Abstract
Converging evidence from the analysis of human brain tumors and genetically engineered mice has revealed that the mammalian target of rapamycin (mTOR) pathway is a central regulator of glial and glioma cell growth. In this regard, mutational inactivation of neurofibromatosis-1 (NF1), tuberous sclerosis complex (TSC), and PTEN genes is associated with glioma formation, such that pharmacologic inhibition of mTOR signaling results in attenuated tumor growth. This shared dependence on mTOR suggests that PTEN and NF1 (neurofibromin) glial growth regulation requires TSC/Rheb (Ras homolog enriched in brain) control of mTOR function. In this report, we use a combination of genetic silencing in vitro and conditional mouse transgenesis approaches in vivo to demonstrate that neurofibromin regulates astrocyte cell growth and glioma formation in a TSC/Rheb-independent fashion. First, we show that Nf1 or Pten inactivation, but not Tsc1 loss or Rheb overexpression, increases astrocyte cell growth in vitro. Second, Nf1-deficient increased mTOR signaling and astrocyte hyperproliferation is unaffected by Rheb shRNA silencing. Third, conditional Tsc1 inactivation or Rheb overexpression in glial progenitors of Nf1+/− mice does not lead to glioma formation. Collectively, these findings establish TSC/Rheb-independent mechanisms for mTOR-dependent glial cell growth control and gliomagenesis relevant to the design of therapies for individuals with glioma.
Keywords: astrocytoma, optic glioma, glia
Mutations in several tumor suppressor genes and receptor tyrosine kinases (RTKs) deregulate cell growth and survival by increasing mammalian target of rapamycin (mTOR) signaling. This convergence on mTOR is well-illustrated in brain tumors (gliomas) in which mutations lead to mTOR-dependent growth deregulation. In this regard, copy-number increases or gain-of-function mutations in key RTK molecules (PDGFR, EGFR, and ERBB2) enhance glioma growth by activating mTOR (1). Similarly, loss-of-function mutations in the PTEN, tuberous sclerosis complex (TSC), and neurofibromatosis-1 (NF1) tumor suppressor genes result in glioma formation, which is reduced by pharmacologic mTOR inhibition. The shared activation of mTOR by PTEN, TSC (tuberin and hamartin), and NF1 (neurofibromin) loss has suggested a model in which protein kinase B (PKB)/AKT hyperactivation following PTEN or neurofibromin loss leads to tuberin phosphorylation (2–5), loss of tuberin/hamartin complex inhibition of Ras homolog enriched in brain (Rheb) activity (6–10), and Rheb-mediated mTOR activation (11–14) relevant to glioma growth and targeted therapy.
However, several experimental findings call this unified TSC/Rheb model of mTOR regulation into question. First, although Nf1 and Pten inactivation cause increased astroglial cell growth in vitro, no such increase in proliferation is observed following Tsc1 loss or Rheb overexpression (15). Second, Tsc1 loss in astrocytes results in increased cell size in the brain, whereas Nf1 inactivation in vitro or using the same Cre driver mouse strain in vivo has no discernable effect on cell size (16, 17). Third, Tsc1 inactivation in neuroglial progenitor cells results in intractable seizures and death, whereas Nf1 loss in these same neuroglial progenitors has no effect on mouse viability or epileptogenesis (16, 17). Importantly, although Pten-, hamartin-, and neurofibromin-deficient glial phenotypes have different biological effects, each is completely inhibited by rapamycin in vitro and in vivo (15, 18–21). Collectively, these observations support a model in which neurofibromin-regulated mTOR-dependent growth regulation is distinct from TSC/Rheb-mediated mTOR-dependent growth regulation in the brain.
To critically examine the requirement for the TSC/Rheb axis in neurofibromin mTOR-dependent growth regulation in the brain, we used a combination of pharmacologic and genetic silencing approaches in vitro coupled with conditional knockout and overexpression transgenesis strategies in vivo. We show that neurofibromin regulation of glial cell growth and glioma formation is TSC/Rheb-independent, and that the functional consequences of TSC/Rheb mTOR regulation in astroglial cells are distinct from those conferred by Nf1 gene inactivation.
