Abstract
Poly(ADP-ribose)polymerase (PARP)14—a member of the B aggressive lymphoma (BAL) family of macrodomain-containing PARPs—is an ADP ribosyltransferase that interacts with Stat6, enhances induction of certain genes by IL-4, and is expressed in B lymphocytes. We now show that IL-4 enhancement of glycolysis in B cells requires PARP14 and that this process is central to a role of PARP14 in IL-4–induced survival. Thus, enhancements of AMP-activated protein kinase activity restored both IL-4–induced glycolytic activity in Parp14−/− B cells and prosurvival signaling by this cytokine. Suppression of apoptosis is central to B-lymphoid oncogenesis, and elevated macro-PARP expression has been correlated with lymphoma aggressiveness. Strikingly, PARP14 deficiency delayed B lymphomagenesis and reversed the block to B-cell maturation driven by the Myc oncogene. Collectively, these findings reveal links between a mammalian ADP ribosyltransferase, cytokine-regulated metabolic activity, and apoptosis; show that PARP14 influences Myc-induced oncogenesis; and suggest that the PARP14-dependent capacity to increase cellular metabolic rates may be an important determinant of lymphoma pathobiology.
Mechanisms promoting cell survival are central to both physiological homeostasis and cancer. The immune system in particular depends on a reservoir of naïve lymphocytes in the G0 stage of the cell cycle. Maintenance of this pool requires regulation of energy balance to promote cell survival while preserving a readiness to mobilize in response to a pathogen (1–3). Such maintenance depends on cytokines to trigger signal transduction pathways that prevent atrophy and subsequent apoptosis (4). Members of the cytokine group binding to hematopoietin receptors in the common γ-chain (γc) family, such as IL-4 and IL-7, play a crucial role in lymphocyte survival through signals downstream from Janus-family (Jak) kinases (5–7). Multiple classes of cytokines and growth factors initiate prosurvival signaling to regulate apoptosis in oncogenesis as well as immunity (1, 2). Tumors also are notable for cellular metabolic programs differing from their normal cellular counterparts (8, 9). These parallels between lymphocyte homeostasis and cancer cell physiology are underscored by findings indicating that the γc cytokine IL-7 promotes lymphocyte survival in G0 and energetics that favor this outcome, but when overexpressed can drive lymphoma formation in mice (1, 7, 10).
Among cytokines in the γc family, interleukin-4 (IL-4) regulates vital cellular processes including lymphocyte differentiation, proliferation, and apoptosis (6). Moreover, IL-4 may parallel IL-7 in its ability to promote tumorigenesis (11), but IL-4 activates signaling components distinct from those induced by other cytokines in the γc family. Thus, IL-4–induced activity of the same Jak kinases as IL-7 causes phosphorylation of a conserved tyrosine residue in an N-P-X-Y motif unique to the IL-4 receptor (6). This site recruits insulin receptor substrate-2 (IRS-2), which associates with the p85α regulatory subunit of phosphatidylinositol 3-kinase (PI3K) and leads to enhanced Akt kinase activity as well as apoptosis inhibition (6). Thus, IL-4 is thought to promote lymphocyte survival through an IRS-2-dependent PI3K-Akt pathway analogous to one used by growth factor receptors.
IL-4 also is unique among γc cytokines in that it mobilizes Jak-mediated tyrosine phosphorylation of latent cytoplasmic Stat6 (signal transducer and activators of transcription 6) proteins in resting lymphocytes rather than Stat5 (5, 12). Tyrosine-phosphorylated Stat6 proteins dimerize, translocate to the nucleus, and bind to specific DNA sequences to regulate gene transcription (12). However, specific transcriptional cofactors and mechanisms used by Stat6 to mediate target gene regulation or specificity under physiological conditions are poorly understood. Furthermore, the extent to which Stat6 is vital for survival, and the mechanisms by which a Stat6 limb of IL-4–activated signaling pathways might function, remains to be elucidated.
Previous work identified a novel Stat6-interacting protein that influenced IL-4–induced gene expression (13). This partner of Stat6 encodes an ADP ribosyltransferase whose activity can participate in amplifying Stat6-mediated promoter induction (13–15). Mice lacking this protein, renamed PARP14 (13), contained normal numbers of B and T lymphocytes, but the proliferative response of anti-IgM-stimulated PARP14-null B cells to IL-4 was decreased, as was the induction of two genes that encode prosurvival proteins (15). These findings suggest that PARP14 is a coactivator of Stat6 at some target genes. Other work in a sarcoma cell line determined that human PARP14 regulates the ubiquitination and levels of phosphoglucose isomerase, a protein involved in intermediary metabolism (16). These latter results suggested a potential link between PARP14 and cellular metabolism. PARP14 and its impact on modifiers of B-lymphoid oncogenesis were particularly notable because PARP14 is overexpressed in mouse B-lymphoma cells and closely related to the human protein BAL/PARP9, heightened expression of which correlated with worse clinical outcome in patients with diffuse large B-cell lymphoma (DLBCL) (17).
We show here that IL-4 regulation of glycolysis and glucose oxidation requires PARP14. The metabolic state of the cells appears vitally linked to the prosurvival effects of IL-4. PARP14 is needed to achieve full prosurvival signaling by IL-4 in B cells. Inhibition of AMP-activated protein kinase (AMPK), a sensor of cellular energy status and metabolism (18), blocked IL-4–induced B-cell survival and glycolysis. Conversely, AMPK-activating interventions coordinately restored the glycolytic and prosurvival signaling of IL-4 in Parp14-null B cells. Strikingly, this macro-PARP facilitated B-lymphoid oncogenesis and alterations in developmental progression driven by c-Myc. Together, the data indicate that PARP14 accelerates lymphoma and regulates cellular energy metabolism to promote B-cell survival.
