Abstract
The bacterial strain TD1 was isolated from Tao Dam hot spring in Thailand. Strain TD1 was Gram positive, rod-shaped, aerobic, motile, and endospore forming. The cell was 2.0–40 μm in length and about 0.4 μm in diameter. The optimum growth occurred at 55–60 °C and at pH 7–8. Strain TD1 was able to grow on medium containing up to 10% NaCl. The DNA G+C content was 38.9 mol%. The cellular fatty acid content was mainly C16:0, which comprised 25.04% of the total amount of cellular fatty acid. 16S rDNA showed 99% identity to Aeribacillus pallidus DSM 3670T. Bayesian tree analysis strongly supported the idea that strain TD1 is affiliated with genus Aeribacillus, as Aeribacillus pallidus strain TD1. Although the 16S rDNA of A. pallidus strain TD1 is similar to that of A. pallidus DSM 3670T, some physiological properties and the cellular fatty acid profiles differ significantly. A. pallidus strain TD1 can produce extracellular pectate lyase, which has not been reported elsewhere for other bacterial strains in the genus Aeribacillus. A. pallidus strain TD1 may be a good candidate as a pectate lyase producer, which may have useful industrial applications.
Keywords: thermophile, halotolerant, Aeribacillus, pectate lyase
1. Introduction
Thermophiles, especially thermophilic enzymes, have attracted much interest as both analytical tools and biocatalysts for applications at a large scale [1]. The application of robust enzymes and microorganisms for the sustainable production of chemicals, biopolymers, materials, and fuels from renewable resources, defined as industrial biotechnology, offers many opportunities for various industries. The most important involve applications to the reduction of waste, energy input, and raw materials, and the development of highly efficient and environmentally friendly processes [2]. Pectate lyase (EC 4.2.2.2), or pectate transeliminases, catalyzes the eliminative cleavage of de-esterified pectin, a major component of the primary cell walls of many higher plants [3]. Potential uses of pectinase include extraction and clarification of fruit juices and wine, maceration of vegetables, scouring of cotton fabric, retting of flax, degumming of plant fibers, fermentation of coffee and tea, as poultry feed, in oil extraction, and pretreating wastewater containing pectinacious material [4]. Pectinases have been isolated from various microbial sources such as bacteria [5–8], yeast [9] and fungi [10,11]. Recently, thermostable pectate lyases were isolated and characterized from many mesophilic and thermophilc bacteria such as Bacillus sp. RN1 [12–14]. The strain was isolated from a hot spring in Thailand and produced pectate lyase (Pel SWU), which can be applied in many industrial fields [13].
Hot springs are a rich source of thermophiles. More than 100 hot springs are distributed throughout Thailand, and the surface temperatures are in the range 40–100 °C at a pH range of 6.4–9.5. Tao Dam hot spring was discovered in 2008. The spring is located in the central part of Thailand. Water in this spring contains a high concentration of sulfide. Surprisingly, this sulfide-rich spring has a pH greater than seven, which is rare in sulfide-rich hot springs in Thailand.
Given the important role of thermophiles in biotechnology, the present study attempted to characterize thermophilic bacteria from Tao Dam hot spring, which contain pectate lyase activity. The study used bacterial culture, microscopic observations, and phenotypic, chemotaxonomic, phylogenic, and molecular systematic analysis to characterize these bacteria.
2. Results and Discussion
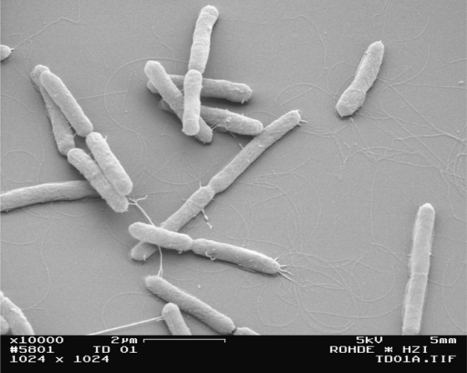
Fourteen bacterial strains were isolated from the spring sediment. Only strain TD1 was able to produce clear halo zones on agar medium containing 1% AZ rhamnogalacturonan. A photometric assay indicated that extracellular pectate lyase was produced by TD1. The morphology of strain TD1 was rod-shaped and contained lophotrichous flagella (Figure 1).