Results
mTOR Activation Differentially Regulates Cell Growth in Astrocytes in Vitro.
Previous studies have demonstrated that Nf1, Pten, and Tsc1 gene inactivation each results in increased mTOR activation in astrocytes in vitro (15, 19, 20). In addition, Rheb overexpression to mimic Tsc1/Tsc2 gene inactivation similarly increases mTOR activation (15). In each case, this mTOR activation is completely inhibited by rapamycin treatment (Fig. 1), suggesting that mTOR signaling is a critical regulator of cell growth in astrocytes. However, mTOR activation as a consequence of Nf1 and Pten loss results in increased astrocyte proliferation in vitro, whereas increased mTOR signaling following Tsc1 loss, Tsc2 loss, or Rheb overexpression (>300-fold) has no such effect. This differential effect does not reflect the magnitude of mTOR activation as measured by S6 phosphorylation on Ser residues 240/244 (Fig. 1 and Fig. S1). Moreover, the difference between TSC/Rheb and neurofibromin/PTEN mTOR regulation of astrocyte proliferation is not due to the activation of other signaling pathways, as both Nf1−/− and Pten−/− astrocyte hyperproliferation is abolished following mTOR inhibition. Together, these findings suggest that mTOR regulation by neurofibromin/PTEN is distinct from TSC/Rheb (Fig. 2A).
Fig. 1.
Neurofibromin and Pten loss in astrocytes results in increased mTOR-dependent proliferation. Whereas Nf1−/− (A) and Pten−/− (B) astrocytes exhibit increased mTOR activation (phospho-S6 Ser-240/244) and proliferation sensitive to 10 nM rapamycin inhibition (C and D), Tsc1-deficient (E) and Rheb-expressing (F) astrocytes exhibit robust mTOR activation but no increase in proliferation in vitro. Total S6 and α-tubulin were included as internal loading controls. Asterisks denote statistically significant differences (P ≤ 0.0001) between WT and Nf1−/− or Pten−/− astrocytes. Bars indicate SEM.
Fig. 2.
Nf1-deficient astrocyte hyperproliferation is not dependent on Rheb expression. (A) Convergence on mTOR pathway by neurofibromin, PTEN, TSC, and Rheb. (B) Rheb shRNA silencing reduces mTOR activation in Tsc1-deficient astrocytes (B; Right), but has no effect on mTOR activity in Nf1−/− astrocytes (B; Left). Representative blots are shown. (C) Rheb shRNA knockdown in Nf1-deficient astrocytes does not reduce cell proliferation. Bars indicate SEM of six replicate wells per condition for the representative experiment shown in B (Left).
Neurofibromin Growth Regulation of Astrocyte Proliferation Does Not Depend on Rheb in Vitro.
Prior analyses of neurofibromin mTOR growth control suggested that the Akt hyperactivation resulting from Nf1 inactivation (Fig. S2A) leads to tuberin phosphorylation and inactivation (21). First, we observed no change in tuberin phosphorylation (residues Thr-1462 and Ser-969) in Nf1−/− astrocytes relative to wild-type astrocytes (Fig. S2B). Similarly, we observed no change in tuberin Ser-969 phosphorylation in Pten−/− astrocytes compared with wild-type astrocytes (Fig. S2C). Second, we found no change in S6 phosphorylation following small hairpin RNA (shRNA) silencing of Rheb in Nf1−/− astrocytes (Fig. 2B and Fig. S2D). In contrast, S6 phosphorylation is attenuated by Rheb silencing in Tsc1-deficient astrocytes. Third, following Rheb knockdown in Nf1-deficient or WT astrocytes, we observed no reproducible changes in proliferation (Fig. 2C and Fig. S2 E and F). Collectively, these observations demonstrate that neurofibromin regulation of astrocyte mTOR signaling and proliferation is not dependent on TSC/Rheb function.
Conditional Rheb Activation in Mice Results in Increased Astrocyte mTOR Signaling and Proliferation in Vivo.
