Abstract
Physiological sensing of O2 tension (partial O2 pressure, pO2) plays an important role in some mammalian cellular systems, but striated muscle generally is not considered to be among them. Here we describe a molecular mechanism in skeletal muscle that acutely couples changes in pO2 to altered calcium release through the ryanodine receptor–Ca2+-release channel (RyR1). Reactive oxygen species are generated in proportion to pO2 by NADPH oxidase 4 (Nox4) in the sarcoplasmic reticulum, and the consequent oxidation of a small set of RyR1 cysteine thiols results in increased RyR1 activity and Ca2+ release in isolated sarcoplasmic reticulum and in cultured myofibers and enhanced contractility of intact muscle. Thus, Nox4 is an O2 sensor in skeletal muscle, and O2-coupled hydrogen peroxide production by Nox4 governs the redox state of regulatory RyR1 thiols and thereby governs muscle performance. These findings reveal a molecular mechanism for O2-based signaling by an NADPH oxidase and demonstrate a physiological role for oxidative modification of RyR1.
Keywords: redox signaling, oxygen sensing, S-nitrosylation
Specialized mammalian sensory cells transduce varying O2 levels, but the mechanisms of O2 sensing and O2-based signaling have not been elucidated fully. In particular, reversible changes in ion channel activity often are implicated in physiological responses to O2, but the molecular bases of these changes are unknown. We previously have described an O2-sensing and -signaling mechanism in mammalian skeletal muscle that operates on the ryanodine receptor–Ca2+-release channel (RyR1), the principal source of Ca2+ release from the sarcoplasmic reticulum (SR) (1, 2). At relatively low partial pressure of O2 (pO2), endogenously generated NO regulates RyR1 activity by S-nitrosylation of a single Cys thiol (1, 3). At higher pO2, RyR1 activity is enhanced independently of NO in association with the oxidation of a separate, small set of Cys thiols (1). Oxidation of RyR1 thiols at high pO2 and reduction following transition from high to low pO2 are observed in isolated SR vesicles (1). However, the molecular mechanisms within the SR that mediate this O2-based redox cycle have not been determined. Here we show that the redox enzyme NADPH oxidase 4 (Nox4) is a constituent of the SR and that hydrogen peroxide (H2O2) produced by Nox4 in proportion to pO2 over a physiological range serves as an essential effector of pO2-dependent regulation of RyR1 redox status and function. These data demonstrate physiological regulation by reactive oxygen species (ROS) of RyR1 and suggest insights into the molecular mechanisms of O2 sensing and O2-based signaling in mammalian cells.
Results
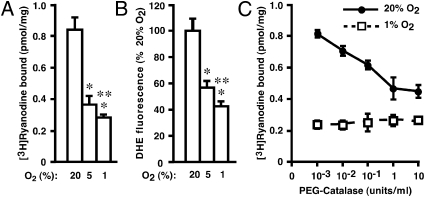
RyR1 activity in an SR-enriched subcellular fraction (SR vesicles) was enhanced progressively at pO2 of 1% O2, 5% O2, and 20% O2 (ambient pO2) (Fig. 1A); these levels largely recapitulate the physiological muscle O2 gradient and extend to oxidative stress (4–7). Production of ROS was enhanced similarly, as assessed by measuring fluorescence resulting from conversion of dihydroethidium (DHE) (8) (Fig. 1B) (or of 2′,7′-dichlorofluorescin; vide infra). The increase in RyR1 activity at high (20% O2) versus low (1% O2) pO2 was largely eliminated by polyethylene glycol (PEG)-catalase (Fig. 1C). Thus, an endogenous SR mechanism generates ROS in a pO2-dependent fashion that regulates RyR1 activity, and H2O2 is apparently the active species.
Fig. 1.
pO2-dependent regulation of RyR1 by endogenous ROS. (A and B) In SR vesicles isolated from rabbit hind-limb skeletal muscle, RyR1 activity assessed by [3H]-ryanodine binding (A) and ROS production assessed by DHE fluorescence (B) were enhanced progressively as pO2 increased from 1% to 5–20% O2. Single and double asterisks indicate significant difference versus 20% O2 and 5% O2, respectively (P < 0.01; n = 3–5). (C) PEG-catalase largely eliminated the enhancement of RyR1 activity at high versus low pO2 (n = 3).
Potential sources of pO2-coupled H2O2 production within the SR include mitochondria, which have been implicated in pO2 sensing in multiple cell types (9–11), xanthine oxidase, which plays a role in ROS generation in cardiac muscle (12), and one or more forms of Nox (13, 14). However, in SR vesicles, enhancement of mitochondrial ROS production by antimycin A had no affect on RyR1 activity (Fig. S1), and inhibition of xanthine oxidase with allopurinol affected neither ROS production nor RyR1 activity (Fig. S2).
