in treated cells (10, 12–14, 37). Other explanations include activation of efflux pumps and induction of novel mutations in the EGFR that prevent drug binding, although such mutations have not been reported in GBM (37–39). Given the importance of local invasion in GBM (40), it is not surprising that the function of uPAR in facilitating activation of extracellular proteases and tissue invasion has been studied in this malignancy (25, 41). The work presented here demonstrates that uPAR-initiated cell signaling regulates the physiology of GBM cells, both in the presence of EGFRvIII and in tumor cells that acquire the ability to grow in vivo in the absence of EGFRvIII. The requirement for uPAR in STAT5b activation downstream of EGFRvIII shows that the capacity of EGFRvIII to promote GBM progression may be inhibited by targeting uPAR. The ability of uPAR to sustain ERK activation when EGFRvIII expression is blocked suggests that, like the PDGF receptor and c-Met (37), uPAR may allow escape of GBM cells from dependency on EGFRvIII.
There is abundant evidence for synergy between the EGFR and SFKs in cancer (31, 32, 42, 43). SFK-dependent STAT5b activation downstream of the EGFR promotes cancer cell proliferation and survival (31, 32, 44). The EGFR may directly activate SFKs (45); however, uPAR also may serve as a major SFK activator (24, 33). Other SFK activators include lysophosphatidic acid, endothelin, and growth hormone (44). In U373MG GBM cells that express EGFRvIII, uPAR was entirely responsible for SFK-dependent Tyr-845 phosphorylation and STAT5b activation, despite the fact that EGFRvIII is constitutively active. Similar results were obtained when we studied EGFRvIII-expressing U87MG cells. By contrast, uPAR did not regulate Tyr-845 phosphorylation in U87MG cells that overexpress full-length EGFR. Why uPAR functions differently with the EGFR and EGFRvIII in the same cell type remains to be determined; however, the conditions of the experiments may be important. In studies with EGFRvIII, the U87MG cells were cultured in SFM for 2 d. In the studies with the EGFR, the cells were treated with EGF for 10 min before analysis. The unique ability of uPAR to control STAT5b activation in GBM cells that express EGFRvIII suggests that targeting uPAR therapeutically may be particularly important in GBMs that express the mutated form of this receptor.
Therapeutic approaches for targeting uPAR in cancer are not well developed but include RNA interference (46) and specific monoclonal antibodies (47). A drug that targets the uPA protease active site is in clinical trials (48); however, it is unclear whether this drug will have any effects on uPAR-initiated cell signaling. uPAR-dependent STAT5b activation requires EGFR activation because Tyr-845 is available for phosphorylation only when the receptor is activated (31). Thus, it is not surprising that erlotinib and gefitinib blocked STAT5b activation in EGFRvIII-expressing U373MG cells, even when uPAR was not silenced. The ability of these drugs to inhibit STAT5b activation indicates that EGFR-selective tyrosine kinase inhibitors may have utility in inhibiting specific uPAR-dependent cell-signaling pathways.
Despite the well-described role of uPAR in ERK activation (28, 29, 33, 49), in U373MG and U87MG cells, the activity of EGFRvIII was dominant in regulating ERK activation, and uPAR gene silencing had no effect. By contrast, in cells derived from escaper tumors, uPAR played a major role in maintaining an elevated level of P-ERK. Unless uPAR is expressed at extremely high levels, ERK activation is uPA-dependent (29, 49). Thus, the increase in uPA levels, associated with EGFRvIII deficiency, may have allowed the GBM cells to activate autocrine uPAR-initiated cell signaling, leading to ERK activation and release of the tumors from dormancy in vivo.
ERK activation downstream of uPAR occurs by EGFR-dependent and -independent pathways (23). Although uPAR activated ERK in Dox-treated cells independently of the EGFR, uPAR did not compensate for the decrease in P-ERK observed in cells treated with erlotinib and gefitinib. We previously reported that inactivated EGFR may have dominant-negative activity and inhibit uPAR-dependent cell signaling to ERK (23). More work is necessary to determine whether erlotinib or gefitinib convert EGFRvIII into a dominant-negative inhibitor of uPAR-initiated cell signaling to ERK; however, if this effect occurs, then the scope of activity of erlotinib and gefitinib as inhibitors of uPAR-activated cell signaling may be broader than STAT5b.