Results
PARP14 Mediates IL-4–Induced Protection Against Apoptosis.
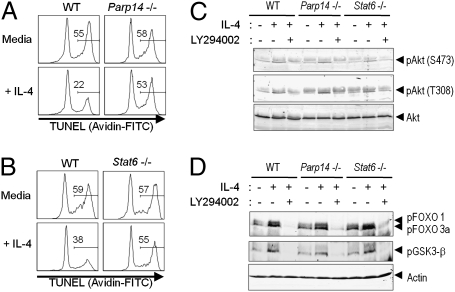
Cytokines such as IL-4 inhibit the atrophy and subsequent apoptosis of lymphocytes removed from their normal microenvironment. There is evidence indicating that IL-4–induced Stat6 contributes to B-cell survival (19, 20). Starting after 9 h in short-term culture, wild-type (WT) B cells removed from their normal environment underwent substantial apoptosis that was decreased by IL-4 (Fig. 1A and Fig. S1 A–C). This rescue was attenuated in both Parp14−/− and Stat6−/− B cells (Fig. 1 A and B and Fig. S1 D and E). In addition to Stat6 induction, IL-4 receptors signal through PI3K and the activating phosphorylation of Akt (5, 6). As expected, IL-4 treatment of WT B cells induced Akt phosphorylation at the T308 and S473 regulatory sites (Fig. 1C and Fig. S2). Parp14−/− and Stat6−/− B cells each showed normal Akt phosphorylation in response to IL-4. Akt acts in part by phosphorylating GSK3β and the transcription factors Foxo1 and 3a (21). Although PARP14 has been reported to influence protein stability (16), absolute levels and IL-4–induced phosphorylation of these downstream targets of Akt were intact in PARP14- and Stat6-null B cells (Fig. 1D). Thus, PARP14 mediates IL-4–induced rescue of B cells from apoptosis without apparent impact on Akt.
Fig. 1.
IL-4 protection of B cells from apoptosis depends on PARP14. (A and B) PARP14 and Stat6 are vital for IL-4–induced suppression of B-cell apoptosis. Spleen cells [WT and knockout (KO) (Parp14−/− and Stat6−/−)] were cultured in the presence or absence of IL-4 and analyzed. Shown are FACS profiles of the TUNEL data (Avidin-FITC) from the B220+ gate in one experiment representative of five independent replicates. Numbers show the % of apoptotic events of the sample. (C and D) IL-4 activation of Akt signaling in Parp14- and Stat6-null B cells. Purified B cells (WT, Parp14−/−, and Stat6−/−) were stimulated with IL-4 (30 min) after pretreatment (30 min) with LY294002 as indicated, or cultured without IL-4. Shown are results of immunoblots using anti-pAkt (S473 or T308) and anti-Akt (C) or anti-pFOXO 1/3a and anti-pGSK-3β Ab along with anti-actin as a loading control (D). Data are from one representative result among three independent experiments.
PARP14 Is Essential for IL-4 Regulation of Glycolysis in B Cells.
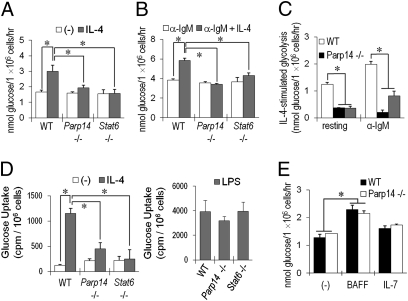
Glucose metabolism plays a critical role in cell survival (22, 23), so glycolytic rates were assayed in B cells from WT, Parp14−/−, and Stat6−/− mice after culture in the presence or absence of IL-4. IL-4 increased rates of glycolysis in WT B cells, but this effect was impaired in B cells lacking either PARP14 or Stat6 (Fig. 2A). The impairment of IL-4–induced glycolysis in Parp14−/− B cells was not due to a global loss of their capacity to increase metabolism, as anti-IgM stimulation enhanced glycolysis in PARP14- and Stat6-null B cells to levels comparable to WT B cells (Fig. 2B). IL-4, which collaborates with B-cell receptor cross-linking to enhance proliferation (5, 6, 15, 24), increased the glycolytic response to anti-IgM in WT, but not PARP14- or Stat6-null, B cells (Fig. 2B). Indeed, the quantitative increase in glycolysis stimulated by IL-4 was greater in anti-IgM-stimulated B cells than in their resting counterparts (Fig. 2C). Glycolytic flux in T cells may be limited by transport of glucose, which can be increased by the γc cytokine IL-7 (7). IL-4 treatment increased glucose uptake into B cells by a PARP14- and Stat6-dependent mechanism, whereas rates of lipopolysaccharide (LPS)-stimulated glucose uptake were comparable for B cells of each genotype (Fig. 2D). IL-7 stimulates increased glycolysis in and survival of T cells by pathways mediated in part by Stat5 (7). The cytokine BAFF promotes mature B-cell glycolysis and survival (25). However, neither PARP14 nor Stat6 influenced the effects of IL-7 or BAFF on B cells (Fig. 2E and Fig. S3). The dramatic IL-4 effect on gluose uptake likely reflects both the increased conversion of hexose through glycolytic demand and IL-4-induced induced increases in surface expression of transporters such as GLUT-1 that are PARP14-dependent (Fig. S4A). We infer that there are specific physiological roles for IL-4, Stat6, and PARP14 in metabolic regulation and survival distinct from the BAFF and IL-7-Stat5 pathways.
Fig. 2.