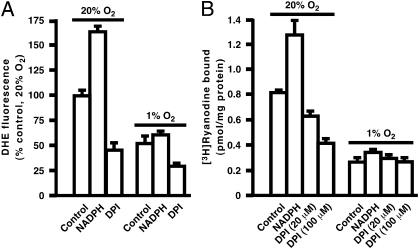
All forms of Nox catalyze electron transfer from NADPH to molecular oxygen to generate superoxide and thereby H2O2 or to generate H2O2 directly in the case of the dual oxidases (Duoxs) and perhaps Nox4 (13–15). In SR vesicles prepared from rabbit (Fig. 2 A and B) or mouse (Fig. S3 A and B) hind-limb skeletal muscle, addition of 1 mM NADPH resulted in substantial increases in both ROS production and RyR1 activity at high pO2. Enhancement of both ROS production and RyR1 activity at high versus low pO2 was largely eliminated by diphenyleneiodonium (DPI), a flavoprotein inhibitor well-characterized as an inhibitor of Nox (Fig. 2 A and B and Fig. S3 A and B), and by the recently described Nox inhibitor, 3-benzyl-7-(benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine (VAS2870) (Fig. S3 C and D) (16). Thus, the pO2-coupled activity within the SR that regulates RyR1 through ROS production exhibits properties of a Nox. Note that ROS production by a Nox in SR vesicles in the absence of added NADPH would require endogenous dinucleotide. We determined that NADPH+NADP is present at 126 ± 12.8 pmol/mg protein and 98.3 ± 10.8 pmol/mg protein in SR vesicles isolated from mouse and rabbit muscle, respectively (n = 3); levels in whole mouse muscle homogenates were 785 ± 83.2 pmol/mg protein (n = 3) (NADP+/NADPH Quantification Kit; Biovision Research Products).
Fig. 2.
Characterization of the ROS-generating activity of SR. In SR vesicles isolated from skeletal muscle of rabbit, (A) ROS production (DHE fluorescence) and (B) RyR1 activity ([3H]-ryanodine binding) were enhanced at high versus low pO2. At high pO2, addition of NADPH (1 mM) enhanced production of O2−, and this enhancement was eliminated by DPI. (n = 4–6).
We then measured free thiols in RyR1 isolated from solubilized SR vesicles (1) after incubation at low or high pO2. RyR1 at low pO2 possessed about 40 free Cys thiols as assessed by monobromobimane labeling, and a small set (5.4 thiols) was lost at high versus low pO2 (39.9 ± 2.8 free thiols at low pO2 versus 34.5 ± 2.4 free thiols at high pO2) (Table 1). DPI and PEG-catalase had no apparent effect on free thiol number at low pO2 (Table 1). However, thiol loss at high versus low pO2 was largely prevented by both DPI (39.6 ± 1.2 free thiols at low pO2 versus 38.7 ± 0.6 free thiols at high pO2 in the presence of DPI) and PEG-catalase (39.4 ± 2.9 free thiols at low pO2 versus 38.4 ± 2.9 free thiols at high pO2 in the presence of PEG-catalase) (Table 1). These results, in combination with those described above, establish a causal relationship between O2-coupled oxidation of RyR1 Cys thiols and RyR1 activation and indicate that H2O2 production intrinsic to the SR is the basis of this pO2-dependent regulation.
Table 1.
pO2- and H2O2-dependent loss of free thiols within RyR1
| 21% O2 | 1% O2 | |
| Control | 34.5 ± 2.4 | 39.9 ± 2.8 |
| PEG-catalase | 38.4 ± 2.0 | 39.4 ± 2.9 |
| DPI | 38.7 ± 0.6 | 39.6 ± 1.2 |
A small set of Cys thiols within RyR1 is oxidized at high versus low pO2 as determined by thiol labeling of SR vesicles (numbers represent free thiols per RyR1 monomer), and the loss of thiols is blocked by removing H2O2 with membrane-permeable PEG-catalase or by treatment with the Nox inhibitor DPI.
Nox2 is associated with transverse tubules of mammalian skeletal muscle (17). However, we saw no effect on ROS production or pO2-regulated RyR1 activity of the Nox2 inhibitor aminoethyl-benzenesulfono-fluoride (Fig. S4A), and pO2-coupled ROS production and RyR1 activation were unaltered in SR vesicles derived from knockout mice deficient in Nox2 (Fig. S4 B and C). Analysis of rat hind-limb skeletal muscle by quantitative real-time PCR (Fig. S5A) indicated that Nox2 and Nox4 and Duox1 and Duox2 were expressed at significant levels and that Nox4 was substantially the most abundantly expressed form [Nox5 apparently is not expressed in rodents (18)]. We detected Nox4 (see below) but not Duox1 or Duox2 in isolated SR vesicles by Western blotting. Nox4 activity does not require cytosolic subunits, and heterologously expressed Nox4 is constitutively active (19–21), consistent with regulation of ROS production by O2 (substrate) level.
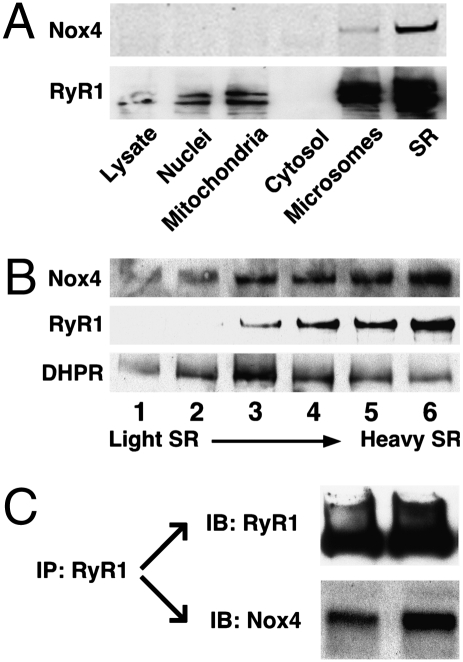
Analysis by Western blotting following subcellular fractionation of rat hind-limb muscle showed that Nox4 and RyR1 colocalize within the junctional SR (Fig. 3 A and B). Fluorescence immunohistochemistry of sections from rat hind-limb extensor muscle directly demonstrated colocalization of Nox4 and RyR1 (Fig. S5B), and RyR1 and Nox4 coimmunoprecipitated from solubilized SR vesicles (Fig. 3C).
