Abstract
Background
We recently reported that the progestagen-associated endometrial protein (PAEP) gene is overexpressed and promotes tumor proliferation and metastasis in human melanoma.
Methods
To identify the molecules that regulate its expression and oncogenic properties, we analyzed the gene microarray profiling of melanoma samples of serial clinical stage.
Results
We found that the expression profile of the PAEP gene parallels that of microphthalmia-associated transcription factor (MITF, r = 0.86), a master regulator of melanocyte development and melanoma progression. This parallelism was further confirmed with semiquantitative reverse transcriptase polymerase chain reaction analysis of melanoma-derived daughter cells. Transfection of melanoma cells with MITF small interfering RNA (siRNA) specifically diminishes PAEP gene expression, whereas PAEP siRNA transfection has no effect on MITF. Furthermore, knockdown of either the MITF or PAEP gene reveals a significant inhibition of tumor cell migration.
Conclusions
Our data indicate that PAEP expression is regulated in part by MITF and may thus play a role in MITF-mediated cell migration in human melanoma.
Keywords: Cell migration, gene regulation, melanoma, microphthalmia-associated transcription factor, progestagen-associated endometrial protein
INTRODUCTION
Melanoma is one of the most aggressive forms of human cancer, with high mortality. Although early diagnosis and appropriate surgical treatment are associated with a high cure rate, its capacity for metastasizing and its overall resistance to current medical treatment modalities have resulted in a poor overall long-term survival for those with advanced disease; only 14% of patients with metastatic melanoma (MM) survive longer than 5 years.1 Moreover, the prognosis for patients with advanced melanoma has shown no improvement over the past 50 years. A better understanding of the molecular mechanisms involved in malignant transformation and metastasis is of great importance for providing effective treatment options in melanoma therapy.
Recently, several groups have identified an increasing number of molecular targets associated with both the molecular and phenotypic characteristics of melanoma from distinct stages of tumor progression.2-4 Riker et al4 identified several putative tumor oncogenes (SPP-1, microphthalmia-associated transcription factor [MITF], CITED-1, GDF-15, c-MET, HOX loci) and suppressor genes (PITX-1, CST-6, PDGFRL, DSC-3, POU2F3, CLCA2, ST7L) as changing expression during the transition period for the emergence of the metastatic phenotype.4 A putative oncogene, the progestagen-associated endometrial protein (PAEP), is overexpressed in human melanoma as well as in gynecologic malignancies and tumors of reproductive organs.5,6
PAEP is a 28-kDa glycoprotein of the lipocalin superfamily, and its functional roles in gynecologic malignancies and tumors of reproductive organs have been described by several groups. Upregulation via the histone deacetylase inhibitors (HDACI) and overexpression of PAEP have been shown to stimulate cell migration in human endometrial adenocarcinoma.7 Song et al8 suggested that PAEP facilitates neovascularization mediated by vascular endothelial growth factor through increasing migration and tube formation of human umbilical vein endothelial cells during embryogenesis and tumor development. Others have suggested that PAEP expression is associated with differentiated epithelia and its expression induces cell differentiation, thereby reducing the malignant characteristics of cancer cells.9,10 They further show that PAEP reduced the tumor growth of MCF-7 breast cancer cells in vivo and suggested that PAEP acts as a tumor suppressor gene in breast cancer.11 These conflicting results were partially explained by a cell-specific role or specific glycosylation pattern of the PAEP protein.
In humans, the PAEP gene is found to be overexpressed in thick primary and metastatic melanoma tissues and cell lines. Small interfering RNA (siRNA) knockdown of PAEP expression in melanoma cell lines inhibited the proliferation, migration, invasion, and colony formation of melanoma cells, suggesting that the PAEP gene plays a critical role in melanoma progression and metastasis.6 However, the mechanisms by which PAEP is regulated or overexpressed in melanoma remain unknown. Here, we show that the PAEP gene interacts with MITF, a known master regulator of melanocyte development and melanoma progression, and may be an important enabling factor for MITF-mediated cell migration in human melanoma.