IL-4 enhancement of glycolysis in B cells depends on PARP14. (A) After culture in the presence or absence of IL-4 (5 ng/mL), glycolysis assays were performed on equal numbers of surviving B cells from WT, Parp14-, or Stat6-null mice as detailed in Materials and Methods. (B) PARP14 and Stat6 influence glycolysis rates responsive to IL-4 treatment of activated B cells. As in A, except that cells were stimulated with anti-IgM (1 μg/mL) ± IL-4 (5 ng/mL). (C) Shown is a comparison of IL-4–stimulated glycolysis, calculated as [(glycolysis after IL-4 treatment) − (basal glycolysis)], for resting and anti-IgM-stimulated B cells. (D) IL-4–induced increase in glucose uptake is mediated by PARP14. Uptake was measured in equal numbers of WT, Parp14−/−, and Stat6−/− viable B cells. (E) PARP14's role is distinct from IL-7 and BAFF. Glycolysis assays were performed with equal numbers of surviving cells after culture (20 h) of WT, Parp14−/−, and Stat6−/− B cells in the presence or absence of IL-7 or BAFF. Shown are mean (±SEM) data from results in replicate experiments. Further data are in SI Materials and Methods.
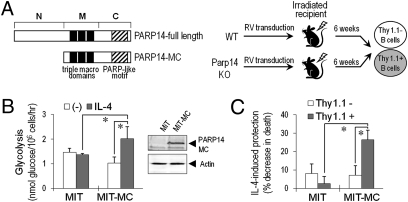
To test whether the defect of PARP14-null B cells in IL-4–induced glycolysis is intrinsic to hematopoietic cells, we performed bone marrow reconstitutions. PARP14 is too large for efficient retrovirion generation, but the middle (three tandem “macrodomains”) and C-terminal (catalytic ADP ribosyltransferase) portions (PARP14-MC) sufficed for its transcriptional function (13, 14). Bone marrow cells lacking PARP14 were transduced with PARP14-MC cDNA or empty vector (“MiT”), and then used to reconstitute recipients (Fig. 3A). Glycolysis and apoptosis were measured in B cells derived from transduced (Thy1.1+) Parp14−/− marrow stem cells and compared with control samples. IL-4 did not increase glycolysis in B cells lacking PARP14 (MiT). In contrast, PARP14-MC-transduced B cells responded to IL-4 by enhanced glycolysis (Fig. 3B) and the ability of IL-4 to rescue B cells from apoptosis (Fig. 3C). We conclude that PARP14 plays a cell-intrinsic role in IL-4–regulated B-cell glycolysis and survival.
Fig. 3.
Reversion of defects of PARP14-deficient B cells by hematopoietic cell transduction with Parp14 cDNA. (A) Schematic of the experimental design and portion of PARP14 used for reconstitution. Parp14−/− bone marrow cells were transduced with empty (MIT) or PARP14-MC-expressing (MIT-MC) vectors and transferred into WT recipient mice. Glycolysis and apoptosis assays were performed on the purified Thy1+ fraction of B cells harvested from these recipients. (B and C) Reconstitution with PARP14-MC enhances IL-4 induction of glycolysis (B) and mitigates the defect of IL-4–induced protection against apoptosis (C) in Parp14−/− B cells. CD3ε-negative Thy1.1+ and Thy1.1− cells were isolated 6 wk postreconstitution and analyzed for glycolysis (B; as in Fig. 2) and apoptosis (C; as in Fig. 1). (B) Shown are mean (±SEM) IL-4–stimulated glycolysis assay results. (Inset) Immunoblot of PARP14-MC expression in transduced lymphoblasts. (C) B220+ gated cells were analyzed by TUNEL assay; bar graphs show mean (±SEM) % apoptosis suppression by IL-4.
Essential Role for PARP14 in Mediating Mitochondrial Activity in B Cells.
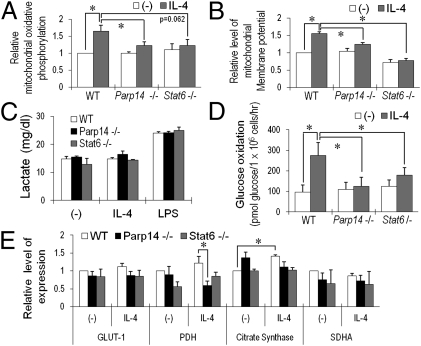
Mitochondrial stability and activity are crucial for cellular metabolism and apoptosis regulation after growth factor withdrawal. PARP14 mediates IL-4 induction of the prosurvival kinase Pim-1, which can impact energetics, and the mitochondrially associated Bcl-2-related protein Mcl-1 (15). IL-4 enhanced mitochondrial respiration and membrane potential in WT B cells, but Parp14−/− and Stat6−/− B cells did not demonstrate this response (Fig. 4 A and B and Fig. S4B). The glycolytic end product pyruvate can be metabolized to lactate or used by mitochondria after conversion to acetyl-CoA by pyruvate dehydrogenase (PDH). LPS stimulation increased lactate release by B cells but IL-4 did not, and lactate production by B cells of each genotype was comparable (Fig. 4C). In contrast, IL-4 accelerated glucose oxidation (conversion of the 6-position carbon of glucose into CO2) by a PARP14- and Stat6-dependent mechanism (Fig. 4D). Consistent with this finding, PDH gene expression was lower in IL-4–stimulated lymphocytes lacking PARP14, and IL-4 increased expression of the citrate synthase gene in WT but not deficient B cells (Fig. 4E, Table S1, and Fig. S4D). This effect on two key steps leading into the Krebs cycle appeared not to be a global defect of mitochondrial biogenesis or energy generation: Genes such as Sdha were unaffected, and fatty acid oxidation in the B cells was neither stimulated by IL-4 nor dependent on PARP14 (Fig. 4E and Fig. S4 C and E). Thus, metabolism in B cells is coordinated after IL-4 stimulation so that a PARP14-dependent increase of glucose flux into pyruvate is used at least in part by greater mitochondrial respiration, with lactate production remaining constant.