Fig. 3.
Colocalization of Nox4 and RyR1 in skeletal muscle. (A) Subcellular fractionation of rat hind-limb muscle showed that Nox4 and RyR1 coenriched and were most abundant in the SR-enriched fraction isolated from the microsomal fraction by densit- gradient centrifugation. (B) Samples taken progressively from the top to the bottom of the SR-enriched gradient segment, which are progressively enriched in junctional SR (heavy SR) (45, 51), were increasingly enriched in both RyR1 and Nox4, whereas the dihydropyridine receptor/Ca2+ channel (DHPR), a constituent of transverse tubule membranes, exhibited a disparate pattern of enrichment. (C) Nox4 coimmunoprecipitates with RyR1 from solubilized SR vesicles; results of two separate experiments are shown.
We used knockdown with siRNA to assess the role of Nox4 using C2C12 cells, a skeletal muscle-derived cell line that provides a well-accepted model system. C2C12 cells were largely differentiated from myoblasts to multinucleated myotubes by 7 d in culture, and in differentiated C2C12 cells (9 d in culture) Nox4 was the most abundantly expressed form of Nox as assessed by PCR (Fig. S6A). Expression of Nox4 and RyR1 increased together as myotubes differentiated (Fig. S6B), and both Nox4 and RyR were most abundant in a microsomal (100,000 × g) fraction following subcellular fractionation of differentiated cells (Fig. S6C).
In the microsomal fraction derived from differentiated C2C12 cells, RyR activity was greater at high pO2 than at low pO2 (Fig. 4). Activity was enhanced at both low and high pO2 by NADPH, and enhancement by NADPH was eliminated by DPI (Fig. 4). Treatment of C2C12 cells with an siRNA specific for Nox4 (but not with scrambled, control siRNA) knocked down most Nox4 expression through at least 11 d in culture, without affecting RyR1 expression (Fig. S6B). Nox4 knockdown both eliminated the enhancement of RyR1 activity by NADPH at high pO2 and reduced basal activity to levels indistinguishable from those observed in the presence of DPI (Fig. 4). Knockdown of Nox4 also eliminated the enhancement of RyR1 activity by NADPH at low pO2 (Fig. 4). Similarly, ROS production by the microsomal fraction was greater at high pO2 than at low pO2, as assessed by DHE fluorescence, and DPI eliminated this difference (Fig. S6D. Nox4 knockdown also eliminated the difference in ROS production at high versus low pO2, and ROS production at high pO2 was indistinguishable from that seen in the presence of DPI (Fig. S6D). Finally, we verified with the fluorescent reporter 2′,7′-dichlorofluorescin (22) that the production of H2O2 by C2C12 microsomes was enhanced at high versus low pO2 and by addition of NADPH (Fig. S6E). Following Nox4 knockdown, basal production of H2O2 at high pO2 was suppressed, and the enhanced production at high versus low pO2 following addition of NADPH was eliminated (Fig. S6E). These results were replicated with a second, nonoverlapping Nox4 siRNA (Fig. S7).Taken together, these results demonstrate that, although there clearly is more than one source of ROS in C2C12 microsomes, Nox4 accounts for NADPH-dependent ROS production and is the necessary and sufficient source of pO2-coupled ROS production that regulates RyR1 activity.
Fig. 4.
ROS generated by Nox4 mediate pO2-dependent regulation of RyR1 in C2C12 cells. RyR activity in the microsomal fraction from differentiated C2C12 cells (assessed by [3H]-ryanodine binding) is enhanced at high versus low pO2 and by the addition of NADPH (1 mM), and enhancement is abrogated by DPI (20 μM) and by siRNA-mediated knockdown of Nox4. For samples at 20% O2, asterisks indicate significant difference versus control (*P < 0.05 re control at 20% O2; n = 4–6).