MATERIALS AND METHODS
Tissue Specimens
We surgically obtained tumor samples from patients with primary cutaneous melanoma (PCM) and MM under an Institutional Review Board–approved tissue procurement protocol (MCC#13448, IRB#101751; PSM# 990914-JM, 020318-JM). As described previously,4 all samples for this study were confirmed to contain > 95% melanoma cells by a dermatopathologist and were cryopreserved in liquid nitrogen and securely deidentified through a centralized database. We analyzed 40 MM samples, composed of 22 bulky, macroscopic lymph node metastases, 16 subcutaneous metastases, and 2 solid organ metastases (adrenal and brain), and compared them with 16 PCM samples, consisting of 2 melanoma in situ (MIS), 2 thin melanomas (< 1 mm in Breslow depth), 3 intermediate-thickness melanomas (1-4 mm), and 9 thick melanomas (> 4 mm). Additionally, we included 4 samples of normal human skin for comparative controls.
RNA Isolation, Hybridization, and Affymetrix GeneChip Analysis
A total of 5 µg of RNA isolated from cryopreserved melanoma tissues was processed using established Affymetrix (Santa Clara, CA) protocols for the generation of biotin, labeled as outlined in the Affymetrix technical manuals. The processed RNA was hybridized to Human Genome U133 Plus 2.0 arrays from Affymetrix and scanned on an Affymetrix GeneChip Scanner 3000 at 2.5 µm resolution. As described in our previous study,4 MicroArray Suite 5.0 analysis software was used to generate signal values for all probe sets based on a mean intensity of 500, subsequently exported, and iteratively normalized as a whole group to create the final normalization based on the most stable gene expression measurements across all samples. The normalized probe set values were log2 transformed and mean-centered across all clustered samples. Hierarchical clustering was then performed using absolute correlation and complete linkage in Eisen's cluster.12 Significance analysis of microarrays identified a more extensive list of genes differentially expressed between PCM and MM. Following all microarray analyses, the identified probe sets were annotated based on the sequence of the probes used on the arrays. The tissue samples were processed in 3 independent groups.
Cell Lines and Tissue Culture
Freshly excised melanoma samples were placed into culture media, and daughter cell lines were expanded using previously published techniques.13 All cell lines were split and passed fewer than 15 times and prepared by flow cytometry and cytospin for cellular confirmation of melanoma cell purity. We used the following cell lines originally procured from melanoma patients: 2 thick primary melanomas (MCC13 and MCC80A), 3 lymph node metastases (MCC67, MCC74, and MCC80B), and 5 distant metastases (MCC12A, MCC12F, MCC69A, MCC69B, and MCC81). Three pairs of cell lines—MCC80A and MCC80B, MCC12A and MCC12F, and MCC69A and MCC69B—were derived from the same patients. Three metastatic melanoma cell lines, 624-Mel, 624.38-Mel, and A375, were provided by the National Cancer Institute. All melanoma cells were maintained in RPMI 1640 culture media (VWR, Radnor, PA) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA). The normal human epidermal melanocyte (NHEM) was obtained from ATCC (Manassas, VA) and cultured according to the manufacturer instructions (Cambrex BioScience, Walkersville, MD).
Semi-quantitative Reverse Transcriptase Polymerase Chain Reaction (semi-qRT-PCR) and Real-Time qRT-PCR
Gene expression profiles of PAEP and MITF in 13 human melanoma cell lines, NHEM, and a skin sample were performed by semi-qRT-PCR analysis as previously described,14 with β-actin serving as an internal control. The primers were PAEP-f-AAG TTG GCA GGG ACC TGG CAC TC; PAEP-r-ACG GCA CGG CTC TTC CAT CTG TT; MITF-f-TTA TAG TAC CTT CTC TTT GCC AGT CC; MITF-r-TC TGC CCT GTT TTG CTC TTC AAA C; β-actin-f-ACA CTG TGC CCA TCT ACG AGG; and β-actin-r-AGG GGC CGG ACT CGT CAT ACT, respectively. Densitometric analysis was performed with AlphaEase FC image analysis software (Alpha Innotech, San Leandro, CA).
