Much has been learned during the past decades about how animals navigate in their local environment. We know that most taxonomic groups, from arthropods to mammals, use multiple mechanisms to find their way (1). Animals may reach goals by following trails or approaching salient beacons or by computationally more sophisticated strategies, such as path integration (1, 2) and the use of geometric “cognitive maps” of the external environment (3, 4). The mammalian brain has specialized systems for many of these functions. Key components include, in the hippocampus, place cells, which are cells that fire only when the animal is in specific positions (4, 5), and, in the entorhinal cortex, grid cells, which provide the animal with a universally applicable metric of local space (5, 6). Together with direction-coding cells (7), place and grid cells are known to form a map-like neural representation of the animal's external environment (4–6). Despite these emerging insights, the growing knowledge of how space is computed in the mammalian brain is based almost exclusively on small-scale laboratory studies in which rodents forage in a test box or on a track. Whether similar mechanisms are used during large-scale navigation in the animal's natural habitat remains to be determined. Most of our knowledge about long-distance navigation comes from studies of nonmammalian species, such as birds, insects, fish, and sea turtles. Those studies have identified sources of information used by animals to determine compass direction as well as spatial location, for example the geomagnetic field, olfactory gradients, and celestial cues such as the sun and the stars (1, 8–11). The physiological mechanisms used to extract the information are not well understood, however, and the relationship to local spatial representation mechanisms described in mammals has almost not been examined at all.
A major obstacle to a better integration of studies on short- and long-distance navigation has been the lack of a species in which known brain mechanisms for small-scale navigation can be investigated in parallel with mechanisms for long-distance navigation. The hippocampal region of birds and turtles may be too different to achieve such convergence in the near future. Recent work points to the bat as an interesting alternative. Many bat species have exceptional navigational skills; some African fruit bats can navigate more than 1,000 km (12). At the same time, the architecture of the hippocampal and parahippocampal cortices of the bat is remarkably similar to that of rodents, despite considerable evolutionary distance, and space seems to be represented by place cells and grid cells in much the same way (13, 14). Thus, it should be possible to determine in bats how place cells and grid cells represent space at larger scales and whether distinct mechanisms have evolved for short- and long-range navigation. A first step toward this goal would be to characterize in quantitative detail how bats navigate between distant locations. A behavioral study by Tsoar et al. reported in PNAS (15) takes this important step.
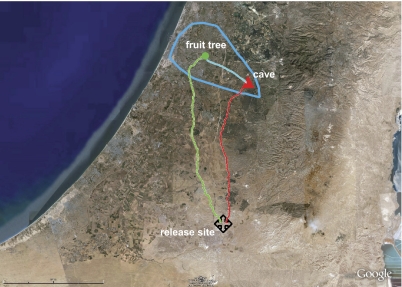
Tsoar et al. ask how cave-dwelling Egyptian fruit bats find their way during long-distance foraging flights. Wild-captured bats were equipped with a global positioning system (GPS) tracking device (16) that allowed the researchers to monitor the animal's location at a frequency of 1 Hz. During the day the bats rested in a cave in central Israel; during the night they foraged on fruit trees up to 25 km further west. The tracking data showed that the bats flew in straight paths between these locations and that they returned to the same tree on consecutive nights. To determine what cues the animals used to navigate with such precision, the authors subsequently released the bats at a remote location, 44 km south of the cave, in the Negev desert, presumably far from anywhere the animal had ever visited. Precision was as striking as before. When fed, the animals flew straight back to the cave. When hungry, they headed to their favorite fruit tree (Fig. 1). In the final experiment the bats were displaced even further, to a location 84 km south of the cave. The release site was in a crater from which distant cues on the horizon were not visible. All bats returned to the cave, but their initial paths out of the crater were considerably less direct than before. Bats that were released at the crater rim flew directly to the cave, suggesting that access to distal sensory cues were both necessary and sufficient for successful homing from a new location.
Fig. 1.
Google Earth map showing straight return paths of bats released far outside their familiar foraging area (+, release location). Blue line indicates the boundaries of the area where the bats were known to forage. Red and green lines indicate tracked trajectories to foraging site and cave, respectively.
Which sensory cues did the bats use to find their way home? Previous studies in birds as well as bats have shown that direction is defined by magnetic north and sun position (8, 9, 11, 17), but the mechanisms for determining location are more enigmatic. We do not know how a directionally calibrated bat calculates the correct flight vector, for example. Tsoar et al. suggest that visual cues play a major role in guiding the bats home. At a flight altitude of a few hundred meters the bats are likely to see large parts of central Israel, including both the desert and the foraging area near the cave. Strong directional cues, such as the Mediterranean Sea and the central mountain range of Israel, as well as city lights near the coast, may be sufficient to indicate the approximate location of the roost from the release site. As acknowledged by the authors, the data do not entirely rule out alternative guidance cues, such as celestial patterns, local magnetic anomalies, and olfactory gradients. The circumvolved flight paths in the crater may reflect miscalibration of the animal's compass, caused for example by the fact that the sun position may not have been available from the crater bottom at sunset, when bats may calibrate their magnetic compass (17). These alternative cues are less likely to provide absolute location coordinates, however; the differences in star constellations and magnetic parameters between start and goal may be too small for that, and wind directions over the complex desert terrain may be too variable to provide reliable olfactory guidance. At present, visual guidance may seem to be the strongest candidate, but in the end direct experimental cue manipulation is unavoidable. One could begin, for example, by testing navigation from remote locations at different atmospheric wind directions and by comparing navigation on nights with clear and cloudy skies.