Our IHC studies identified uPAR and P–Tyr-845 as potentially valuable biomarkers in GBM. Our model in which phosphorylation of P–Tyr-845 in EGFRvIII results from crosstalk between EGFRvIII and uPAR is supported by IHC studies of tumors formed by U87MG cells, BA/F3 cells, and human GBMs that were propagated as xenografts. These studies justify a clinical trial to test our model correlating uPAR expression with P–Tyr-845 in GBMs statistically. Overall, the studies presented here demonstrate that crosstalk between uPAR and the EGFR is important even when the EGFR is mutated and constitutively active. The activity of drugs currently in use for GBM may be influenced by the effects of uPAR on GBM cell physiology. Understanding crosstalk between uPAR and EGFRvIII offers the potential to reveal new opportunities for therapeutics development. uPAR is a justified target in GBM.
Materials and Methods
Cell Lines.
U373MG cells, which express EGFRvIII under the control of Dox, have been previously described (15). Escaper tumor cell lines also have been previously described (15). These cells were maintained in DMEM supplemented with 10% tetracycline-approved FBS (Clontech), Dox (1 μg/mL), puromycin (1 μg/mL), and Geneticin (200 μg/mL). U87MG cells that express EGFRvIII have been previously described (8).
Tumor Processing and IHC.
U87MG orthotopic xenografts were prepared by stereotactically implanting cells in the striatum of athymic nude mice as previously described (50). Intracerebral implants of BA/F3 cells that express EGFRvIII were prepared in SCID mice as previously described (51) and provided by Santosh Kesari (Department of Neurosciences, University of California at San Diego). Human GBMs (nos. 8 and 39), which were propagated as xenografts, were kindly provided by C. David James (Department of Neurological Surgery, University of California, San Francisco, CA).
Xenograft-implanted brains were fixed in buffered paraformaldehyde, dissected, and routinely processed in paraffin. Tissue sections (5 μm) were immunostained with a Ventana Discovery Ultra automated IHC system (Ventana Medical Systems). For detection of P–Tyr-845, primary antibody was applied at a final concentration of 0.84 μg/mL for 1 h after antigen retrieval. For detection of uPAR, primary antibody was applied at a final concentration of 1.5 μg/mL for 1 h after antigen retrieval. UltraMap multimer was used for antibody detection. Phospho-epitope labeling was validated by elimination of staining in lambda phosphatase-pretreated sections.
For additional details on antibodies and reagents, real-time qPCR, immunoblot analysis, siRNA transfection, cell growth, BrdU incorporation, and cell-death assays, please see SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01 CA-94900 (to S.L.G.) and P01 CA95616 (to W.K.C. and F.F.) and an award from the Goldhirsh Foundation (to F.F.). W.K.C. is a fellow of the National Foundation for Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113416108/-/DCSupplemental.
References
- 1.Kanu OO, et al. Glioblastoma multiforme: A review of therapeutic targets. Expert Opin Ther Targets. 2009;13:701–718. doi: 10.1517/14728220902942348. [DOI] [PubMed] [Google Scholar]
- 2.Mrugala MM, Chamberlain MC. Mechanisms of disease: Temozolomide and glioblastoma—look to the future. Nat Clin Pract Oncol. 2008;5:476–486. doi: 10.1038/ncponc1155. [DOI] [PubMed] [Google Scholar]
- 3.Libermann TA, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 4.Schlegel J, et al. Amplification and differential expression of members of the erbB-gene family in human glioblastoma. J Neurooncol. 1994;22:201–207. doi: 10.1007/BF01052920. [DOI] [PubMed] [Google Scholar]
- 5.Schlegel J, et al. Amplification of the epidermal-growth-factor-receptor gene correlates with different growth behaviour in human glioblastoma. Int J Cancer. 1994;56:72–77. doi: 10.1002/ijc.2910560114. [DOI] [PubMed] [Google Scholar]
- 6.Sugawa N, Ekstrand AJ, James CD, Collins VP. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci USA. 1990;87:8602–8606. doi: 10.1073/pnas.87.21.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldape KD, et al. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004;63:700–707. doi: 10.1093/jnen/63.7.700. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa R, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagane M, et al. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 10.Zhu H, et al. Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. Proc Natl Acad Sci USA. 2009;106:2712–2716. doi: 10.1073/pnas.0813314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minniti G, Muni R, Lanzetta G, Marchetti P, Enrici RM. Chemotherapy for glioblastoma: Current treatment and future perspectives for cytotoxic and targeted agents. Anticancer Res. 2009;29:5171–5184. [PubMed] [Google Scholar]