For real-time qRT-PCR, gene-specific primers (unlabeled) and TaqMan minor groove binder probes (6-FAM dye labeled) were purchased from Applied Biosystems (Foster City, CA): PAEP-Hs00171462_m1, MITF-Hs01117294_m1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-Hs99999905_m1. The relative amounts of each transcript of the tested genes were normalized to GAPDH. Real-time qRT-PCR was carried out in triplicate and run on a Bio-Rad (Hercules, CA) IQ5 multicolor real-time PCR detection system. Semi-qRT-PCR and real-time qRT-PCR were independently performed 3 times for all cell lines and skin samples.
siRNA Knockdown
One siGLO green transfection indicator, 1 siControl nontargeting pool, 3 duplex PAEP siRNAs (target sequences: siPAEP10-GGAAGAGCCGUGCCGUUUUU, siPAEP11-CCACGCUGCUCGAUACUGAUU, and siPAEP12-ACAGCUGUGUUGAGAAGAAUU), and 4 duplex MITF siRNAs (target sequences: siMITF5-UGGCUAUGCUUACGCUUAA, siMITF6-AGAACUAGGUACUUUGAUU, siMITF7-AGACGGAGCACACUUGUUA, and siMITF8-GAACACACAUUCACGAGCG) were synthesized by ThermoFisher Dharmacon (Lafayette, CO). Transfection conditions were optimized by transfecting melanoma cells with siGLO of varying concentrations followed by fluorescence microscope examination and flow cytometric analysis. For knockdown experiments, melanoma cells (1-2 × 105) were cultured in a 12-well plate in RPMI 1640 culture media supplemented with 10% FBS. Gene-specific siRNAs or nonspecific siControl were transfected into tumor cells using DharmaFECT1 (Dharmacon) according to the manufacturer's instructions. To assess the knockdown efficiency, transfected cells were collected for subsequent real-time qRT-PCR and Western blot analysis at 48 h and 72 h after transfection, respectively. Each experiment was independently performed 3 times.
Western Blotting
A total of 30 µg of each protein sample from cell lysate or serum-free culture medium was loaded within sodium dodecyl sulfate polyacrylamide gel electrophoresis gel followed by electrophoresis. Immunostaining was performed with a PAEP (2 µg/mL; Invitrogen, South San Francisco, CA), MITF (1∶500; Abcam, Cambridge, MA), or α-tubulin (1∶1000; Cell Signaling Technology, Danvers, MA) antibody followed by secondary antibody conjugated to horseradish peroxidase, as described previously.6 The blot was visualized with a Fujifilm Luminescent image analyzer LAS-3000 (Fujifilm, Valhalla, NY) and analyzed using Multi Gauge V3.1 software (Fujifilm). The target bands of PAEP, MITF, and α-tubulin were visualized at 30 kDa, 59 kDa, and 52 kDa, respectively. The experiment was independently performed 3 times for each sample.
Transwell Migration Assay
Melanoma cells with or without transfection of gene-specific siRNA or siControl were suspended in complete culture medium at 2 × 105 cells/mL. Each 100 µL of cells was then applied onto the upper migration chambers of the transwell plate (6.5 mm, 8.0 µm pore size; Corning, Acton, MA) and allowed to migrate for 6 h. The cells were fixed with 2% paraformaldehyde and then stained by 0.5% crystal violet. Using standard light microscopy, migrating cells were counted in 10 randomly chosen fields, with the relative migration ratio of wild-type cells set as 100%. The migration assay was independently performed 3 times for each cell line.
Statistical Analysis
Statistical analyses were performed with the SAS statistical software (SAS Institute, Cary, NC). We assessed correlation by performing a Pearson correlation analysis on gene expression level of PAEP and MITF observed by microarray or semi-qRT-PCR. If the r value exceeded 0.5, the significance of P value differences was then calculated. PAEP was considered to be strongly correlated with MITF at a value of P < .05. The statistical tests were two sided. Statistical differences between groups were assessed using analysis of variance and Dunnett t-tests for each group. The minimal level of significance was set at a value of P < .05, with the values presented as the mean ± standard deviation.