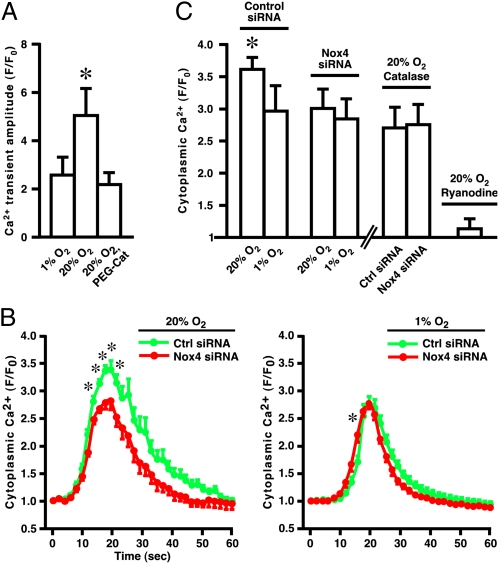
We next explored the role of Nox4 in regulating stimulus-induced Ca2+ flux. In primary skeletal muscle myocytes derived from mouse flexor digitorum brevis, the amplitudes of Ca2+ transients induced by electrical depolarization (23), known to reflect RyR1 activation, were greater at 20% O2 than at 1% O2, and this difference was largely eliminated by treatment with PEG-catalase (Fig. 5A and Fig. S8A). In differentiated C2C12 cells, depolarization with KCl (50 mM) resulted in rapid increases in cytoplasmic Ca2+ levels (Fig. 5 B and C and Fig. S8B) that were eliminated by preincubation with ryanodine (Fig. 5C) and which therefore could be ascribed to RyR activation. Depolarization-induced Ca2+ release through RyR was greater at high versus low pO2, and this difference was eliminated by Nox4 knockdown (Fig. 5 B and C). Ca2+ release at low pO2 was not affected by Nox4 knockdown (Fig. 5 B and C). In addition, we verified that the effects of pO2 on KCl-induced Ca2+ release through RyR1 in C2C12 cells were mediated by H2O2: the enhancement of Ca2+ release at high pO2 was eliminated by PEG-catalase (Fig. 5C). Thus, Nox4 mediates regulation by O2-derived H2O2 of stimulus-induced Ca2+ release through RyR1.
Fig. 5.
Nox4 regulates stimulus-induced Ca2+ release through RyR1. (A) In primary skeletal muscle myocytes, electrically induced Ca2+ transients are greater at high than at low pO2, and this difference is largely eliminated by PEG-catalase (n = 3–4; asterisk indicates P < 0.05). (B) In C2C12 cells, maximal cytoplasmic Ca2+ levels following depolarization by KCl (50 mM) are greater at high than at low pO2, and this difference is eliminated by Nox4 knockdown with Nox4-specific siRNA. Nox4 knockdown does not affect Ca2+ levels at low pO2 or the time to peak amplitude at high pO2. Each data point was obtained by integrating fluorescence emission over three to five entire myofibers within a single microscopic field of view during a single experiment (myofibers were depolarized only once); n = 6–9. Asterisks indicate significant differences (P < 0.05). (C) For each condition, maximal depolarization-induced Fluo 3 fluorescence was measured by integrating within 12 subfields distributed within three to five fibers, in each of six to nine experiments. As indicated by an asterisk, ANOVA indicated a significant difference (P ≤ 0.016) between the magnitude of Ca2+ release at high pO2 in control siRNA-treated samples and all other conditions; there were no significant differences (P ≥ 0.465) between any other pair of conditions (excluding the effects of ryanodine). Note that ryanodine eliminated K+-induced Ca2+ release (n = 3), which therefore may be ascribed to RyR activity, and that elimination of H2O2 with PEG-catalase also eliminated the enhancement of Ca2+ release at high pO2 (n = 5).
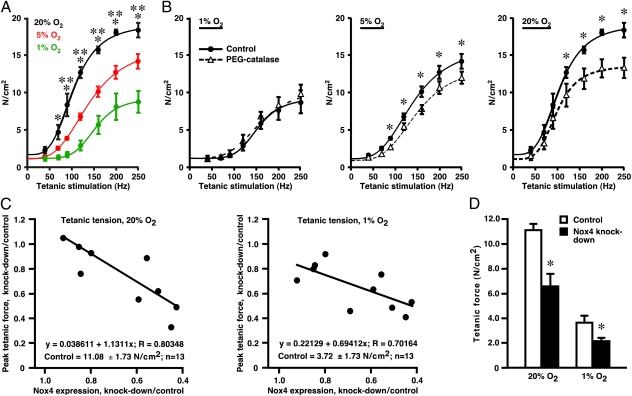
We then examined in bioassays the effects of pO2 on contractility of isolated, intact mouse extensor digitorum longus (EDL), a predominantly fast-twitch hind-limb muscle. We reported previously that incubation of EDL at 1% O2 and at 20% O2 results in muscle core pO2 of about 3.5 mm Hg and 37 mm Hg, respectively (2), levels that broadly recapitulate the pO2 gradient in skeletal muscle across the continuum of resting muscle to moderate exercise (4–7). The curve describing the relationship between tetanic stimulation frequency and evoked force (force-frequency curve) was progressively left-shifted at 5% O2 and 20% O2 versus 1% O2 (Fig. 6A). Enhanced contractility at higher pO2 was reduced by incubation with PEG-catalase (Fig. 6B). Thus, excitation–contraction coupling in skeletal muscle is pO2 sensitive, and H2O2 conveys at least a significant part of the pO2-coupled regulatory signal.