Figure 1.
Scanning electron micrograph of strain TD1. Bar, 2 μm.
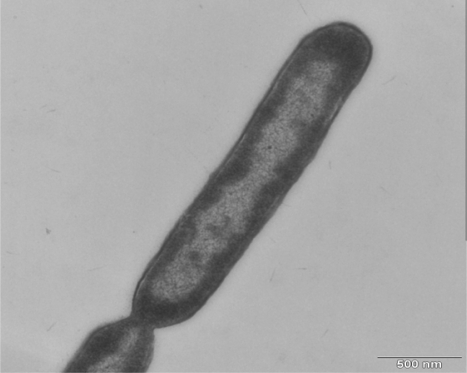
Gram staining and type were positive. The cell was motile. Each cell was 2.0–40 μm in length and about 0.4 μm in diameter (Figure 2). The endospore was ellipsoidal and was located in the subterminal swollen sporangium. The temperature range for growth was 30–67 °C, and the optimum growth conditions were 55–60 °C and pH 7–8. Strain TD1 was able to grow in medium containing 0–10% NaCl. The physiological characteristics of the strain TD1 are shown in Table 1.
Figure 2.
Electron micrograph of ultrathin-sectioned of strain TD1.
Table 1.
Physiological characteristics of A. pallidus strain TD1 and A. pallidus DSM 3670T [15].
| Characteristic | A. pallidus TD1 | A. pallidus DSM 3670T |
|---|---|---|
| Cell width (μm) | 0.4 | 0.8–0.9 |
| Cell length (μm) | 2–>40 | 2–5 |
| Motility | + | + |
| Temperature range (°C) | 45–67 | 30–70 |
| DNA G+C content (mol%) | 38.9 | 39–41 |
| Producing of pectate lyase | + | − |
| Acid produced from: | ||
| Cellobiose | + | − |
| Maltose | + | d |
| Mannose | + | − |
| Sucrose | + | ND |
| Trehalose | + | d |
| Xylose | + | − |
| Arabinose | − | − |
| Ribose | + | − |
| Citrated used | + | − |
| Hydrolysis of: | ||
| Casein | − | − |
| Gelatin | − | − |
| Strach | − | +w |
| Alkane utilization | − | ND |
+, positive;
−, negative;
+w, weakly positive;
d, variable between strains;
ND = not determined.
The utilization of carbohydrates differed markedly between strain TD1 and the A. pallidus type strain (Table 1). Only strain TD1 was able to produce acid from arabinose, cellobiose, mannose, ribose, and xylose. Strain TD1 could utilize citrate and propionate. Hydrolysis of starch was not detected in strain TD1.
The cellular fatty acid profile of strain TD1 is shown in Table 2. The cellular fatty acid was mainly C16:0, which comprised 25.04% of the total amount of cellular fatty acid. The cellular fatty acid profiles differed significantly between strain TD1 and A. pallidus DSM 3670T. A. pallidus DSM 3670T contained more than twice the amount of C16:0 compared with strain TD1 (Table 2).
Table 2.
Cellular fatty acid profiles of A. pallidus TD1 and A. pallidus DSM 3670T [15].