Fig. 4.
PARP14 mediates IL-4–enhanced mitochondrial activity in B cells. (A) As detailed in Materials and Methods, mitochondrial oxidative phosphorylation was measured in B cells (WT, Parp14-, and Stat6-null) using a flow cytometric assay after culture (20 h) in medium ± IL-4. Specific mean fluorescent intensities (MFI) of mitochondrial signals for B220+ cells in matched samples of each independent experiment were normalized to the MFI of the WT sample cultured without added cytokine. Bar graphs represent the means (±SEM) of normalized values from these independent replicates. (B) PARP14 dependence of the IL-4–enhanced mitochondrial membrane potential in B cells. Mitochondrial membrane potential in samples as in A was measured using DiOC6 (3) dye; shown are mean (±SEM) data from independent replicate experiments. Signals for B220+ cells in matched samples of each independent experiment were normalized as in A. Bar graphs represent the means (±SEM) of three independent replicates. (C) Lactate in the medium was measured for purified B cells (WT, Parp14−/−, and Stat6−/−) after culture (20 h) in the presence or absence of IL-4 (5 ng/mL) or LPS (1 μg/mL). (D) PARP14- and Stat6-dependent enhancement of glucose oxidation by IL-4. Using B cells as in glycolysis assays, glucose oxidation was assayed as detailed in Materials and Methods. (E) PARP14 impacts PDH gene expression in IL-4–treated B cells. After first determining an optimal time for measuring induction by IL-4, RNA was isolated from B cells cultured for 4 h with and without IL-4; levels of mRNAs encoding metabolic genes were analyzed by quantitative real-time RT-PCR. Bar graphs show mean (±SEM) data from three independent experiments, with mRNA levels in each experiment normalized to WT B cells without IL-4 treatment and then averaged, as in A (*P < 0.05).
AMPK Activation Reverts Defects of IL-4–Treated Parp14-Null B Cells.
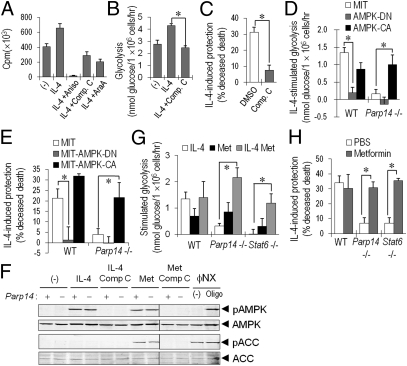
IL-4 enlarges resting B cells (24), so we tested its effect on biosynthesis that would increase ATP consumption. IL-4 increased protein synthesis in B cells within 3 h, whereas treatment with the protein synthesis inhibitor anisomycin blocked this effect (Fig. 5A). AMPK senses cellular energy status and signals increased glucose uptake, glycolysis, and mitochondrial activity (18). AMPK inhibitors (compound C or araA) reduced rates of protein synthesis in IL-4–treated cells to below those of unstimulated controls (Fig. 5A). AMPK inhibition either with compound C or a well-established dominant-negative mutant decreased glycolysis rates of IL-4–stimulated B cells to control levels and reduced IL-4 protection from apoptosis (Fig. 5 B–E). Thus, IL-4–induced B-cell survival required adequate AMPK activity and an ability to increase glycolytic metabolism.
Fig. 5.
PARP14, the metabolic response, and survival. (A) IL-4 increases protein synthesis in B cells. Mean (±SEM) incorporation of [35S]methionine into WT B cells after a 3-h culture in the presence or absence of IL-4; where indicated, cells were pretreated 30 min with inhibitor. (B and C) AMPK inhibition attenuates glycolysis (B) and survival (C) of IL-4–treated B cells. Shown are the mean (±SEM) results of glycolysis and TUNEL assays of B cells pretreated with AMPK inhibitor (compound C; 1 μM) and then cultured ±IL-4. (D–H) AMPK activation in Parp14-null B cells reverses their defects of glycolysis and B-cell rescue by IL-4. (D and E) Activated B cells were transduced with MIT, MIT-AMPK-DN, or MIT-AMPK-CA retrovectors. Glycolysis (D) or apoptosis (E) of purified Thy1.1+ (transduced) B cells was then assayed after culture ±IL-4 (20 h). (F) Activation of AMPK and ACC in WT and Parp14-null B cells. Purified B cells (WT and Parp14−/−) were stimulated with IL-4 (2 h) or metformin, after pretreatment (30 min) with compound C as indicated. ΦNX cells treated with oligomycin (30 min) were used as a positive control. Shown are results of immunoblots using anti-pAMPK (T172) and anti-pACC (S79) along with anti-AMPK and anti-ACC as loading controls. (G and H) Effect of metformin on IL-4–enhanced glycolysis (G) and survival (H) of Parp14 KO B cells. After 20 h culture in IL-4, metformin, or both, glycolysis and apoptosis were assayed; data shown are as in B and C.