Fig. 6.
pO2-dependent regulation of muscle contractility by endogenous ROS. (A) Contractile force evoked by tetanic stimulation (40–250 Hz) of isolated, intact mouse EDL muscles at 1% O2, 5% O2, or 20% O2 and (B) the effects of PEG-catalase (500 U/mL). (A) Plots of force generation versus stimulus frequency reveal a leftward (facilitatory) shift as a function of increasing pO2. *P < 0.05, 1% and 20% O2; **P < 0.05, 1% versus 5% O2 (two-way ANOVA; n = 3–4). (B) Force generation is reduced by PEG-catalase at 20% and at 5% O2 but not at 1% O2. *P < 0.05, control values versus values in the presence of PEG-catalase (two-way ANOVA; n = 3–4). (C) Contractile force induced by maximal tetanic stimulation (250 Hz) of isolated, intact mouse EDL muscle decreases in proportion to the degree of shRNA-mediated Nox4 knockdown. (D) A histogram illustrates the decrease in tetanic force generation for muscles in which knockdown of Nox4 was ≥60% (*P < 0.05 re control; n = 5).
To verify a role for Nox4 in pO2-coupled regulation of contractility, we used local administration of an adeno-associated viral vector, AAV6, to express Nox4-directed shRNA in intact EDL muscle, followed after 4 wk by bioassay. As used (Materials and Methods), percutaneous hind-limb injection of vector resulted in variable degrees of Nox4 knockdown in EDL as assessed by Western blotting (Fig. S9), but the extent of Nox4 knockdown was correlated strongly with the observed decrease in tetanic force generation (Fig. 6C), and knockdown decreased force production to a greater extent at 20% O2 than at 1% O2 (Fig. 6C). For EDL muscles in which Nox4 knockdown was ≥60%, tetanic force production at 20% O2 was decreased by about 70% (Fig. 6D).
Discussion
Our results may provide insights into the molecular mechanisms of O2 sensing and O2-based signaling in mammalian cells and into the nature and functional significance of redox-based regulation of skeletal muscle function. In particular, although a potential role for Noxs in O2 sensing has long been considered (24, 25), and a role for Nox4 has received support from in vitro evidence derived from heterologous expression (26) and recently from analysis of hypoxic pulmonary vasoconstriction (27), no molecular mechanisms have been adduced. In addition, although oxidation of Cys thiols within RyR1 is associated with multiple disease states (28–30), a physiological role for Cys thiol modification by ROS has not been suggested heretofore, and in situ sources of ROS that might act upon RyR1 under physiological conditions have not been identified. Here we demonstrate a role for H2O2 that is derived from SR-localized Nox4 in O2-coupled regulation of RyR1 under physiologically relevant conditions.
We reported previously that RyR1 is activated by oxidation of a small set of Cys thiols at higher pO2, and that oxidation also prevents S-nitrosylation of a separate Cys thiol that activates RyR1 at low pO2 (1, 2). However, the molecular mechanism responsible for oxidation of these allosteric thiols and thus for pO2-dependent regulation of RyR1 activity remained unknown. We now show that this mechanism is provided by SR-resident Nox4. We also reported previously that RyR2, the principal form of RyR in the SR of cardiac striated muscle, is activated at high versus low pO2 in association with loss of a small set of Cys thiols (31). The present results suggest that H2O2 produced by Nox4 likely serves to regulate RyR2 in cardiac muscle as well. The relationship between RyR1 Cys residues modified in a Nox4- and pO2-dependent fashion and those identified as redox sensitive in previous studies (32, 33) remains to be determined. In situ, muscle activation is associated with a decline in pO2 (and therefore in Nox4-derived ROS) and also with enhanced NO production (34). The present results thus suggest that the generation of H2O2 by Nox4 provides a molecular basis for the coordinated actions of NO and O2 on RyR1 that subserve redox regulation of skeletal muscle contractility over the physiological range of pO2.
Specialized oxygen-sensing cells apparently share a common approach to transducing alterations in pO2: Regulation of K+-channel activity (hypoxia-coupled inhibition) results in modulation (enhancement) of Ca2+ influx through voltage-gated Ca2+ channels (35–37). Consistent with a potential role for Nox(s) (24, 25), the reported effects on K+ channel activity and hypoxic signaling of exogenous thiol oxidants (enhancement) and reductants (suppression) generally have been consistent with coupling between endogenous ROS production and channel activity (38). However, the evidence bearing on a role for Nox(s) in O2 sensing is contradictory (10, 11, 35, 37), and in general studies in knockout mice have not supported a role for Nox2 (39–41). Our present and previous (1) findings indicate that, in the case of RyR1, O2-based signaling is mediated by reversible channel oxidation/reduction coupled to H2O2 production by Nox4 that results in channel activation/deactivation. Thus, in at least some other cell types, hypoxia-coupled decreases in K+ channel activity may represent a disfacilitation of channel activation otherwise maintained by pO2- and Nox4-coupled channel oxidation.
Aberrant oxidation of Cys thiols within RyR1 and RyR2, which may be linked to dysregulated S-nitrosylation, contributes to Ca2+ leak through RyR1 in muscle pathophysiologies including extreme exercise-induced fatigue, central core disease, malignant hypothermia, and muscular dystrophy (28–30) and in the case of RyR2 may contribute to aberrant cardiac contractility (30, 42–44). All these disorders are characterized by tissue O2 deficits and/or aberrant O2 processing, as are a wide range of additional diseases, including sickle cell disease, sepsis, diabetes, and heart failure, in which myopathy is an established but poorly understood feature. It may be worthwhile to examine the role of Nox4 in muscle disorders and more generally in pathophysiological conditions characterized by disordered tissue oxygenation.