- 12.Voelzke WR, Petty WJ, Lesser GJ. Targeting the epidermal growth factor receptor in high-grade astrocytomas. Curr Treat Options Oncol. 2008;9:23–31. doi: 10.1007/s11864-008-0053-5. [DOI] [PubMed] [Google Scholar]
- 13.Cheng CK, Fan QW, Weiss WA. PI3K signaling in glioma—animal models and therapeutic challenges. Brain Pathol. 2009;19:112–120. doi: 10.1111/j.1750-3639.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo HW. EGFR-targeted therapy in malignant glioma: Novel aspects and mechanisms of drug resistance. Curr Mol Pharmacol. 2010;3:37–52. doi: 10.2174/1874467211003010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukasa A, et al. Mutant EGFR is required for maintenance of glioma growth in vivo, and its ablation leads to escape from receptor dependence. Proc Natl Acad Sci USA. 2010;107:2616–2621. doi: 10.1073/pnas.0914356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salajegheh M, Rudnicki A, Smith TW. Expression of urokinase-type plasminogen activator receptor (uPAR) in primary central nervous system neoplasms. Appl Immunohistochem Mol Morphol. 2005;13:184–189. doi: 10.1097/01.pai.0000138448.85231.da. [DOI] [PubMed] [Google Scholar]
- 17.Blasi F, Carmeliet P. uPAR: A versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 18.Mazar AP. Urokinase plasminogen activator receptor choreographs multiple ligand interactions: Implications for tumor progression and therapy. Clin Cancer Res. 2008;14:5649–5655. doi: 10.1158/1078-0432.CCR-07-4863. [DOI] [PubMed] [Google Scholar]
- 19.Kjøller L, Hall A. Rac mediates cytoskeletal rearrangements and increased cell motility induced by urokinase-type plasminogen activator receptor binding to vitronectin. J Cell Biol. 2001;152:1145–1157. doi: 10.1083/jcb.152.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: Vitronectin provides the key. J Cell Biol. 2007;177:927–939. doi: 10.1083/jcb.200612058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jo M, Takimoto S, Montel V, Gonias SL. The urokinase receptor promotes cancer metastasis independently of urokinase-type plasminogen activator in mice. Am J Pathol. 2009;175:190–200. doi: 10.2353/ajpath.2009.081053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1:445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 23.Jo M, Thomas KS, O'Donnell DM, Gonias SL. Epidermal growth factor receptor-dependent and -independent cell-signaling pathways originating from the urokinase receptor. J Biol Chem. 2003;278:1642–1646. doi: 10.1074/jbc.M210877200. [DOI] [PubMed] [Google Scholar]
- 24.Jo M, et al. Urokinase receptor primes cells to proliferate in response to epidermal growth factor. Oncogene. 2007;26:2585–2594. doi: 10.1038/sj.onc.1210066. [DOI] [PubMed] [Google Scholar]
- 25.Mohanam S, et al. In vitro inhibition of human glioblastoma cell line invasiveness by antisense uPA receptor. Oncogene. 1997;14:1351–1359. doi: 10.1038/sj.onc.1200963. [DOI] [PubMed] [Google Scholar]
- 26.Krishnamoorthy B, et al. Glioma cells deficient in urokinase plaminogen activator receptor expression are susceptible to tumor necrosis factor-α-related apoptosis-inducing ligand-induced apoptosis. Clin Cancer Res. 2001;7:4195–4201. [PubMed] [Google Scholar]
- 27.Chandrasekar N, et al. Downregulation of uPA inhibits migration and PI3k/Akt signaling in glioblastoma cells. Oncogene. 2003;22:392–400. doi: 10.1038/sj.onc.1206164. [DOI] [PubMed] [Google Scholar]
- 28.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Z, Webb DJ, Jo M, Gonias SL. Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci. 2001;114:3387–3396. doi: 10.1242/jcs.114.18.3387. [DOI] [PubMed] [Google Scholar]