| Fatty Acid | A. pallidus TD1 (%) | A. pallidus DSM 3670T (%) |
|---|---|---|
| 14:0 ISO | 0.36 | 1.6 |
| 14:0 ANTEISO | – | 1.6 |
| 14:0 | 1.87 | 8.5 |
| 15:0 ISO | 16.30 | 6.2 |
| 15:0 ANTEISO | 4.50 | 4.9 |
| 15:0 | 0.33 | 1.2 |
| 16:1 w7c alcohol | 0.14 | – |
| 16:0 ISO | 11.15 | 9.3 |
| 16:1 w11c | 0.65 | – |
| 16:0 | 25.04 | 50.0 |
| ISO 17:1 w10c | 0.30 | – |
| 17:0 ISO | 17.95 | 4.0 |
| 17:0 ANTEISO | 19.74 | 6.5 |
| 17:0 | 0.19 | – |
| 18:0 ISO | 0.29 | – |
| 18:0 | 1.03 | 2.1 |
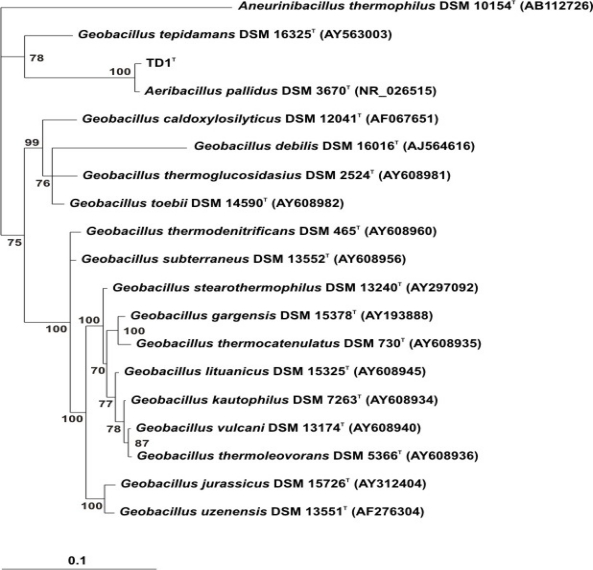
Strain TD1 contained linear and branched fatty acids, and some unsaturated fatty acids; branched saturated fatty acids were dominant. By contrast, no unsaturated fatty acids were detected in strain A. pallidus DSM 3670 and linear fatty acids were dominant. The G+C content was 38.9 mol%. The 16S rDNA of strain TD1 (GQ355275) was similar to that of A. pallidus DSM 3670T (99% identity). The Bayesian tree is shown in Figure 3. Strain TD1 clustered with A. pallidus DSM 3670T (100% clade credibility).
Figure 3.
The Bayesian tree was constructed using the Bayesian inference algorithm with the GTR+I+G model of nucleotide substitution. The values associated with nodes correspond to the clade credibility support in %.
The Bayesian tree strongly supports the idea that strain TD1 is affiliated with genus Aeribacillus as Aeribacillus pallidus strain TD1. At the time of writing, A. pallidus DSM 3670T is the type and sole species of genus Aeribacillus [16]. This strain was first described in 1987 by Scholz and colleagues as “Bacillus pallidus” [15] and was renamed in 2004 by Banat and colleagues as “Geobacillus pallidus” [17]. Based on 16S rDNA sequence divergence and the presence of unique phenotypic characteristics, the strain was then transferred to the new genus Aeribacillus as A. pallidus in 2010 by Minana-Galbis and colleagues [18].
3. Experimental Section
3.1. Isolation of Bacterial Strains
Bacterial strains were isolated from Tao Dam hot spring, Khluang Wangchao National Park, Thailand, which is located in a remote region of Kampangpetch province. The spring was discovered recently and contains mineral (sulfide-rich) water with a surface temperature range of 50–75 °C and pH 8.0. The sediment was 60 °C at the time of sampling. The bacteria were isolated using tenfold serial dilutions of sediment in phosphate buffer (0.1 M K2HPO4 and 0.1 M KH2PO4, pH 8). The dilutions were spread on R2A agar (Difco, NJ, USA), and the plates were incubated overnight at 55 °C.
3.2. Pectate Lyase Assay
Bacterial isolates and the type strain of A. pallidus DSM 3670 were grown on R2A agar which contained one percent of AZ rhamnogalacturonan (Megazyme, Ireland). The plates were incubated overnight at 55 °C. A clear halo zone should appear when pectate lyase is produced. Positive clones were inoculated in 100 mL of M9 broth (pH 8) plus 1 g sodium pectate (Sigma, Germany). The flask was incubated at 55 °C with shaking at 150 rpm overnight. Bacterial cells were removed by centrifugation at 8,000 rpm for 10 min, and the supernatant was collected for the photometric assay, in which 0.5 mL of supernatant was added to 2.5 mL of substrate (60 mM Tris-HCl, pH 8.0, 0.6 mM CaCl2, and 0.24% (w/v) sodium pectate). The reaction was incubated at 55 °C for 2 h. Pectate lyase activity was measured on a Genesys 10 uv spectrophotometer (Thermo Fisher Scientific, Madison, WI, USA) at 232 nm as described by Collmer and colleagues [19]. Pectate lyase from Aspergillus sp. (Megazyme) was used as the positive control, and sterile water and substrate were used as the negative control.