Materials and Methods
Subcellular Fractionation of Skeletal Muscle, Preparation of SR Vesicles, and Purification of RyR1.
SR vesicles were prepared essentially as described (1, 45). Briefly, hind-limb muscle from rabbit or mouse was homogenized in buffer containing 20 mM Hepes (pH 7.4), 2 mM EDTA, 0.2 mM EGTA, 0.3 M sucrose, and protease inhibitors (100 nM aprotinin, 20 μM leupeptin, 1 μM pepstatin, 0.2 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine). Homogenates then were subjected to differential centrifugation: 100 × g for 10 min (to remove unbroken cells and debris); 1,000 × g for 10 min (to pellet nuclei); 10,000 × g for 20 min (to pellet mitochondria); and 100,000 × g for 1 h (to generate a membrane-enriched microsomal pellet and cytosol-enriched supernatant).
To isolate SR vesicles, the pellet generated at 100,000 × g (the membrane fraction) was resuspended and fractionated on a continuous 20–45% sucrose gradient (without KCl) by centrifugation at 100,000 × g for 14 h. Heavy and light SR vesicle fractions were eluted separately and, after collection by centrifugation at 120,000 × g, were resuspended, aliquoted, and stored in liquid nitrogen. RyR1 was purified from SR vesicles solubilized with CHAPS by sucrose density gradient centrifugation as described (1, 46). Protein concentrations were determined with a bicinchoninic acid-based assay.
Assay of RyR1 Activity by 3H-Ryanodine Binding.
RyR1 activity was assayed essentially as described (1). Isolated SR vesicles or microsomal membranes were incubated overnight with 5 nM [3H]-ryanodine at room temperature in medium containing 20 mM imidazole/125 mM KCl (pH 7.0), 0.3 mM Pefabloc (Roche), 30 μM leupeptin, and 10 μM free Ca2+. The medium was bubbled continuously with a gas mixture containing a fixed concentration of O2 as specified, 5% CO2, remainder N2. Nonspecific binding was determined using a 1,000-fold excess of unlabeled ryanodine. After incubation, samples were diluted with 20 vol H2O at 4 °C and placed on Whatman GF/B filters soaked with 2% (wt/wt) polyethyleneimine. Filters were washed three times by vacuum with 5 mL buffer per wash (1 mM Pipes, 0.1 M KCl, pH 7.0), and the radioactivity remaining on the filters was quantified by liquid scintillation counting.
Quantification of Protein Sulfhydryls (Free Thiols).
The free thiol content of RyR1 was quantified by monobromobimane fluorescence (MBB; Molecular Probes) (47). As described (1), MBB labeling was carried out in SR vesicle preparations in the presence of 10 μM Ca2+.
Assay of ROS Production by DHE Conversion.
Isolated SR vesicles or C2C12 microsomal fractions were incubated with 10 μM DHE (Molecular Probes) (8) for 20 min at room temperature and controlled pO2 (glove box) in 200 μL buffer per sample [20 mM imidazole/125 mM KCl (pH 7.0), 10 μM Ca2+] in 96-well microplates (0.4 μg protein/μL), and plates were read with a fluorescence microplate reader (excitation 510 nm; emission 590 nm). When used, DPI, NADPH, VAS2870, or PEG-catalase was added 30 min before DHE.
Assay of H2O2 with 2′,7′-Dichlorofluorescein.
H2O2 production was measured essentially as described (22). Isolated C2C12 microsomes were incubated with 10 μM 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA; Molecular Probes) for 20 min at room temperature and controlled pO2 (glove box) in 200 μL buffer per sample [20 mM imidazole/125 mM KCl (pH 7.0), 10 μM Ca2+, and 100 mg/mL HRP, ± 1 mM NADPH) in 96-well microplates (0.3 μg protein/μL), and plates were read with a fluorescence microplate reader (excitation 485 nm; emission 520 nm).
Nox4 Expression.
RNA isolation, quantitative PCR, immunoprecipitation, Western blot analysis, and immunohistochemistry were carried out as described in SI Materials and Methods.
Analysis of Nox4/RyR1 in C2C12 Cells.
Culture of C2C12 cells, siRNA-mediated knockdown of Nox4, and assay of intracellular Ca2+ release with Fluo 3-AM were carried out as described in SI Materials and Methods.
Assay of Intracellular Ca2+ Release: Primary Myocytes.
Single fibers were isolated from the flexor digitorum brevis hind-limb muscle of mice essentially as described (48, 49). Muscle bundles were dissected and incubated in Tyrode's solution containing 2 mg/mL collagenase (type II; Worthington) for 3 h at 37 °C and then were transferred to DMEM supplemented with BSA as well as 50 U/mL penicillin and 50 mg/mL streptomycin. Individual myofibers were generated by trituration, collected by centrifugation at 1,000 × g for 3 min, and resuspended in DMEM. Myofibers then were incubated at 37 °C under 5% CO2, remainder room air, for 2–3 d.