RESULTS AND DISCUSSION
PAEP Overexpression Is Correlated With MITF Gene Expression in Human Melanoma Tissues
Our previous findings revealed that PAEP gene overexpression in melanoma is linked to an increased metastatic potential, sparking further interest in deciphering the mechanisms governing its expression and regulation in human melanoma. It has been suggested that a significant correlation exists between the expression of a specific gene with that of its transcription factor or downstream target genes. Hoek and colleagues15 have identified 13 previously recorded targets of MITF-mediated upregulation, as well as 71 novel targets in human melanoma using a dual DNA microarray-based approach. They first identified genes upregulated by MITF-transfection of SK-Mel-28 melanoma cells and then compared them to genes that correlated with MITF expression in 7 different published data sets.
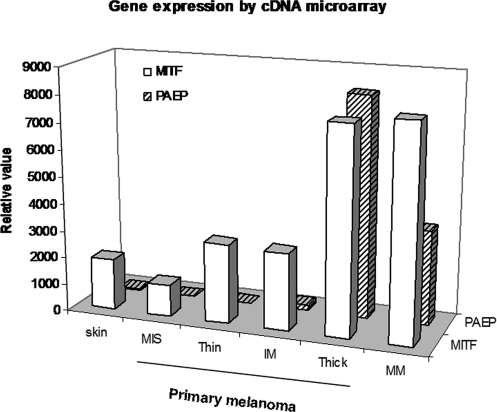
In this study, we analyzed the gene expression data of 16 primary and 40 metastatic melanoma tissue specimens using an Affymetrix human genome U133 plus 2.0 array platform. Pearson correlation analysis assessed each probe set in comparison to PAEP across all samples of each clinical stage. The analysis revealed a close correlation between PAEP and several genes, such as MITF, c-Met, HOXA10, and HOXA3. The MITF gene is of the most significance, and the calculated Pearson correlation coefficient (r) for PAEP (206859_s_at) and MITF (207233_s_at) probe sets was 0.86 (P = .027). The gene expressions of PAEP and MITF in both thick primary and metastatic lesions were markedly higher compared to those of MIS primary lesions (574-, 245-fold for PAEP and 6.5-, 6.7-fold for MITF overexpressed, respectively). The relative high level of MITF transcript in skin, MIS, and thin and intermediate thickness primary lesions is partially explained by its executing diverse functions and regulating numerous target genes in melanocyte development and melanoma progression (Figure 1).
Figure 1. .
Correlation of expression of progestagen-associated endometrial protein (PAEP) with microphthalmia-associated transcription factor (MITF) in human melanoma tissues. The gene expression profiles of 16 primary (MIS, melanoma in situ; thin, < 1 mm; IM, intermediate melanoma, 1-4 mm; thick, > 4 mm) and 40 metastatic melanoma (MM) specimens were examined utilizing an Affymetrix Human Genome U133 Plus 2.0 Array platform. Normalized signal intensity values for PAEP (206859_s_at) and MITF (207233_s_at) genes are analyzed by MAS 5.0 analysis software and the calculated Pearson correlation coefficient (r) is 0.86 (P < .05).
PAEP and MITF Are Overexpressed in Human Melanoma Daughter Cell Lines
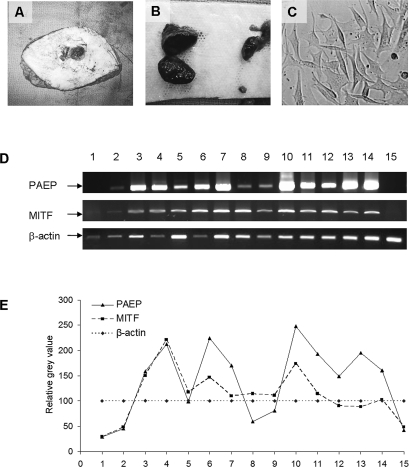
To validate the expression correlation of these 2 genes, we generated melanoma daughter cell lines from freshly procured tumor samples and compared the gene expression profile of MITF and PAEP by semi-qRT-PCR analysis. Two thick primary melanomas, 3 lymph node metastases, and 5 distant metastases were derived from patient samples using previously published techniques.12 We achieved similar gene expression profiles of PAEP and MITF genes in 13 melanoma daughter cell lines, 1 NHEM cell line, and 1 normal human skin sample by semi-qRT-PCR and subsequent densitometric analysis (Figure 2). The calculated r value was 0.75 (P = .001), confirming the presence of concomitant overexpression of PAEP and MITF genes in human melanoma–derived daughter cells.