3.3. Morphology
The morphology was examined under a Zeiss Gemini DSM852 field emission scanning electron microscope (Zeiss, Oberkochen, Germany) and Zeiss transmission electron microscope TEM910 (ProScan, 1024 × 1024, Scheuring, Germany). To prepare the samples for field emission scanning electron microscopy, the samples were fixed with a solution containing 2% glutaraldehyde and 5% formaldehyde in cacodylate buffer (0.1 M cacodylate, 0.09 M sucrose, 0.01 M MgCl2, and 0.01 M CaCl2, pH 6.9) for 1 h on ice and then washed with TE buffer (20 mM Tris and 2 mM EDTA, pH 6.9). The fixed bacterial cells were placed onto poly-l-lysine-coated cover slips, left for 5 min, washed once in TE buffer, and fixed with 2% glutaraldehyde. After washing with TE buffer, the samples were dehydrated with a graded series of acetone (10%, 30%, 50%, 70%, 90%, 100%) for 10 min at each step on ice, except that the final 100% acetone step was performed at room temperature. Samples were critical-point dried with liquid CO2 (CPD 030, Bal-Tec, Liechtenstein), mounted onto aluminum stubs, sputter coated with gold (SCD 500, Bal-Tec), and examined in the field emission scanning electron microscope at an acceleration voltage of 5 kV using an Everhart–Thornley SE-detector and in-lens SE-detector at a 50:50 ratio. Images were stored onto a 230 MB MO-disk.
To embed the bacteria for transmission electron microscopy, the samples were fixed in a fixation solution containing 5% formaldehyde and 2% glutaraldehyde in cacodylate buffer (0.1 M cacodylate, 0.01 M CaCl2, 0.01 M MgCl2, 0.09 M sucrose, pH 6.9), washed with cacodylate buffer, fixed with 1% aqueous osmium for 1 h at room temperature, and washed. The pellets were embedded in 2% water agar and cut into small cubes. The samples were dehydrated in a graded series of acetone (10%, 20%, 50%) for 30 min on ice followed by contrast staining with 2% uranyl acetate in 70% acetone overnight at 4 °C, and the samples were dehydrated further with 90% and 100% acetone. At the 100% acetone step, the samples were allowed to reach room temperature and were then infiltrated with the epoxy resin according to Spurr’s formula for a medium resin [20]: 1 part 100% acetone/1 part resin overnight; 1 part 100% acetone/2 parts resin for 8 h; and pure resin overnight and several changes in the following 2 d. The samples were then transferred to resin-filled gelatin capsules and polymerized for 8 h at 75 °C. Ultrathin sections were cut with a diamond knife, picked up onto Formvar-coated copper grids (300 mesh), and counterstained with 2% aqueous uranyl acetate and lead citrate. After air-drying, the samples were examined in a Zeiss TEM910 transmission electron microscope at an acceleration voltage of 80 kV. The images were recorded digitally with a Slow-Scan CCD-Camera (ProScan, 1024 × 1024, Scheuring, Germany) with ITEM-Software (Olympus Soft Imaging Solutions, Germany).
3.4. Phenotypic Characterization
The temperatures for growth of the strain TD1 were examined from 30–70 °C on R2A medium (Difco, NJ, USA). The salt tolerance was also assessed on R2A medium with 0–10% (w/v) NaCl at 55 °C. The phenotype of the strain TD1 was investigated using API 20E and API 50 CHB tests (bioMérieux, Nürtingen, Germany) at 55 °C, according to the manufacturer’s instructions.
3.5. Chemotaxonomy
Analysis of cellular fatty acids was performed at the German Collection of Microorganisms and Cell Cultures (DSMZ) (Braunschweig, Germany) according to the Sherlock Microbial Identification System manual (version 4.5; Microbial ID, Newark, DE). The profiles of cellular fatty acids were compared using the TSBA40 library database version 4.10 (Microbial ID, Newark, DE, USA).