To obtain measurements of Ca2+ transients, myofibers were loaded with Fluo 3-AM (5 μM) for 45 min with or without PEG-catalase (10 units/mL). Myofibers were suspended in Tyrode's solution and placed in a sealed chamber designed for field stimulation (RC-21BRFS; Warner Instruments) that was superfused with a continuous linear flow of Tyrode's solution externally sparged with room air or 1% O2/5% CO2 (remainder nitrogen). Myofibers were visualized using an inverted Axiovert microscope (Zeiss) and simulated at 1 Hz (1-ms duration; ∼40–50 V). Ca2+ signals (Fluo 3-AM fluorescence emission) were recorded with a CCD camera system (PentaMAX; Princeton Instruments). Ca2+ transient amplitude was measured as the difference between peak systolic and baseline diastolic levels and was normalized to baseline fluorescence (F/Fo).
Intact Muscle Bioassay.
Muscle bioassays were carried out essentially as described (2). EDL muscles were obtained from male mice at 8–10 wk of age. Each assay comprised both EDL muscles from two mice. Individual muscles were suspended from proximal and distal tendons, in series with force transducers, in organ baths containing Kreb's solution (pH 7.4) at 37 °C and were continuously gassed with 20% O2 and 5% CO2 (remainder NO2). After equilibration (see below), each muscle was adjusted to the length at which isometric twitch-force generation was maximal (optimal muscle length, Lo) and subjected to a single train of tetanic stimulation (160 Hz) to assess force generation qualitatively. Stimulation consisted of trains of pulses (train duration 75 ms; individual pulse duration, 2 ms; pulse frequency, 40–250 Hz; pulse amplitude, 60 V, which is about 75% of the amplitude that elicited maximal twitch force) delivered through platinum electrodes (7.0 mm wide) placed parallel to the long axis of the muscle. At least 2 min elapsed between trains of tetanic stimuli. For each muscle, Lo was measured after testing, the muscle was weighed wet, and the effective cross-sectional area was calculated by approximating the muscle as a cylinder of length Lo and a density of 1.06 g⋅cm−3 (2). Force production was normalized with respect to EDL cross-sectional area (N/cm2).
After the determination of Lo, muscles were allowed to equilibrate for ∼10 min at 20% O2, after which pO2 was maintained at 20% O2 or was switched to 1% O2 or 5% O2. Force production was assessed 10 min or 90 min later. When used, PEG-catalase was added to the bath after the initial 10-min equilibration, and muscles were incubated for 90 min at 20% O2, followed by 10-min exposure at 1% O2, 5% O2, or 20% O2 before testing.
Nox4 Knockdown in Intact Muscle.
Construction and administration of the Nox4 shRNA-AAV vector were as described in ref. 50 and in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. D. Lambeth for supplying antibody to Nox4 and Dr. H. Schmidt for providing VAS2870. This work was supported by National Institutes of Health RO1 Grants HL0591130 and AR018687.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109546108/-/DCSupplemental.
References
- 1.Eu JP, Sun J, Xu L, Stamler JS, Meissner G. The skeletal muscle calcium release channel: Coupled O2 sensor and NO signaling functions. Cell. 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 2.Eu JP, et al. Concerted regulation of skeletal muscle contractility by oxygen tension and endogenous nitric oxide. Proc Natl Acad Sci USA. 2003;100:15229–15234. doi: 10.1073/pnas.2433468100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J, Xin C, Eu JP, Stamler JS, Meissner G. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc Natl Acad Sci USA. 2001;98:11158–11162. doi: 10.1073/pnas.201289098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorczynski RJ, Duling BR. Role of oxygen in arteriolar functional vasodilation in hamster striated muscle. Am J Physiol. 1978;235:H505–H515. doi: 10.1152/ajpheart.1978.235.5.H505. [DOI] [PubMed] [Google Scholar]
- 5.Molé PA, et al. Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am J Physiol. 1999;277:R173–R180. doi: 10.1152/ajpregu.1999.277.1.R173. [DOI] [PubMed] [Google Scholar]
- 6.Richardson RS, Newcomer SC, Noyszewski EA. Skeletal muscle intracellular PO(2) assessed by myoglobin desaturation: Response to graded exercise. J Appl Physiol. 2001;91:2679–2685. doi: 10.1152/jappl.2001.91.6.2679. [DOI] [PubMed] [Google Scholar]
- 7.Richardson RS, et al. Human skeletal muscle intracellular oxygenation: The impact of ambient oxygen availability. J Physiol. 2006;571:415–424. doi: 10.1113/jphysiol.2005.102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarpey MM, Fridovich I. Methods of detection of vascular reactive species: Nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ Res. 2001;89:224–236. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]
- 9.Weir EK, López-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sommer N, et al. Regulation of hypoxic pulmonary vasoconstriction: Basic mechanisms. Eur Respir J. 2008;32:1639–1651. doi: 10.1183/09031936.00013908. [DOI] [PubMed] [Google Scholar]
- 11.Weissmann N, et al. Oxygen sensors in hypoxic pulmonary vasoconstriction. Cardiovasc Res. 2006;71:620–629. doi: 10.1016/j.cardiores.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Khan SA, et al. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci USA. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 14.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takac I, et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stielow C, et al. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem Biophys Res Commun. 2006;344:200–205. doi: 10.1016/j.bbrc.2006.03.114. [DOI] [PubMed] [Google Scholar]