Figure 2. .
Correlation of expression of progestagen-associated endometrial protein (PAEP) with microphthalmia-associated transcription factor (MITF) in melanoma daughter cells. Tumor cells isolated from freshly excised thick primary (A) and metastatic melanoma samples (B) were passaged and expanded in vitro (C). (D) The expression of PAEP and MITF genes was examined by semi-qRT-PCR in 10 melanoma daughter cell lines (No. 3-12), 3 National Cancer Institute (NCI) melanoma cell lines (No. 13-15), 1 human normal skin sample (No. 1) and 1 normal human epidermal melanocyte (NHEM; No. 2). (E) The densitometry of specific bands for PAEP and MITF genes was analyzed according to β-actin with AlphaEase FC image analysis software. 1. skin; 2. NHEM, thick primaries; 3. MCC13; 4. MCC80A, lymph node metastases; 5. MCC67; 6. MCC74; 7. MCC80B, distant metastases; 8. MCC12A; 9. MCC12F; 10. MCC69A; 11. MCC69B; 12. MCC81, NCI metastatic cell lines; 13. 624-Mel; 14. 624.38-Mel; 15. A375. The calculated Pearson correlation coefficient (r) of these 2 genes was 0.75 (P = .001).
The MITF gene has been previously shown to function as an oncogene and master regulator in human melanoma.16,17 Ectopic MITF gene expression in conjunction with the BRAF (V600E) mutant has the capacity to transform primary human melanocytes into melanoma, with the expression of MITF conferring soft-agar clonogenic growth.15 MITF regulates the transcription of multiple genes by binding specific sequences, as subset of E-boxes, present in promoter or enhancer elements containing the consensus CATGTG, CACATG, or CACGTG sequences.18-20 Analysis of the PAEP promoter region in linear genomics (GeneBank: M34046.1, GI: 190216) revealed a CACGTG consensus at position −2818 and three CACATG sequences at −2547, −1203, and −72. These analyses may indicate that PAEP has the capacity to be one of several target genes for MITF gene regulation.
siRNA Silencing of MITF Gene Expression Inhibits PAEP Gene Expression in Melanoma Cells
The master melanocyte regulator, MITF, has also been implicated in the coordination of melanocyte development as an essential factor in melanoblast differentiation and coat color pigmentation.21 More recently, MITF has been shown to be involved in melanoma progression, controlling cell survival, proliferation, and metastasis/migration through its direct regulation of a broad variety of target genes.