To explore the relationship among IL-4, glycolysis, and AMPK, and to validate the effects of pharmacological agents used to probe the relationships between metabolism and antiapoptotic signaling by IL-4, we analyzed phosphorylation of AMPK and its substrate ACC. These analyses revealed that IL-4 induced p-AMPK(T172) but not p-ACC(S79), and activation of the pathway was effectively blocked by compound C (Fig. 5F). In contrast, inhibition of AMPK did not affect IL-4–induced p-Akt or p-Stat6 (Fig. S5 B and C). We further explored the relationship of PARP14 and the metabolic response to survival signaling by using the AMPK activator metformin (18) to test its impact on the defects observed in PARP14-deficient B cells. In the presence of IL-4, metformin reverted the glycolytic, mitochondrial, and survival defects of Parp14- and Stat6-null B cells (Fig. 5 G and H and Fig. S5D). Although metformin alone increased basal glycolysis rates in WT, Parp14, and Stat6−/− B cells slightly, IL-4 was essential for full recovery. Consistent with AMPK impacting the PARP14 role in glycolytic regulation, transduction of constitutively activated AMPK into anti-IgM-stimulated PARP14-deficient B cells largely reversed their defect of IL-4–enhanced glycolysis (Fig. 5D), paralleled by a similar impact on IL-4–induced survival (Fig. 5E). Thus, AMPK activation did not suffice to reverse defects in the absence of IL-4, but increased AMPK activity in IL-4–stimulated Parp14−/− B cells coordinately restored glycolysis, mitochondrial activity, and protection against apoptosis. Together with the results on AMPK inhibition, the findings indicate that an ADP ribosyltransferase, PARP14, helps to orchestrate a coordinate metabolic response with increased glucose flux through glycolysis and mitochondrial oxidative metabolism. This metabolic response is vital for the prosurvival signaling of IL-4.
Absence of PARP14 Protects Against Myc-Induced Developmental Block and Lymphoma.
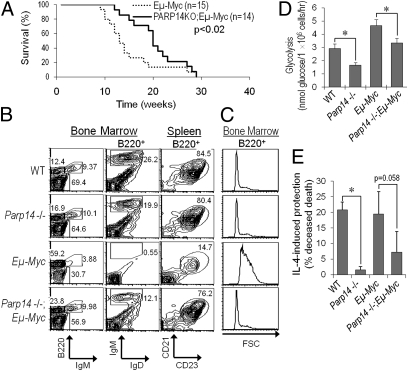
PARP14 is a member of the BAL family of macrodomain proteins implicated in the clinical aggressiveness of human B-cell lymphomas (17). Moreover, the capacity to reprogram metabolism may be central to oncogenesis, and suppression of apoptosis accelerates B-lymphoid malignancy (8, 9, 26). When we tested whether the absence of PARP14 from B cells influenced the prognosis for cancer-prone Eμ-myc transgenic mice (27), the absence of PARP14 delayed lymphomagenesis (Fig. 6A). Strikingly, PARP14 was required for the dramatic Myc-induced decrease in mature-phenotype B cells in premalignant marrow and deficit of follicular B cells in spleen (Fig. 6B and Table 1), although Myc in the absence of PARP14 induced pre-B lymphomas similar to the main phenotype of Eμ-myc tumors (Fig. S6). Myc expression in this system forces increased cell size on B-lineage cells (27, 28), but the absence of PARP14 from the B220+ population countered this in each subset (Fig. 6C and Table S2). These effects were associated with decreased glycolysis in freshly isolated Parp14−/− B cells compared with controls and a quantitatively similar blunting of the glycolytic response to Eμ-myc in premalignant B-lineage cells lacking PARP14 compared with Eμ-myc controls (Fig. 6D). We further observed that survival signaling in Parp14−/− B220+ Myc-transgenic cells was impaired compared with Eμ-myc controls (Fig. 6E), whereas the in vivo BrdU-labeling indices of Eμ-myc-transgenic B-lineage cells were unaffected by the lack of PARP14 (Fig. S7). These analyses of the premalignant bone marrow and spleen further highlight a striking mitigation of the impact of sustained Myc expression when PARP14 is absent. We conclude that expression of this B aggressive lymphoma family macro-PARP is vital for oncogenic and developmental effects of Myc as well as normal metabolic regulation of B cells.
Fig. 6.
PARP14 facilitates Myc-induced developmental distortion and lymphoma. (A) PARP14 deficiency protects against Myc-induced lymphoma. Survival curves for Eμ-myc, Parp14 WT and Eμ-myc, Parp14−/− mice are shown. (B) PARP14 and the Myc-induced distortion of B-lymphoid development. B-lineage subsets in the premalignant bone marrow and spleen of mice (age 6 wk) were analyzed. Shown are results from one experiment representative of four independent replicates. (C) Myc depends on PARP14 for increasing cell size. Shown are forward scatter (FSC) profiles of B220+ events. (D) Glycolysis in prelymphomatous Eμ-myc+ B cells. Glycolysis was assayed in freshly purified B220+ cells from WT, Parp14 KO, Eμ-myc, and Eμ-myc, Parp14−/− mice (age 6 wk). Shown are mean (±SEM) data from three independent experiments (*P < 0.05). (E) PARP14 impacts IL-4 inhibition of Eμ-myc+ B-cell apoptosis. Splenocytes from mice as in D were analyzed by TUNEL assay after culture in the presence or absence of IL-4. Bar graphs show mean (±SEM) IL-4–induced rescue from apoptosis in three independent replicate experiments.
Table 1.
PARP14 vital for Myc-induced hyper-cellularity and developmental distortions
| Bone marrow |
Spleen |
|||||||
| B220+ cells |
Pre/proB (B220+ IgMlo) |
Mature B (B220+ IgD+ IgMlo) |
FOB (B220+ CD23+ CD21+) |
|||||
| % of total bone marrow cells | Number (×105)* | % among bone marrow cells | Number (×105) | % among bone marrow cells | Number (×105) | % of B220+ spleen cells | Number (×106)† | |
| WT | 30.3 ± 3.0‡ | 13 ± 1.5‡ | 18.4 ± 3.5 | 8.1 ± 2.2 | 13.3 ± 3.6 | 5.3 ± 1.2 | 75.9 ± 2.7 | 41.6 ± 15.6 |
| PARP14 KO | 35.5 ± 2.0 | 14 ± 0.7 | 20.4 ± 1.7 | 8.4 ± 1.3 | 13.4 ± 3.3 | 5.3 ± 1.3 | 77.1 ± 1.1 | 35.7 ± 12.7 |
| Eμ-myc | 55.6 ± 1.2 | 31 ± 2.5 | 48.1 ± 7.5 | 26.7 ± 4.6 | 0.8 ± 0.4 | 0.5 ± 0.2 | 15.7 ± 2.1 | 7.2 ± 3.2 |
| PARP14 KO/Eμ-myc | 48.9 ± 9.4 | 20 ± 6.1 | 26.0 ± 2.7 | 10.6 ± 2.5 | 8.7 ± 1.2 | 3.2 ± 0.3 | 67.7 ± 3.7 | 28.2 ± 9.7 |
FOB, follicular B cells.