To extend the above in vitro studies, we next generated conditional Rheb-expressing mice (Fig. 3A). The conditional Rheb transgene was engineered into the Rosa locus by homologous recombination, resulting in two independent strains of LSL-Rheb mice (lines 13431 and 13433). In addition, the transgene contained a reverse tetracycline control element, allowing doxycycline regulation of Rheb expression as well as a downstream enhanced green fluorescent protein (eGFP) driven from an internal ribosomal entry site (IRES). We verified the inducible and regulatable expression of Rheb in primary forebrain astrocytes from these mice. Following infection with Ad5-Cre virus, robust GFP and Rheb expression was observed, which was abrogated following doxycycline treatment (line 13431; Fig. 3B). No GFP or Rheb expression was observed in identical astrocytes infected with Ad5-LacZ. Similar results were also obtained using line 13433 (Fig. S3). Moreover, as seen following viral Rheb expression (Fig. 1F), transgenic expression of Rheb resulted in mTOR activation (S6 Ser-240/244 and Ser-235/236 phosphorylation; Fig. 3C), but no increase in astrocyte proliferation (Fig. 3D).
Fig. 3.
Conditional expression of Rheb in astrocytes does not increase cell growth in vitro. (A) Conditional targeting strategy for the generation of LSL-Rheb mice (line 13431). (B) Ad5 virus-mediated Cre delivery results in eGFP (Left; immunofluorescence microscopy) and Rheb (Right; Western blot) expression compared with LacZ delivery. Both eGFP and Rheb expression in Cre-transduced astrocytes is eliminated with doxycycline. (C) Cre-mediated Rheb expression results in increased mTOR signaling (phospho-S6 Ser-240/244 and Ser-235/236). (D) No change in astrocyte proliferation was observed following Cre-mediated Rheb expression as assessed by [3H]thymidine incorporation. Bars indicate SEM.
Next, we sought to determine whether Rheb overexpression in GFAP+ cells had similar effects as Tsc1 or Nf1 inactivation in vivo. For these experiments, both LSL-Rheb lines were intercrossed with GFAP-Cre mice. In contrast to our previous results in Tsc1GFAPCKO mice, we found no increase in body or brain weight (Fig. 4A). However, similar to Tsc1GFAPCKO mice (17), Rheb overexpression resulted in increased mTOR signaling (number of S6 phospho-Ser-240/244-immunoreactive cells; Fig. 4B), proliferation (Fig. 4C), and astrocytes (GFAP-immunoreactive cells; Fig. 4D) without any change in apoptosis (Fig. S4). Similar results were obtained with both LSL-Rheb strains. These findings demonstrate that Rheb overexpression, similar to Tsc1 inactivation, is sufficient to increase astrocyte numbers in vivo.
Fig. 4.
Conditional Rheb expression in astroglial cells results in increased glial numbers and proliferation in vivo. (A) No effect of Rheb expression on body size or brain weight was observed in RhebGFAP mice. (B) GFAP-Cre-mediated Rheb expression results in increased numbers of pS6 (Ser-240/244) cells, (C) Ki67+ proliferating cells, and (D) GFAP+ cells in vivo. Magnification = 10×. (Insets) Representative pS6+ (B) and Ki67+ (C) cells. (Scale bars, 100 μm.) Bars indicate SEM.
Rheb Activation Is Insufficient for Gliomagenesis in Vivo.
Previous studies from our laboratory have shown that Nf1 loss in astrocytes alone is not sufficient for glioma formation unless coupled with reduced Nf1 expression in the brain (Nf1+/− mice) (16, 22). The requirement for both an Nf1+/− brain environment and Nf1 loss in astrocytes provides a unique in vivo platform to assess the impact of neurofibromin downstream signaling pathway activity on gliomagenesis. Consistent with the known role of neurofibromin as a negative regulator of KRas in astrocytes, Nf1+/− mice with astrocyte expression of an activated KRas allele develop optic glioma similar to Nf1+/−GFAPCKO mice. The ability of activated KRas to replace neurofibromin loss and result in optic glioma formation in Nf1+/− mice was exploited to evaluate the impact of other mTOR-activating events on gliomagenesis. First, we used an LSL-myr-Akt strain (23) to determine whether constitutive Akt activation is sufficient for optic glioma formation in Nf1+/− mice. Unfortunately, due to tissue mosaicism, the LSL-Akt transgene was not expressed in the optic nerve. This limitation precluded our ability to directly examine the impact of Akt hyperactivation on optic nerve gliomagenesis.