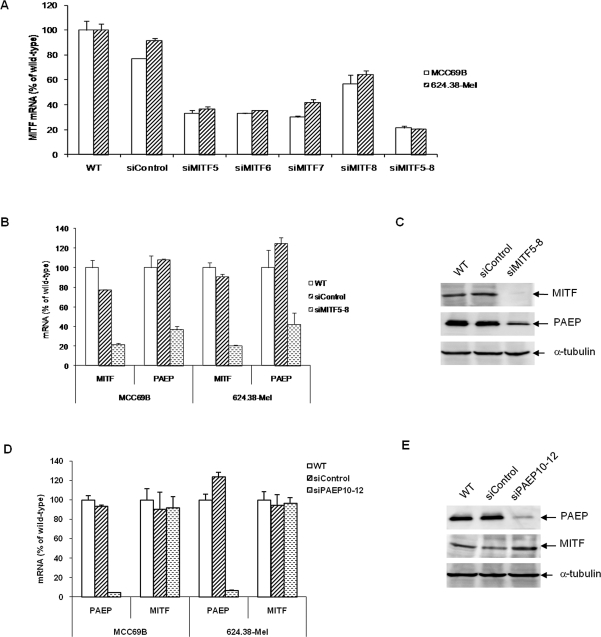
We wanted to further examine whether MITF regulates the expression of the PAEP gene via RNA interference methods to silence MITF gene expression in melanoma cells. Transfection conditions were optimized by transfecting melanoma cells with siGLO green transfection indicator of varying concentrations followed by fluorescence microscope examination and flow cytometric analysis. A high transfection efficiency (80%-90%) was achieved for 624.38-Mel and MCC69B cells (data not shown). To find the sequences that inhibit MITF gene expression most efficiently, 4 duplex siRNA; siMITF 5, 6, 7, and 8; and a pool of siMITF5-8 were separately transfected into melanoma cells. Real-time qRT-PCR analysis revealed siMITF5-8 showed the best overall gene silencing of MITF, with a silencing efficiency of ∼ 80% (Figure 3). Therefore, we utilized the siMITF5-8 pool to silence MITF expression in MCC69B and 624.38-Mel cells. Subsequent real-time qRT-PCR and Western blotting assays (Figure 3) indicated that the knockdown of MITF gene expression resulted in a marked decrease, by 60%, for both mRNA and protein expression of the PAEP gene.
Figure 3. .
Transfection of MCC69B and 624.38-Mel metastatic melanoma cells with microphthalmia-associated transcription factor (MITF) small interfering RNA (siRNA) specifically diminishes progestagen-associated endometrial protein (PAEP) gene expression. (A) Four duplex siRNA; siMITF5, 6, 7, and 8; and a pool of siMITF5-8 were separately transfected into melanoma cells. MITF expression was examined by real-time qRT-PCR at 48 h after transfection. The expression of MITF and PAEP genes was examined by real-time qRT-PCR (B) and Western blotting (C) after siMITF5-8 transfection in melanoma cells at 48 h and 72 h after transfection, respectively. The expression of PAEP and MITF genes was examined by real-time qRT-PCR (D) and Western blotting (E) after siPAEP10-12 transfection in melanoma cells. Mock (wild-type) and nonspecific siControl transfection served as negative controls.
To explore whether the gene expression of PAEP plays a role in MITF gene expression, we silenced PAEP gene expression by RNA interference as described previously.6 However, upon PAEP gene silencing by ∼ 95%, it did not appear to influence MITF gene expression at the mRNA or protein levels (Figure 4). These collective data may indicate that PAEP is partially regulated by MITF, while the reverse (PAEP as a regulator of MITF gene expression) is not a significant interaction.
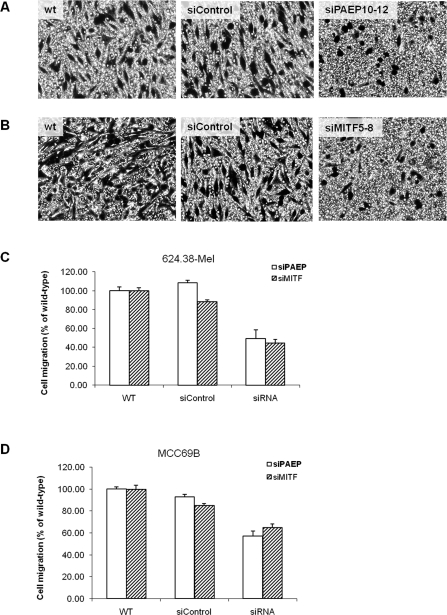
Figure 4. .