*(Number of recovered bone marrow cells) × (% in B220+ gate).
†(Number of recovered spleen cells) × (% in B220+ CD23+ CD21+ gate).
‡Mean ± SEM.
Discussion
A fundamental insight from this study pertains to the biological functions of ADP ribosyltransferases, in particular those recently identified as intracellular monotransferases (29). ADP ribosylation by endogenous activities of mammalian cells was first identified with PARP1, but 17–18 mammalian genes encode a module similar to the catalytic domain of PARP1 (30, 31). Unlike PARP1, PARP14 appears to catalyze mono- and not poly-ADP ribosylation (29). Furthermore, PARP14 is one of three members of the BAL family of PARPs containing macrodomains (“macro-PARPs”) that bind (poly-)ADP ribose (32, 33). PARP1's roles in DNA repair, chromatin, and inflammation have been identified (30, 31), but the physiological functions of monotransferase or macro-PARP family members are not known.
Increased macro-PARP gene expression is associated with worse clinical outcomes of DLBCL (17), but there has been no direct evidence of an impact of these proteins on B-cell malignancy or of contributory mechanisms that could account for such a role. Our data indicate that PARP14 contributes to Myc-induced lymphoma pathobiology and provide evidence that PARP14 impacts two processes pertinent to B-lymphoid oncogenesis. First, PARP14 is vital for a coordinated metabolic effect whereby glycolysis and aspects of mitochondrial function are increased in a manner vital for IL-4–induced survival signaling of normal and premalignant Eμ-myc-expressing B-lineage cells. This role of PARP14 intersects with AMPK such that this central regulator of glycolysis coordinately restored glycolytic fitness and survival signaling when combined with IL-4. Second, this macro-PARP is vital for the impairment of developmental progression imposed by dysregulated c-Myc in the premalignant phase (27), so that the pool of target cells (pro-, pre-, and overall numbers of B lineage-committed cells) is expanded by Eμ-myc far less if PARP14 is absent. This effect, along with the lack of impact on IL-7-treated B cells, distinguishes PARP14 facilitation of Eμ-myc perturbations of B-lymphoid physiology from recent findings on an IL-7 receptor-Stat5 pathway important for c-Myc-driven lymphomagenesis (10, 34).
Reorganization of metabolism and a substantial increase in demands on the glycolytic pathway are features common among cancer cells, but the underlying molecular mechanisms effecting these changes are not clear. We found substantially increased glycolysis in premalignant B lineage-committed Eμ-myc bone marrow cells, consistent with the increased size of these cells and analyses of cell lines showing that c-Myc directly binds regulatory elements in the chromatin of genes encoding glycolytic enzymes to increase their expression (35). Of note, we found that PARP14 is crucial for Eμ-myc cells fully to increase their glycolysis and size. Thus, the proliferative drive and faster protein synthesis forced by sustained c-Myc overexpression (27, 28) must contend with a restriction on the rate of glycolysis when PARP14 is absent. Rapid engulfment of apoptotic cells in vivo precludes accurately measuring rates of apoptosis in situ. However, BrdU incorporation into all B220+ subsets in vivo was similar in Parp14+/+ and Parp14−/− Eμ-myc mice, and the ex vivo data collectively indicate that PARP14 is vital for sustaining normal glycolytic rates in vivo and mitigates the apoptotic stress of Myc. Perturbations that decrease apoptotic susceptibility commonly potentiate Myc-induced lymphomagenesis (26, 36). This suggests that the role of PARP14 in mediating regulation of B-cell metabolism and thereby enhancing survival is a significant component of its impact on oncogenesis.
The work also provides evidence of an interaction between AMPK activity and the prosurvival role of PARP14. The findings are intriguing in light of conflicting evidence from prior explorations of the relationship between AMPK and cell survival, and evidence of a metformin effect on cancer in patients with diabetes (37), discussed further in SI Discussion. AMPK mediates a prosurvival signal initiated by adiponectin treatment of cardiomyocytes after ischemia and reperfusion and is similarly protective against neuronal apoptosis (38, 39). Conversely, sustained AMPK activation by high concentrations of an AMP analog caused apoptosis of normal and cancerous B cells and can similarly impact other cell types (40, 41). These opposite outcomes were due either to cell type-specific differences or the degree of AMPK activation, its duration, and cross-talk in which AMPK inhibits signaling via mTOR or exercises ACC-independent functions (42). In contrast to AMPK activation by stresses such as glucose deprivation, IL-4 stimulates mTOR (12). Of note, in the absence of IL-4, constitutively increased AMPK neither improved survival signaling nor raised rates of glycolysis to the level of IL-4–stimulated cells. Although the main role of AMPK may be to promote metabolic fitness on a pathway parallel to the IL-4–PARP14 mechanism, such findings indicate that coordination of at least two distinct mechanisms downstream from the IL-4 receptor is crucial. Akt activation by IL-4 was unaffected by the absence of PARP14, and this kinase can regulate survival by a mechanism that involves enhancement of glycolysis in coordination with mitochondrial stabilization (1, 22). Thus, although further study will be required to establish the nature of the PARP14-independent component(s), a contribution of Akt that cannot be replaced by AMPK is a straightforward model based on the data.