If the predominant cues were visual, how would the animals use them for homing? Were the cues used merely as beacons, or was the flight guided by an internal map of the visual surface landscape? The data are probably consistent with both possibilities. The remote location of the release site rules out straight navigation to a single beacon, but it remains possible that animals use a sequence of landmarks to find their way home, without taking into account the spatial relationship between the landmarks. Alternatively, as suggested by the authors, the animals may have formed a cognitive map of the space that they can see from flying altitude in their normal foraging area. To the extent that such maps preserve information about spatial relationships in the external world, the bats may use them to infer optimal travel routes from anywhere in the mapped space. In agreement with the postulated existence of such map-like representations (3), rats and honey bees have been reported to take short cuts and detours between familiar places when alternative routes become available (3, 18), and hippocampal place cells have been claimed to represent short cuts that have never been traveled (19). The straight flight paths of the bats in the Tsoar et al. study are consistent with the use of geometrically accurate maps. If the bats used beacons only, they would probably change direction several times. Nevertheless, the suggestion that bats use geometric maps for navigation is based on a number of unproven assumptions. First, it assumes that bats can triangulate their position from angles between landmarks and that they do so from places and angles that they have never visited. Moreover, the animals must be able to extend this ability to the
Tsoar et al. ask how cave-dwelling Egyptian fruit bats find their way during long-distance foraging flights.
periphery of the visual map—to areas seen only at very shallow angles from the familiar foraging range—and they must be capable of mentally rotating the familiar landmark configuration. Experimental studies will be required to test these rather strong assumptions. It will be important to determine whether the internal map preserves the euclidian geometry of the external environment or whether information is rather stored in small-world networks primarily as associations between beacons that appear near each other, without detailed information about direction and distance. The extent to which spatial maps replicate the geometry of the environment is currently not well understood in any species, neither the bat nor the laboratory rat.
Finally, do the mechanisms for long-distance navigation in bats differ from those of other mammals? Long-distance navigation is not unique to bats, birds, and sea animals. Large-scale ungulate migrations, caused by seasonal variations, were once common on all continents. Some of the longest distances traveled by extant terrestrial mammals have been reported in Alaskan caribou. Satellite tracking data suggest that herds of caribou migrate more than 2,500 km twice per year between their summer and winter habitats (20). A common factor in each of these migration patterns is that the animals return to the same place year after year, suggesting that long-distance navigation follows highly specific landmark cues also in large terrestrial animals. Whether the migration is guided by similar mechanisms as in flying mammals has not been determined, but with a perspective from several hundred meters altitude the bats may have evolved capacities for more accurate 2D representation of the Earth surface.
The Tsoar et al. study provides a foundation for studies of the neural basis of long-distance navigation. The study extends modern GPS-based tracking technology to the study of long-distance navigation in mammals and shows that flight paths of homing bats can be reconstructed with extreme precision, far beyond what has been achieved in previous studies with traditional low-frequency radiotransmitters. The straightness of the reconstructed paths is remarkable when the animals are released from such remote locations. The next step will be to determine what neuronal mechanisms enable these levels of precise navigation and to relate these processes to the known properties of the short-range spatial representation system of the mammalian brain.
Footnotes
References
- 1.Gallistel CR. The Organization of Learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- 2.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 3.Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 4.O'Keefe J, Nadel L. The Hippocampus As a Cognitive Map. Oxford, UK: Clarendon; 1978. [Google Scholar]
- 5.Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- 6.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 7.Taube JS. The head direction signal: Origins and sensory-motor integration. Annu Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- 8.Baker RR. Bird Navigation: The Solution of a Mystery? New York: Holmes and Meier; 1984. [Google Scholar]
- 9.Cochran WW, Mouritsen H, Wikelski M. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science. 2004;304:405–408. doi: 10.1126/science.1095844. [DOI] [PubMed] [Google Scholar]
- 10.Lohmann KJ, Lohmann CM, Ehrhart LM, Bagley DA, Swing T. Animal behaviour: Geomagnetic map used in sea-turtle navigation. Nature. 2004;428:909–910. doi: 10.1038/428909a. [DOI] [PubMed] [Google Scholar]
- 11.Wallraff HG. Avian olfactory navigation: Its empirical foundation and conceptual state. Anim Behav. 2004;67:189–204. [Google Scholar]
- 12.Richter HV, Cumming GS. First application of satellite telemetry to track African straw-coloured fruit bat migration. J Zool (Lond) 2008;275:172–176. [Google Scholar]
- 13.Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nat Neurosci. 2007;10:224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- 14.Yartsev MM, Witter M, Ulanovsky N. Spatial maps in the medial entorhinal cortex of the Egyptian fruit bat. Soc Neurosci Abstr. 2010;30:203.15. [Google Scholar]
- 15.Tsoar A, et al. Large-scale navigational map in a mammal. Proc Natl Acad Sci USA. 2011;108:E718–E724. doi: 10.1073/pnas.1107365108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipp HP, et al. Pigeon homing along highways and exits. Curr Biol. 2004;14:1239–1249. doi: 10.1016/j.cub.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Holland RA, Thorup K, Vonhof MJ, Cochran WW, Wikelski M. Navigation: Bat orientation using Earth's magnetic field. Nature. 2006;444:702. doi: 10.1038/444702a. [DOI] [PubMed] [Google Scholar]
- 18.Menzel R, et al. Honey bees navigate according to a map-like spatial memory. Proc Natl Acad Sci USA. 2005;102:3040–3045. doi: 10.1073/pnas.0408550102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fancy SG, Pank LF, Whitten KR, Regelin WL. Seasonal movements of caribou in arctic Alaska as determined by satellite. Can J Zool. 1989;67:644–650. [Google Scholar]