Because mice with GFAP conditional Pten inactivation die in the early postnatal period (18), we focused on experiments using Tsc1 conditional knockout mice. For these studies, we intercrossed Tsc1flox/flox mice with Nf1+/− and GFAP-Cre mice to develop Nf1+/−; Tsc1GFAPCKO mice. Similar to our previous studies using Tsc1GFAPCKO mice, Tsc1 inactivation resulted in seizures and death by 6–10 wk of age. Whereas the vast majority of Nf1+/−; Tsc1GFAPCKO mice died before 3 mo of age (n = 12), a time when Nf1+/−GFAPCKO mice and Nf1+/−; KRasGFAP transgenic mice develop optic glioma, three mice survived to 12 wk of age. None of these mice (11.5, 12, and 13 wk of age) were found to have optic gliomas on gross histologic analysis despite loss of Tsc1 expression.
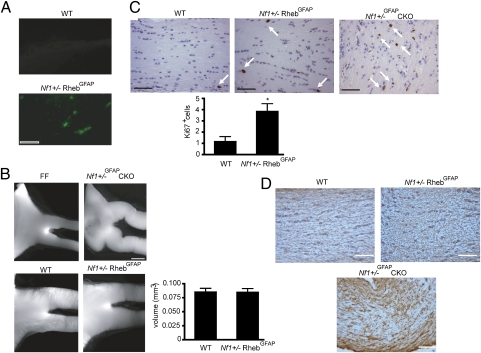
Because RhebGFAP mice appear healthy and do not develop seizures, we intercrossed LSL-Rheb mice with Nf1+/− and GFAP-Cre mice to generate Nf1+/−; RhebGFAP mice. Unlike LSL-Akt mice, there was robust Rheb transgene expression in the optic nerve (Fig. 5A). However, optic gliomas were not observed in Nf1+/−; RhebGFAP mice from either line at 3 mo of age (n = 5 mice analyzed). In this regard, optic nerves in Nf1+/−; RhebGFAP mice were indistinguishable from their wild-type counterparts on gross examination and had optic nerve volumes similar to control mice (Fig. 5B). As observed in the brain, Nf1+/−; RhebGFAP mouse optic nerves had more proliferating cells (Fig. 5C) but no optic glioma, as judged by focal collections of GFAP-immunoreactive cells (Fig. 5D). In contrast, Nf1+/−GFAPCKO mice developed optic gliomas with kinking of the prechiasmatic optic nerves and chiasmal enlargement (Fig. 5B), increased numbers of proliferating cells (average number of Ki67+ cells = 6 per nerve; Fig. 5C), and more GFAP immunostaining (Fig. 5D). These observations demonstrate that Tsc1 loss or Rheb overexpression is not equivalent to neurofibromin loss in vivo, and establishes TSC/Rheb-independent mechanisms of mTOR regulation in the brain relevant to glial cell growth and tumor formation.
Fig. 5.
Conditional Rheb expression in Nf1+/− mice is insufficient for glioma formation. (A) Robust eGFP expression is seen in the optic nerves of RhebGFAP mice. Magnification, 10×. (Scale bar, 100 μm.) (B) Nf1+/−; RhebGFAP mice have normal-appearing optic nerves and no increase in optic nerve volume, whereas Nf1+/−GFAPCKO mice develop prechiasmatic optic nerve and chiasmal gliomas. FF, Nf1flox/flox(WT). Magnification, 10×. (Scale bar, 100 μm.) (C and D) Nf1+/−; RhebGFAP mice have increased numbers of Ki67+ proliferating cells in the optic nerve but no focal areas of GFAP+ cells indicative of optic glioma, whereas Nf1+/−GFAPCKO mice demonstrated both increased numbers of Ki67+ cells and GFAP immunostaining. Magnification, 20×. (Scale bars, 100 μm.) The arrows denote Ki67+ cells. Bars indicate SEM.