Finally, this work reveals that the capacity of Myc to impede B-cell maturation is strikingly dependent on PARP14. A block to B-lineage maturation in the bone marrow of Eμ-myc-transgenic mice has long been known (27), but the basis for this effect is not understood and conditional loss of myc also led to a deficit of mature B cells (43). Sustained c-Myc drives increased cell size along with persistent cell cycling and the attendant demand for increased energy production; the absence of PARP14 abrogated this increased size of B lineage-committed Eμ-myc cells. Developmental progression may require being able to exit the cell cycle (44), so it is tempting to speculate that the limitation in glycolysis prevented constant cycling, thereby allowing pre-B cells to mature. In addition, it is intriguing that bone marrow pre-B cells appeared more resistant to inhibition of glycolysis than their more mature IgM+ progeny (45). In any case, the emergence of lymphoma is thought to be due in part to the accumulation of an expanded pre-B population in Eμ-myc mice (27, 28). Thus, the combined changes may account for the contribution of this BAL-family ADP ribosyltransferase to Myc-induced lymphomagenesis.
Materials and Methods
Mice and Bone Marrow Reconstitutions.
B6, B6.Eμ-myc, B6.PL-Thy1a mice (The Jackson Laboratory), Parp14−/− mice (15), backcrossed to C57BL/6 (B6; Thy1b) for 12 generations, and B6/129-intercrossed Stat6−/− mice were housed in ventilated microisolators under specified pathogen-free conditions. Bone marrow cells were harvested after induction of donors with 5-fluorouracil, transduced with MiT vector or MiT-PARP14(MC) as described (13), and transferred into irradiated recipients (46). After 6 wk, B cells were isolated using magnetic beads, divided, cultured overnight ±IL-4, and assayed for apoptosis and glycolysis (modified by using 0.3 × 106 cells per sample for the triplicates). Further details are in SI Materials and Methods.
Reagents, Cell Purification, Cytokines, Cell Culture, Flow Cytometry, and Apoptosis Assays.
Mouse B cells were purified (90–95%) by depleting Thy1+ cells from spleen cells using anti-Thy1.2, and these primary lymphocytes or bone marrow cells were cultured in the presence or absence of IL-4 as described (13). Further details and standard reagents from commercial suppliers are discussed in SI Materials and Methods. Published methods and commercial reagents detailed further in SI Materials and Methods were used for flow cytometric analyses including TUNEL assays (15).
RNA and Protein Measurements.
Immunoblot and quantitative real-time RT-PCR analyses were performed using commercial antibodies and reagents by published methods (15) detailed further in SI Materials and Methods. Data are presented as values normalized to WT control and averaged over three independent experiments.
Glucose Uptake, Glycolysis Assays, Measurement of Mitochondrial Parameters, and Lactate Production.
B cells (106 per sample) were cultured in the presence or absence of IL-4 or analyzed immediately after purification. As detailed in SI Materials and Methods, glycolysis in equal numbers of viable cells was then assayed by minor modifications of a standard technique measuring the conversion of 5-[3H]glucose into diffused and undiffused 3HOH. Glucose uptake was measured using 2-[1,2-3H]deoxyglucose after depleting intracellular glucose stores (details in SI Materials and Methods). As detailed in SI Materials and Methods, purified B cells recounted after culture were assayed by measuring rates of mitochondrial oxidation of fluorescent compounds or determination of 14CO2 generation from [6-14C]glucose. Lactate levels in the medium afterward were determined using a commercial kit.
Supplementary Material
Acknowledgments
We are indebted to D. Carling (AMPK mutant cDNAs) and N. Taylor and M. Sitbon (H2RBD-EGFP protein preparations) for generous gifts of reagents. This work was initiated with support from Cancer Center Support Grant CA68485 and National Institutes of Health (NIH) Grants AI068149 (to M.B.), GM071735, and cores supported by DK020593, followed by Institutional Bridge funding from Vanderbilt University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017082108/-/DCSupplemental.