- 17.Hidalgo C, Sánchez G, Barrientos G, Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S -glutathionylation. J Biol Chem. 2006;281:26473–26482. doi: 10.1074/jbc.M600451200. [DOI] [PubMed] [Google Scholar]
- 18.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 19.Ago T, et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 20.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Serrander L, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black MJ, Brandt RB. Spectrofluorometric analysis of hydrogen peroxide. Anal Biochem. 1974;58:246–254. doi: 10.1016/0003-2697(74)90464-3. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Carroll SL, Klein MG, Schneider MF. Calcium transients and calcium homeostasis in adult mouse fast-twitch skeletal muscle fibers in culture. Am J Physiol. 1997;272:C1919–C1927. doi: 10.1152/ajpcell.1997.272.6.C1919. [DOI] [PubMed] [Google Scholar]
- 24.Acker H. PO2 chemoreception in arterial chemoreceptors. Annu Rev Physiol. 1989;51:835–844. doi: 10.1146/annurev.ph.51.030189.004155. [DOI] [PubMed] [Google Scholar]
- 25.Acker H, Dufau E, Huber J, Sylvester D. Indications to an NADPH oxidase as a possible pO2 sensor in the rat carotid body. FEBS Lett. 1989;256:75–78. doi: 10.1016/0014-5793(89)81721-1. [DOI] [PubMed] [Google Scholar]
- 26.Lee YM, et al. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal. 2006;18:499–507. doi: 10.1016/j.cellsig.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad M, Kelly MR, Zhao X, Kandhi S, Wolin MS. Roles for Nox4 in the contractile response of bovine pulmonary arteries to hypoxia. Am J Physiol Heart Circ Physiol. 2010;298:H1879–H1888. doi: 10.1152/ajpheart.01228.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durham WJ, et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellinger AM, et al. Remodeling of ryanodine receptor complex causes “leaky” channels: A molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellinger AM, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J, et al. Regulation of the cardiac muscle ryanodine receptor by O(2) tension and S-nitrosoglutathione. Biochemistry. 2008;47:13985–13990. doi: 10.1021/bi8012627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voss AA, Lango J, Ernst-Russell M, Morin D, Pessah IN. Identification of hyperreactive cysteines within ryanodine receptor type 1 by mass spectrometry. J Biol Chem. 2004;279:34514–34520. doi: 10.1074/jbc.M404290200. [DOI] [PubMed] [Google Scholar]
- 33.Aracena-Parks P, et al. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem. 2006;281:40354–40368. doi: 10.1074/jbc.M600876200. [DOI] [PubMed] [Google Scholar]
- 34.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 35.Dinger B, et al. The role of NADPH oxidase in carotid body arterial chemoreceptors. Respir Physiol Neurobiol. 2007;157:45–54. doi: 10.1016/j.resp.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2007;157:55–64. doi: 10.1016/j.resp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Kemp PJ. Detecting acute changes in oxygen: Will the real sensor please stand up? Exp Physiol. 2006;91:829–834. doi: 10.1113/expphysiol.2006.034587. [DOI] [PubMed] [Google Scholar]
- 38.Weir EK, Archer SL. Counterpoint: Hypoxic pulmonary vasoconstriction is not mediated by increased production of reactive oxygen species. J Appl Physiol. 2006;101:995–998, discussion 998. doi: 10.1152/japplphysiol.00480a.2006. [DOI] [PubMed] [Google Scholar]
- 39.Roy A, et al. Mice lacking in gp91 phox subunit of NAD(P)H oxidase showed glomus cell [Ca(2+)](i) and respiratory responses to hypoxia. Brain Res. 2000;872:188–193. doi: 10.1016/s0006-8993(00)02458-6. [DOI] [PubMed] [Google Scholar]
- 40.He L, et al. Characteristics of carotid body chemosensitivity in NADPH oxidase-deficient mice. Am J Physiol Cell Physiol. 2002;282:C27–C33. doi: 10.1152/ajpcell.2002.282.1.C27. [DOI] [PubMed] [Google Scholar]
- 41.Archer SL, et al. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci USA. 1999;96:7944–7949. doi: 10.1073/pnas.96.14.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci USA. 2007;104:20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fauconnier J, et al. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem. 2010;285:28938–28945. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson K, Cohn AH, Meissner G. High-affinity [3H]PN200-110 and. [3H]ryanodine binding to rabbit and frog skeletal muscle. Am J Physiol. 1994;266:C462–C466. doi: 10.1152/ajpcell.1994.266.2.C462. [DOI] [PubMed] [Google Scholar]
- 46.Meissner G, Henderson JS. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987;262:3065–3073. [PubMed] [Google Scholar]
- 47.Kosower NS, Kosower EM. Thiol labeling with bromobimanes. Methods Enzymol. 1987;143:76–84. doi: 10.1016/0076-6879(87)43015-2. [DOI] [PubMed] [Google Scholar]
- 48.Shefer G, Yablonka-Reuveni Z. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol Biol. 2005;290:281–304. doi: 10.1385/1-59259-838-2:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wozniak AC, et al. C-Met expression and mechanical activation of satellite cells on cultured muscle fibers. J Histochem Cytochem. 2003;51:1437–1445. doi: 10.1177/002215540305101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- 51.Lai FA, Erickson HP, Rousseau E, Liu QY, Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988;331:315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.