- 30.Chumbalkar V, et al. Analysis of phosphotyrosine signaling in glioblastoma identifies STAT5 as a novel downstream target of ΔEGFR. J Proteome Res. 2011;10:1343–1352. doi: 10.1021/pr101075e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kloth MT, et al. STAT5b, a mediator of synergism between c-Src and the epidermal growth factor receptor. J Biol Chem. 2003;278:1671–1679. doi: 10.1074/jbc.M207289200. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen DH, et al. Urokinase-type plasminogen activator stimulates the Ras/extracellular signal-regulated kinase (ERK) signaling pathway and MCF-7 cell migration by a mechanism that requires focal adhesion kinase, Src, and Shc. Rapid dissociation of GRB2/Sps-Shc complex is associated with the transient phosphorylation of ERK in urokinase-treated cells. J Biol Chem. 2000;275:19382–19388. doi: 10.1074/jbc.M909575199. [DOI] [PubMed] [Google Scholar]
- 34.Smith HW, Marra P, Marshall CJ. uPAR promotes formation of the p130Cas–Crk complex to activate Rac through DOCK180. J Cell Biol. 2008;182:777–790. doi: 10.1083/jcb.200712050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jo M, et al. Dynamic assembly of the urokinase-type plasminogen activator signaling receptor complex determines the mitogenic activity of urokinase-type plasminogen activator. J Biol Chem. 2005;280:17449–17457. doi: 10.1074/jbc.M413141200. [DOI] [PubMed] [Google Scholar]
- 36.Sarkaria JN, et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6:1167–1174. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- 37.Wykosky J, Mukasa A, Furnari F, Cavenee WK. Escape from targeted inhibition: The dark side of kinase inhibitor therapy. Cell Cycle. 2010;9:1661–1662. doi: 10.4161/cc.9.9.11592. [DOI] [PubMed] [Google Scholar]
- 38.Li J, et al. Association of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients. Cancer Biol Ther. 2007;6:432–438. doi: 10.4161/cbt.6.3.3763. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 40.Teodorczyk M, Martin-Villalba A. Sensing invasion: Cell surface receptors driving spreading of glioblastoma. J Cell Physiol. 2010;222:1–10. doi: 10.1002/jcp.21901. [DOI] [PubMed] [Google Scholar]
- 41.Rao JS, et al. Role of plasminogen activator and of 92-KDa type IV collagenase in glioblastoma invasion using an in vitro Matrigel model. J Neurooncol. 1994;18:129–138. doi: 10.1007/BF01050419. [DOI] [PubMed] [Google Scholar]
- 42.Luttrell DK, et al. Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc Natl Acad Sci USA. 1994;91:83–87. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: Implications for the etiology of multiple human cancers. Proc Natl Acad Sci USA. 1995;92:6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boerner JL, Biscardi JS, Silva CM, Parsons SJ. Transactivating agonists of the EGF receptor require Tyr 845 phosphorylation for induction of DNA synthesis. Mol Carcinog. 2005;44:262–273. doi: 10.1002/mc.20138. [DOI] [PubMed] [Google Scholar]
- 45.Ricono JM, et al. Specific cross-talk between epidermal growth factor receptor and integrin αvβ5 promotes carcinoma cell invasion and metastasis. Cancer Res. 2009;69:1383–1391. doi: 10.1158/0008-5472.CAN-08-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gondi CS, et al. Intraperitoneal injection of a hairpin RNA-expressing plasmid targeting urokinase-type plasminogen activator (uPA) receptor and uPA retards angiogenesis and inhibits intracranial tumor growth in nude mice. Clin Cancer Res. 2007;13:4051–4060. doi: 10.1158/1078-0432.CCR-06-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabbani SA, et al. An anti-urokinase plasminogen activator receptor antibody (ATN-658) blocks prostate cancer invasion, migration, growth, and experimental skeletal metastasis in vitro and in vivo. Neoplasia. 2010;12:778–788. doi: 10.1593/neo.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein LJ, et al. Phase II, two-arm, double-blind, multicenter, randomized study of the combination of oral WX-671 plus capecitabine versus capecitabine in first-line HER2-negative metastatic breast cancer (MBC) J Clin Oncol. 2010;28(Suppl):15s. (abstr TPS131) [Google Scholar]
- 49.Nguyen DH, et al. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J Cell Biol. 1999;146:149–164. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherjee B, et al. EGFRvIII and DNA double-strand break repair: A molecular mechanism for radioresistance in glioblastoma. Cancer Res. 2009;69:4252–4259. doi: 10.1158/0008-5472.CAN-08-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji H, et al. Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc Natl Acad Sci USA. 2006;103:7817–7822. doi: 10.1073/pnas.0510284103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.