References
- 1.Plas DR, Rathmell JC, Thompson CB. Homeostatic control of lymphocyte survival: Potential origins and implications. Nat Immunol. 2002;3:515–521. doi: 10.1038/ni0602-515. [DOI] [PubMed] [Google Scholar]
- 2.Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 3.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: Energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 4.Lum JJ, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 5.Boothby M, Mora AL, Stephenson LM. Lymphokine-dependent proliferation of T-lymphoid cells: Regulated responsiveness and role in vivo. Crit Rev Immunol. 2001;21:487–522. [PubMed] [Google Scholar]
- 6.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 7.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroemer G, Pouyssegur J. Tumor cell metabolism: Cancer's Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Osborne LC, Duthie KA, Seo JH, Gascoyne RD, Abraham N. Selective ablation of the YxxM motif of IL-7Rα suppresses lymphomagenesis but maintains lymphocyte development. Oncogene. 2010;29:3854–3864. doi: 10.1038/onc.2010.133. [DOI] [PubMed] [Google Scholar]
- 11.Bruns HA, Kaplan MH. The role of constitutively active Stat6 in leukemia and lymphoma. Crit Rev Oncol Hematol. 2006;57:245–253. doi: 10.1016/j.critrevonc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Leonard WJ, O'Shea JJ. Jaks and STATs: Biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 13.Goenka S, Boothby M. Selective potentiation of Stat-dependent gene expression by collaborator of Stat6 (CoaSt6), a transcriptional cofactor. Proc Natl Acad Sci USA. 2006;103:4210–4215. doi: 10.1073/pnas.0506981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goenka S, Cho SH, Boothby M. Collaborator of Stat6 (CoaSt6)-associated poly(ADP-ribose) polymerase activity modulates Stat6-dependent gene transcription. J Biol Chem. 2007;282:18732–18739. doi: 10.1074/jbc.M611283200. [DOI] [PubMed] [Google Scholar]
- 15.Cho SH, et al. PARP-14, a member of the B aggressive lymphoma family, transduces survival signals in primary B cells. Blood. 2009;113:2416–2425. doi: 10.1182/blood-2008-03-144121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanagawa T, et al. Regulation of phosphoglucose isomerase/autocrine motility factor activities by the poly(ADP-ribose) polymerase family-14. Cancer Res. 2007;67:8682–8689. doi: 10.1158/0008-5472.CAN-07-1586. [DOI] [PubMed] [Google Scholar]
- 17.Aguiar RC, et al. BAL is a novel risk-related gene in diffuse large B-cell lymphomas that enhances cellular migration. Blood. 2000;96:4328–4334. [PubMed] [Google Scholar]
- 18.Hardie DG. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 19.Wurster AL, Rodgers VL, White MF, Rothstein TL, Grusby MJ. Interleukin-4-mediated protection of primary B cells from apoptosis through Stat6-dependent up-regulation of Bcl-xL. J Biol Chem. 2002;277:27169–27175. doi: 10.1074/jbc.M201207200. [DOI] [PubMed] [Google Scholar]
- 20.Dufort FJ, et al. Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism. J Immunol. 2007;179:4953–4957. doi: 10.4049/jimmunol.179.8.4953. [DOI] [PubMed] [Google Scholar]
- 21.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majewski N, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Gottlob K, et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabin EM, Ohara J, Paul WE. B-cell stimulatory factor 1 activates resting B cells. Proc Natl Acad Sci USA. 1985;82:2935–2939. doi: 10.1073/pnas.82.9.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patke A, Mecklenbräuker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC β- and Akt-dependent mechanism. J Exp Med. 2006;203:2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 27.Langdon WY, Harris AW, Cory S, Adams JM. The c-myc oncogene perturbs B lymphocyte development in E-μ-myc transgenic mice. Cell. 1986;47:11–18. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- 28.Iritani BM, Eisenman RN. c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc Natl Acad Sci USA. 1999;96:13180–13185. doi: 10.1073/pnas.96.23.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleine H, et al. Substrate-assisted catalysis by PARP10 limits its activity to mono-ADP-ribosylation. Mol Cell. 2008;32:57–69. doi: 10.1016/j.molcel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Hassa PO, Haenni SS, Elser M, Hottiger MO. Nuclear ADP-ribosylation reactions in mammalian cells: Where are we today and where are we going? Microbiol Mol Biol Rev. 2006;70:789–829. doi: 10.1128/MMBR.00040-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnakumar R, Kraus WL. The PARP side of the nucleus: Molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karras GI, et al. The macro domain is an ADP-ribose binding module. EMBO J. 2005;24:1911–1920. doi: 10.1038/sj.emboj.7600664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dani N, et al. Combining affinity purification by ADP-ribose-binding macro domains with mass spectrometry to define the mammalian ADP-ribosyl proteome. Proc Natl Acad Sci USA. 2009;106:4243–4248. doi: 10.1073/pnas.0900066106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abraham N, et al. Haploinsufficiency identifies STAT5 as a modifier of IL-7-induced lymphomas. Oncogene. 2005;24:5252–5257. doi: 10.1038/sj.onc.1208726. [DOI] [PubMed] [Google Scholar]
- 35.Kim JW, et al. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol Cell Biol. 2004;24:5923–5936. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eischen CM, Woo D, Roussel MF, Cleveland JL. Apoptosis triggered by Myc-induced suppression of Bcl-X(L) or Bcl-2 is bypassed during lymphomagenesis. Mol Cell Biol. 2001;21:5063–5070. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jalving M, et al. Metformin: Taking away the candy for cancer? Eur J Cancer. 2010;46:2369–2380. doi: 10.1016/j.ejca.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, et al. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119:835–844. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- 40.Campàs C, et al. Acadesine activates AMPK and induces apoptosis in B-cell chronic lymphocytic leukemia cells but not in T lymphocytes. Blood. 2003;101:3674–3680. doi: 10.1182/blood-2002-07-2339. [DOI] [PubMed] [Google Scholar]
- 41.Kefas BA, et al. AICA-riboside induces apoptosis of pancreatic β cells through stimulation of AMP-activated protein kinase. Diabetologia. 2003;46:250–254. doi: 10.1007/s00125-002-1030-3. [DOI] [PubMed] [Google Scholar]
- 42.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 43.de Alboran IM, et al. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity. 2001;14:45–55. doi: 10.1016/s1074-7613(01)00088-7. [DOI] [PubMed] [Google Scholar]
- 44.Miller JP, Yeh N, Vidal A, Koff A. Interweaving the cell cycle machinery with cell differentiation. Cell Cycle. 2007;6:2932–2938. doi: 10.4161/cc.6.23.5042. [DOI] [PubMed] [Google Scholar]
- 45.Kojima H, et al. Differentiation stage-specific requirement in hypoxia-inducible factor-1α-regulated glycolytic pathway during murine B cell development in bone marrow. J Immunol. 2010;184:154–163. doi: 10.4049/jimmunol.0800167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otsu M, et al. Reconstitution of lymphoid development and function in ZAP-70-deficient mice following gene transfer into bone marrow cells. Blood. 2002;100:1248–1256. doi: 10.1182/blood-2002-01-0247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.