Silencing of microphthalmia-associated transcription factor (MITF) or progestagen-associated endometrial protein (PAEP) gene expression decreases the migration of MCC69B and 624.38-Mel metastatic melanoma cells. 624.38-Mel cells with or without transfection of small interfering (si) PAEP10-12 or siControl (A), or with or without transfection of siMITF5-8 or siControl (B), were plated onto the upper transwell chambers and allowed to migrate for 6 h. 624.38-Mel (C) or MCC69B (D) cells that migrated to the underside of the chamber were counted in 10 randomly chosen fields under a microscope and were analyzed by Dunnett t-test, with the relative migration ratio of wild-type cells set as 100% (P < .05).
Silencing of MITF or PAEP Gene Expression Decreases the Migration of Melanoma Cells
Cellular motility is one of the primary characteristics of tumor cells with metastatic potential. Previous studies indicated that MITF enhances tumor cell migration by regulating a panel of downstream target genes. Hepatocyte growth factor (HGF)/c-Met signaling, which is thought to be a key pathway in both melanocyte development and melanoma metastasis, is reportedly directly regulated by MITF. McGill et al22 have shown that MITF directly binds to the c-MET promoter and is essential for the homeostatic upregulation of c-Met expression following activation of the receptor by its ligand HGF.22 They also observed that MITF regulates c-Met gene expression in primary melanocytes and that the ability of HGF to stimulate invasive growth of melanocytes and melanoma cells in culture was abolished on suppression of endogenous MITF.
PAEP is another gene with oncogenic characters that is reportedly linked to the metastatic potential of melanoma. In our previous study, we observed a decreased cell migration of melanoma cells transfected with siPAEP10-12 compared to wild-type and nontargeting controls (25%-50% less, P < .05).6 Furthermore, an invasion assay revealed that melanoma cells with higher PAEP gene expression were more capable of penetrating the artificial Matrigel (BD Biosciences, Franklin Lakes, NJ) barrier. Consistent with this study, tumor cell migration enhanced by the PAEP gene is also observed in human endometrial adenocarcinoma cells. Uchida et al7 described the use of HDACI to stimulate the migration of tumor cells, mediated by the upregulation and overexpression of PAEP, supporting the role of the PAEP gene in cellular migratory capacity in tumor cells.
To explore whether PAEP gene expression influences MITF to mediate cell migration in human melanoma, we silenced MITF gene expression in MCC69B and 624.38-Mel cells. The transfected cells were then seeded in a transwell chamber and allowed to migrate for 6 h. As shown in Figures 4A-C, cell migration of 624.38-Mel cells was significantly suppressed by 55% after siMITF5-8 transfection compared to wild-type and nontargeting controls (P < .05), consistent with that of siPAEP10-12–transfected 624.38-Mel cells. A similar result of migration suppression (about 40%) was achieved in siMITF5-8– or siPAEP10-12–transfected MCC69B cells (Figure 4D). Thus, it appears that the inhibition of either MITF or PAEP gene expression results in a significant suppression of melanoma cell migration.
In conclusion, PAEP is a progesterone-regulated immunosuppressive molecule with a variety of diverse functions that also is overexpressed and capable of promoting tumor cell migration in human melanoma. It appears to be at least partially regulated by the MITF gene, a known master regulator of melanocyte differentiation and melanoma development. We are just beginning to understand the exact molecular and cellular mechanisms involved during melanocyte transformation and melanoma cell progression and metastasis. Currently, several research groups are examining the role of targeted therapeutics to block MITF gene function, possibly disrupting PAEP gene expression and further inhibiting melanoma cell metastatic potential.
Footnotes
This study was supported by a grant from the National Natural Science Foundation of China (PDRC-81071709) to Suping Ren, MD, PhD, and the Abraham A. Mitchell Clinical Research Scholarship to Adam I. Riker, MD, FACS.