Discussion
Numerous studies have highlighted the central role of Akt axis signaling in astrocyte differentiation and growth. Astrocyte differentiation from neural stem cells is controlled by Akt signaling through STAT3, N-CoR, or p27 deregulation (24–26), whereas Akt/mTOR hyperactivation is observed in reactive astrocytes responding to spinal cord injury (27). Similarly, Akt drives astrocyte survival and proliferation initiated by RTK activation (28, 29). The importance of Akt/mTOR signaling to astrocyte proliferation is also underscored by glioma-associated tumor mutations, including the PTEN, TSC, and NF1 tumor suppressor genes. Investigations using genetically engineered mutant mice and derivative astrocytes demonstrate that growth control is dependent on mTOR activation, such that Nf1-deficient and Pten-deficient astrocytes exhibit increased astrocyte proliferation in vitro and in vivo, which is inhibited by pharmacologic inhibition of mTOR pathway function (18, 20, 30).
In contrast to PTEN and neurofibromin loss, Tsc1 loss and Rheb overexpression do not increase astrocyte growth in vitro. Moreover, Rheb silencing did not reduce Nf1-deficient astrocyte proliferation or mTOR activation (pS6) in vitro, whereas Rheb silencing attenuated mTOR activity in Tsc1-deficient astrocytes. Following Rheb overexpression or Tsc1 loss in astrocytes in vivo, there was a modest increase in cell proliferation and astrocyte numbers. This increase in astrocyte number prompted us to determine whether hamartin loss or Rheb overexpression could substitute for neurofibromin loss in the genesis of optic glioma. In contrast to Nf1 loss, gliomas were not observed in Nf1+/−; RhebGFAP or Nf1+/−; Tsc1GFAPCKO mice. These findings demonstrate that whereas TSC/Rheb signaling is important for mTOR activation and glial cell proliferation, it is not equivalent to neurofibromin loss, and establish two independent pathways to mTOR-regulated cell growth with different functional consequences.
Importantly, our results do not support previous conclusions that neurofibromin growth control mediated by mTOR is TSC/Rheb-dependent (21). In this report, the TSC dependence was largely based on increased tuberin phosphorylation on residues Thr-1462 and Ser-939 in Nf1-deficient mouse embryonic fibroblasts and tumor cells as well as the use of a phospho-Thr-1462/Ser-939 double mutant tuberin molecule. In contrast, we used a combination of Rheb silencing in vitro, Tsc1 inactivation in vivo, and conditional Rheb overexpression in vivo to show that neurofibromin astrocyte growth control and gliomagenesis is TSC/Rheb-independent. Our findings are consistent with previous studies in Drosophila demonstrating that tuberin is not a critical substrate of Akt relevant to fly development (31). In this regard, we have also shown that the adhesion molecule on glia regulates Akt/mTOR/S6K activation independently of Rheb (32).
The observation that neurofibromin regulates glial cell proliferation in a TSC/Rheb-independent manner raises several issues. First, there are likely differences in signaling pathways activated following Nf1 loss in distinct cell types. We have recently shown that neural stem cells from different brain regions have varying responses to Nf1 inactivation, attributable to changes in mTOR complex protein (rictor versus raptor) levels (25). Similarly, PDK1-dependent Akt activation is differentially regulated in neurons versus astrocytes as a result of cell type-specific activation of regulatory feedback pathways (33). In addition, there are differences in mTOR pathway activation and proliferation between astrocytes and fibroblasts in response to Nf1, Tsc1, and Pten inactivation (7). For example, 4EBP1 phosphorylation is unaltered following Nf1 and Pten inactivation in astrocytes, whereas Tsc1−/− astrocytes exhibit increased 4EBP1 phosphorylation relative to their wild-type counterparts (Fig. S5).