3.6. Molecular Systematics
DNA was extracted using MasterPure™ Gram Positive DNA Purification Kit (Biozym Scientific, Germany) according to the manufacturer’s instructions. The genomic G+C ratio was examined using HPLC at DSMZ. Primers 27f (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492r (5′-TACGGYTACCTTGTTACGACTT-3′) [21] were used for bacterial 16S rDNA amplification. The 50 μL total volume of PCR reaction contained 10 ng of DNA, 20 pmol of each primer, 80 μM dNTP, 1 × Taq reaction buffer, and 1.25 U Taq DNA polymerase (EURx, Poland). The PCR was started by heating the reactions to 94 °C for 3 min, followed by 30 cycles of 30 s at 94 °C, 30 sec at 52 °C, and 1 min at 72 °C, and ending with a final step at 72 °C for 3 min. The PCR product was purified using a MinElute PCR Purification Kit (QIAGEN, Germany). DNA sequencing was performed directly on the purified PCR product. The sequencing primers were 27f and 1492r. Big Dye v. 1.1 reagents (Applied Biosystems, Inc.) were used for the sequencing, and the analysis was performed on an ABI Genetic Analyzer sequencer (AB3130XL). The sequence was compared with the nonredundant database of sequences deposited at GenBank using BLAST [22]. 16S rDNA of the strain TD1 was aligned with other bacterial strains in the genera Geobacillus and Aeribacillus using MUSCLE 3.7 [23]. The size of the 16S rDNA for the alignment was about 1,500 bp. The evolutionary model was calculated using Modeltest 3.8 [24]. Bayesian inference was performed using MrBayes 3.1.2 [25]. The Bayesian posterior probabilities were obtained by performing two separate runs with four Markov chains. Each run was conducted with 2 × 106 generations and sampled every 100 generations. Convergence was checked by examining the generation plot visualized with TRACER 1.4 [26]. Potential scale reduction factor was computed by MrBayes 3.1.2. A consensus tree was calculated after discarding the first 25% of the iterations as burn-in.
4. Conclusions
A. pallidus strain TD1 is a thermophilic and halotolerant bacterium. The physiological properties of strain TD1 are promising for biotechnology research. A. pallidus strain TD1 can produce extracellular pectate lyase, which has not been reported for other bacterial strains in the genus Aeribacillus. A. pallidus strain TD1 may be a good candidate as a pectate lyase producer and may have useful industrial applications.
Acknowledgments
This research was supported by the Royal Golden Jubilee Ph.D. Program No. PHD/0019/2548. We thank Manfred Rohde for the electron microscorpic experiments.
References
- 1.Turner P, Mamo G, Karlsson EN. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Fact. 2007;6:9. doi: 10.1186/1475-2859-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niehaus F, Bertoldo C, Kahler M, Antranikian G. Extremophiles as a source of novel enzymes for industrial application. Appl. Microbiol. Biotechnol. 1999;51:711–729. doi: 10.1007/s002530051456. [DOI] [PubMed] [Google Scholar]
- 3.Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoondal GS, Tiwari RP, Tewari R, Dahiya N, Beg QK. Microbial alkaline pectinases and their industrial applications: A review. Appl. Microbiol. Biotechnol. 2002;59:409–418. doi: 10.1007/s00253-002-1061-1. [DOI] [PubMed] [Google Scholar]
- 5.Bruhlmann F. Purification and characterization of an extracellular pectate lyase from an Amycolata sp. Appl. Environ. Microbiol. 1995;61:3580–3585. doi: 10.1128/aem.61.10.3580-3585.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi T, Hatada Y, Higaki N, Lusterio DD, Ozawa T, Koike K, Kawai S, Ito S. Enzymatic properties and deduced amino acid sequence of a high-alkaline pectate lyase from an alkaliphilic Bacillus isolate. Biochim. Biophys. Acta. 1999;1427:145–154. doi: 10.1016/s0304-4165(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T, Higaki N, Suzumatsu A, Sawada K, Hagihara H, Kawai S, Ito S. Purification and properties of a high-molecular-weight, alkaline exopolygalacturonase from a strain of Bacillus. Enzyme Microb. Technol. 2001;29:70–75. doi: 10.1016/s0141-0229(01)00355-6. [DOI] [PubMed] [Google Scholar]