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54(3):131–149; quiz 182-184. doi: 10.3322/canjclin.54.3.131. [DOI] [PubMed] [Google Scholar]
- 2.Haqq C, Nosrati M, Sudilovsky D, et al. The gene expression signatures of melanoma progression. Proc Natl Acad Sci U S A. 2005;102(17):6092–6097. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winnepenninckx V, Lazar V, Michiels S, et al. Melanoma Group of the European Organization for Research and Treatment of Cancer. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006;98(7):472–482. doi: 10.1093/jnci/djj103. [DOI] [PubMed] [Google Scholar]
- 4.Riker AI, Enkemann SA, Fodstad O, et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics. 2008;1:13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren S, Liu S, Howell PM, Jr, Riker AI. Identification of a putative oncogene in human melanoma: progesterone-associated endometrial protein [abstract] Ann Surg Oncol. 2008;15(Suppl 2):10. [Google Scholar]
- 6.Ren S, Liu S, Howell PM, Jr, et al. Functional characterization of the progestagen-associated endometrial protein gene in human melanoma. J Cell Mol Med. 2010;14(6B):1432–1442. doi: 10.1111/j.1582-4934.2009.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchida H, Maruyama T, Ono M, et al. Histone deacetylase inhibitors stimulate cell migration in human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology. 2007;148(2):896–902. doi: 10.1210/en.2006-0896. [DOI] [PubMed] [Google Scholar]
- 8.Song M, Ramaswamy S, Ramachandran S, et al. Angiogenic role for glycodelin in tumorigenesis. Proc Natl Acad Sci U S A. 2001;98(16):9265–9270. doi: 10.1073/pnas.151151198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandelin E, Lassus H, Seppälä M, et al. Glycodelin in ovarian serous carcinoma: association with differentiation and survival. Cancer Res. 2003;63(19):6258–6264. [PubMed] [Google Scholar]
- 10.Seppälä M, Koistinen H, Koistinen R, Hautala L, Chiu PC, Yeung WS. Glycodelin in reproductive endocrinology and hormone-related cancer. Eur J Endocrinol. 2009;160(2):121–133. doi: 10.1530/EJE-08-0756. [DOI] [PubMed] [Google Scholar]
- 11.Hautala LC, Koistinen R, Seppälä M, et al. Glycodelin reduces breast cancer xenograft growth in vivo. Int J Cancer. 2008;123(10):2279–2284. doi: 10.1002/ijc.23773. [DOI] [PubMed] [Google Scholar]
- 12.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riker AI. Isolation and culture of melanoma cell lines. Methods Mol Med. 2004;88:93–99. doi: 10.1385/1-59259-406-9:93. [DOI] [PubMed] [Google Scholar]
- 14.Horowitz IR, Cho C, Song M, et al. Increased glycodelin levels in gynecological malignancies. Int J Gynecol Cancer. 2001;11(3):173–179. doi: 10.1046/j.1525-1438.2001.01017.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoek KS, Schlegel NC, Eichhoff OM, et al. Novel MITF targets identified using a two-step DNA microarray strategy. Pigment Cell Melanoma Res. 2008;21(6):665–676. doi: 10.1111/j.1755-148X.2008.00505.x. [DOI] [PubMed] [Google Scholar]
- 16.Garraway LA, Widlund HR, Rubin MA, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436(7047):117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 17.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12(9):406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Hemesath TJ, Steingrímsson E, McGill G, et al. microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 1994;8(22):2770–2780. doi: 10.1101/gad.8.22.2770. [DOI] [PubMed] [Google Scholar]
- 19.Bentley NJ, Eisen T, Goding CR. Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol. 1994;14(12):7996–8006. doi: 10.1128/mcb.14.12.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasumoto K, Yokoyama K, Shibata K, Tomita Y, Shibahara S. Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol Cell Biol. 1994;14(12):8058–8070. doi: 10.1128/mcb.14.12.8058. Erratum in: Mol Cell Biol. 1995;15(3):1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steingrímsson E, Arnheiter H, Hallsson JH, Lamoreux ML, Copeland NG, Jenkins NA. Interallelic complementation at the mouse Mitf locus. Genetics. 2003;163(1):267–276. doi: 10.1093/genetics/163.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGill GG, Haq R, Nishimura EK, Fisher DE. c-Met expression is regulated by Mitf in the melanocyte lineage. J Biol Chem. 2006;281(15):10365–10373. doi: 10.1074/jbc.M513094200. [DOI] [PubMed] [Google Scholar]