Second, TSC and Rheb may have additional functions that influence Akt/mTOR signaling. For example, loss of TSC expression disrupts Akt signaling through down-regulation of the platelet-derived growth factor receptor (PDGFR) (34). In addition, phosphodiesterase-4B (PDE4B) can bind to and inhibit Rheb function in a cAMP-dependent manner (35). Previous studies from our laboratory have revealed reduced cAMP levels in Nf1-deficient astrocytes (36). These lower cAMP levels would be predicted to reduce mTOR signaling as a result of enhanced PDE4B–Rheb binding; however, we observed no changes in mTOR activity following pharmacologic treatments that raise cAMP levels in Nf1-deficient astrocytes (Fig. S6A). Whereas tuberin/Rheb has been reported to regulate MAPK activity via B-RAF (37), we observed no change in MAPK activation in either Tsc1-deficient or Rheb-expressing astrocytes (Fig. S6B). The increase in MAPK activity in Nf1-deficient astrocytes results from Ras hyperactivation and is not attenuated by PI3K inhibition, as might be predicted if Akt activation silenced TSC function by tuberin phosphorylation to increase Rheb/BRAF-mediated MAPK signaling.
Third, although neurofibromin astrocyte growth regulation does not require TSC/Rheb signaling, the precise mechanism underlying Nf1−/− astrocyte mTOR activation is unclear. Although neurofibromin activation of mTOR is Akt-dependent (Fig. S6C), it does not involve direct mTOR phosphorylation (Fig. S6D), cAMP signaling (Fig. S6A), or changes in either DEPTOR expression (38) (Fig. S6E) or phospholipase D activity (39) (Fig. S6F). Interestingly, we found that PRAS40 phosphorylation was increased in Nf1−/−, but not Tsc1−/−, astrocytes (Fig. S7 A and B), suggesting that neurofibromin loss might lead to Akt-mediated PRAS40 phosphorylation (inactivation) and reduced mTOR inhibition (40). However, shRNA-mediated PRAS40 silencing in WT astrocytes, to mimic PRAS40 inactivation in Nf1-deficient astrocytes, decreased S6 activation (Fig. S7C) and reduced astrocyte proliferation (Fig. S7D), arguing against differential PRAS40 phosphorylation as the likely mechanism underlying mTOR activation in Nf1−/− glial cells. The identification of Akt signaling intermediates responsible for neurofibromin mTOR regulation will require further studies aimed at discovering new potential regulators of mTOR or mTOR complex molecules in astrocytes.
In summary, our results establish Akt-mediated TSC/Rheb-dependent and -independent mechanisms for mTOR growth regulation in the brain and demonstrate that neurofibromin controls astrocyte growth in an Akt-dependent, but TSC/Rheb-independent, fashion relevant to gliomagenesis. As we move into an era of targeted therapeutics, these differences in mTOR growth regulation will need to be considered to develop the most effective therapies for cancers with deregulated TOR signaling.
Methods
Mice.
Nf1flox/flox, Tsc1flox/flox, and Ptenflox/flox mice were generated as previously described (16–18), whereas Nf1+/−; LSL-Rheb; GFAP-Cre and Nf1+/−; Tsc1flox/flox; GFAP-Cre mice were generated by successive intercrossing (16, 22, 41) with age-matched mice as controls. GFAP-Cre and LSL-Rheb littermates were indistinguishable from C57BL/6 mice. All mice were maintained on a C57BL/6 background and used in accordance with active animal studies protocols at Washington University School of Medicine.
Generation of Conditional Rheb-Expressing Mice.
LSL-Rheb mice were generated by a knock-in mouse strategy using a backbone knock-in ROSA-26-tetracycline-regulated transcriptional activator (tTA) vector provided by S. Miyazaki (Osaka University Graduate School of Medicine, Osaka, Japan). This construct included a splice acceptor sequence, a loxP-flanked neomycin phosphotransferase gene with a polyA sequence, the pUHD15-1 tTA gene, a separate polyA sequence, an insulator sequence, the CMV*1 promoter responsive to tTA, the rabbit β-globin second intron, the rat Rheb2 gene (HA-Rheb generated by PCR), an IRES, the eGFP gene, and another polyA sequence (42) (Fig. 3A). Three ES cell lines were chosen, verified by karyotyping, and used to generate LSL-Rheb knock-in mice by blastocyst injection. Two chimeric founder mice with germ-line transmission (lines 13431 and 13433) were identified.