- 8.Sawada K, Suzumatsu A, Kobayashi T, Ito S. Molecular cloning and sequencing of the gene encoding an exopolygalacturonase of a Bacillus isolate and properties of its recombinant enzyme. Biochim. Biophys. Acta. 2001;1568:162–170. doi: 10.1016/s0304-4165(01)00213-6. [DOI] [PubMed] [Google Scholar]
- 9.Blanco P, Sieiro C, Villa TG. Production of pectic enzymes in yeasts. FEMS Microbiol. Lett. 1999;175:1–9. doi: 10.1111/j.1574-6968.1999.tb13595.x. [DOI] [PubMed] [Google Scholar]
- 10.Guo W, Gonzalez-Candelas L, Kolattukudy PE. Cloning of a novel constitutively expressed pectate lyase gene pelB from Fusarium solani f. sp. pisi (Nectria haematococca, mating type VI) and characterization of the gene product expressed in Pichia pastoris. J. Bacteriol. 1995;177:7070–7077. doi: 10.1128/jb.177.24.7070-7077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huertas-Gonzalez MD, Ruiz-Roldan MC, Garcia Maceira FI, Roncero MI, Di Pietro A. Cloning and characterization of pl1 encoding an in planta-secreted pectate lyase of Fusarium oxysporum. Curr. Genet. 1999;35:36–40. doi: 10.1007/s002940050430. [DOI] [PubMed] [Google Scholar]
- 12.Ouattara HG, Reverchon S, Niamke SL, Nasser W. Biochemical properties of pectate lyases produced by three different Bacillus strains isolated from fermenting cocoa beans and characterization of their cloned genes. Appl. Environ. Microbiol. 2010;76:5214–5220. doi: 10.1128/AEM.00705-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sukhumsiirchart W, Kawanishi S, Deesukon W, Chansiri K, Kawasaki H, Sakamoto T. Purification, characterization, and overexpression of thermophilic pectate lyase of Bacillus sp. RN1 isolated from a hot spring in Thailand. Biosci. Biotechnol. Biochem. 2009;73:268–2673. doi: 10.1271/bbb.80287. [DOI] [PubMed] [Google Scholar]
- 14.Tonouchi A, Hara Y, Umehara R, Sanuki T, Fukusawa T, Miyairi K. Cloning of the gene encoding an endo-acting pectate lyase from Streptomyces thermocarboxydus. Biosci. Biotechnol. Biochem. 2010;74:433–436. doi: 10.1271/bbb.90693. [DOI] [PubMed] [Google Scholar]
- 15.Scholz T, Demharter W, Hensel R, Kandler O. Bacillus pallidus sp. nov., a new thermophilic species from sewage. Syst. Appl. Microbiol. 1987;9:91–96. [Google Scholar]
- 16.Euzeby JP. List of Bacterial Names with Standing in Nomenclature: A folder available on the Internet. Int. J. Syst. Bacteriol. 1997;47:590–592. doi: 10.1099/00207713-47-2-590. [DOI] [PubMed] [Google Scholar]
- 17.Banat IM, Marchant R, Rahman TJ. Geobacillus debilis sp. nov., a novel obligately thermophilic bacterium isolated from a cool soil environment, and reassignment of Bacillus pallidus to Geobacillus pallidus comb. nov. Int. J. Syst. Evol. Microbiol. 2004;54:2197–2201. doi: 10.1099/ijs.0.63231-0. [DOI] [PubMed] [Google Scholar]
- 18.Minana-Galbis D, Pinzon DL, Loren JG, Manresa A, Oliart-Ros RM. Reclassification of Geobacillus pallidus (Scholz et al. 1988) Banat et al. 2004 as Aeribacillus pallidus gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2010;60:1600–1604. doi: 10.1099/ijs.0.003699-0. [DOI] [PubMed] [Google Scholar]
- 19.Collmer A, Ried JL, Mount MS. Assay methods for pectic enzymes. Methods Enzymol. 1988;161:329–335. [Google Scholar]
- 20.Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 21.Lane D. 16S/23S rRNA Sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematics. John Wiley & Sons; New York, NY, USA: 1991. pp. 115–175. [Google Scholar]
- 22.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 23.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Posada D, Crandall KA. MODELTEST: Testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 25.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 26.Rambaut A, Drummond A. Tracer v1.4. 2007. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 9 August 2011).