Primary Astrocytes.
PN2 forebrain astrocyte cell cultures from LSL-Rheb, Nf1flox/flox, Tsc1flox/flox, and Ptenflox/flox mice were generated as previously described (16, 17, 20). Tsc2-deficient and control astrocytes were provided by Michael Wong (Washington University). Adenovirus type 5 containing β-galactosidase (Ad5-LacZ) and Cre recombinase (Ad5-Cre) (University of Iowa Gene Transfer Core, Iowa City, IA) were used to produce wild-type and Nf1−/−, Tsc1−/−, and Pten−/− or Rheb-expressing astrocytes, respectively. Neurofibromin, hamartin, and Pten loss or Rheb overexpression was confirmed by immunoblot analysis 5 d after infection (Fig. S1). All experiments were performed on passage-1 or -2 cultures at least three times with similar results.
Retroviral and Lentiviral Constructs and Viral Delivery.
Mouse-specific shRNA lentivirus against Rheb (Sigma; GenBank accession no. NM_053075; TRCN0000075607) was used to silence Rheb in Tsc1−/− and Nf1−/− astrocytes following two infections (25). Empty pLKO.1 virus was used as a control. Murine stem cell virus (MSCV)-containing rat Rheb2 was generated following 293T cell transfection with τ-helper DNA using Fugene HD (Roche) (20). Forty-eight hours later, virus-containing supernatants were filtered through 0.45-μm syringe filters, and astrocytes were infected thrice and harvested 72 h later. MSCV-GFP was used as a control.
Pharmacologic Inhibitors.
Rapamycin (LC Laboratories) was used at a concentration of 10 nM for treating the cells for 16–18 h unless otherwise indicated.
Cell Proliferation.
Cell proliferation was performed using [3H]thymidine (1 μCi/mL) as previously described (43).
Immunohistochemistry.
Mice were perfused transcardially with 4% paraformaldehyde in PBS. Following vibratome sectioning, immunohistochemistry was performed on adjacent paraffin sections with GFAP (Invitrogen), Ki67 (BD PharMingen), and pS6 (Cell Signaling) antibodies (16). Immunoreactivity was visualized with the Vectastain avidin–biotin complex and 3,3′-diaminobenzidine (Vector Laboratories). TUNEL labeling was performed as previously described (44). Representative sections were photographed with a digital camera (Optronics) attached to an inverted microscope (Nikon).
Western Blotting.
Cells were lysed in Nonidet P-40 lysis buffer with protease and phosphatase inhibitors. All antibodies were used at the stated dilutions (Table S1). Western blotting was performed as previously described (43).
Optic Glioma Measurements.
The entire optic nerve with the eye and intact chiasm was microdissected and photographed with a scale bar. Prechiasmatic optic nerve volumes were calculated as previously reported (44).
Supplementary Material
Acknowledgments
We thank Dr. Renate Lewis for generating the LSL-Rheb targeting construct, Dr. Joshua Rubin for additional Nf1flox/flox astrocytes, and Dr. Michael Wong for the Tsc2−/− astrocytes. We also thank Ms. Cori DeSanto for expert technical assistance. This study was partially funded by Grant NF050176 from the Department of Defense (to D.H.G.), National Cancer Institute Grant CA127008 (to D.H.G.), and the Hope Center Transgenic Vector Core Facility at the Washington University School of Medicine.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019012108/-/DCSupplemental.
References
- 1.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai SL, et al. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan HC, et al. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364–35370. doi: 10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- 4.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 5.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 6.Castro AF, Rebhun JF, Clark GJ, Quilliam LA. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem. 2003;278:32493–32496. doi: 10.1074/jbc.C300226200. [DOI] [PubMed] [Google Scholar]
- 7.Garami A, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 8.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 11.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 12.Patel PH, et al. Drosophila Rheb GTPase is required for cell cycle progression and cell growth. J Cell Sci. 2003;116:3601–3610. doi: 10.1242/jcs.00661. [DOI] [PubMed] [Google Scholar]
- 13.Saucedo LJ, et al. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 14.Stocker H, et al. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- 15.Uhlmann EJ, et al. Loss of tuberous sclerosis complex 1 (Tsc1) expression results in increased Rheb/S6K pathway signaling important for astrocyte cell size regulation. Glia. 2004;47:180–188. doi: 10.1002/glia.20036. [DOI] [PubMed] [Google Scholar]
- 16.Bajenaru ML, et al. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol. 2002;22:5100–5113. doi: 10.1128/MCB.22.14.5100-5113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlmann EJ, et al. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 18.Fraser MM, et al. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64:7773–7779. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- 19.Neshat MS, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65:2755–2760. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- 21.Johannessen CM, et al. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci USA. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajenaru ML, et al. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–8577. [PubMed] [Google Scholar]
- 23.Elghazi L, et al. Generation of a reporter mouse line expressing Akt and EGFP upon Cre-mediated recombination. Genesis. 2008;46:256–264. doi: 10.1002/dvg.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermanson O, Jepsen K, Rosenfeld MG. N-CoR controls differentiation of neural stem cells into astrocytes. Nature. 2002;419:934–939. doi: 10.1038/nature01156. [DOI] [PubMed] [Google Scholar]
- 25.Lee DY, Yeh TH, Emnett RJ, White CR, Gutmann DH. Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes Dev. 2010;24:2317–2329. doi: 10.1101/gad.1957110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de la Iglesia N, et al. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev. 2008;22:449–462. doi: 10.1101/gad.1606508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Codeluppi S, et al. The Rheb-mTOR pathway is upregulated in reactive astrocytes of the injured spinal cord. J Neurosci. 2009;29:1093–1104. doi: 10.1523/JNEUROSCI.4103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SG, et al. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 29.Li B, et al. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol-3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene. 2004;23:4594–4602. doi: 10.1038/sj.onc.1207602. [DOI] [PubMed] [Google Scholar]
- 30.Johannessen CM, et al. TORC1 is essential for NF1-associated malignancies. Curr Biol. 2008;18:56–62. doi: 10.1016/j.cub.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 31.Dong J, Pan D. Tsc2 is not a critical target of Akt during normal Drosophila development. Genes Dev. 2004;18:2479–2484. doi: 10.1101/gad.1240504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheidenhelm DK, et al. Akt-dependent cell size regulation by the adhesion molecule on glia occurs independently of phosphatidylinositol 3-kinase and Rheb signaling. Mol Cell Biol. 2005;25:3151–3162. doi: 10.1128/MCB.25.8.3151-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalhoub N, Zhu G, Zhu X, Baker SJ. Cell type specificity of PI3K signaling in Pdk1- and Pten-deficient brains. Genes Dev. 2009;23:1619–1624. doi: 10.1101/gad.1799609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HW, et al. Cyclic AMP controls mTOR through regulation of the dynamic interaction between Rheb and phosphodiesterase 4D. Mol Cell Biol. 2010;30:5406–5420. doi: 10.1128/MCB.00217-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dasgupta B, Dugan LL, Gutmann DH. The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. J Neurosci. 2003;23:8949–8954. doi: 10.1523/JNEUROSCI.23-26-08949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karbowniczek M, et al. Regulation of B-Raf kinase activity by tuberin and Rheb is mammalian target of rapamycin (mTOR)-independent. J Biol Chem. 2004;279:29930–29937. doi: 10.1074/jbc.M402591200. [DOI] [PubMed] [Google Scholar]
- 38.Peterson TR, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y, et al. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci USA. 2008;105:8286–8291. doi: 10.1073/pnas.0712268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sancak Y, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyazaki S, Yamato E, Miyazaki J. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53:1030–1037. doi: 10.2337/diabetes.53.4.1030. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee S, et al. The neurofibromatosis type 1 tumor suppressor controls cell growth by regulating signal transducer and activator of transcription-3 activity in vitro and in vivo. Cancer Res. 2010;70:1356–1366. doi: 10.1158/0008-5472.CAN-09-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hegedus B, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68:1520–1528. doi: 10.1158/0008-5472.CAN-07-5916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.