Abstract
Background
Allergic fungal rhinosinusitis (AFRS) is a relatively new and incompletely understood clinical entity with characteristic clinical, radiographic, and histopathologic findings. AFRS is often misdiagnosed. Recognition and understanding of this unique disease will lead to efficient diagnosis and treatment of this curable process.
Methods
The following is a review, conducted via a PubMed English language search, of the current diagnosis, pathogenesis, and treatment of AFRS.
Results
AFRS is an immune-modulated disease entity. The Bent and Kuhn diagnostic criteria are the standard for diagnosis of this disease that occurs because of an incompletely understood allergic mechanism. Multimodality treatment relies heavily on surgical therapy along with corticosteroid use and immunotherapy.
Conclusions
AFRS is a unique disease process that differs from other forms of sinusitis and as such requires that physicians understand its diagnosis and management to provide care for patients with this condition.
Keywords: Allergic fungal sinusitis, allergic mucin, Aspergillus, Bent and Kuhn, Bipolaris, dematiaceous fungi, rhinosinusitis, sinusitis, type I and III hypersensitivity
INTRODUCTION
Allergic fungal rhinosinusitis (AFRS) was first reported as a distinct clinical entity in 1976.1 AFRS is coupled with the clinical entity of fungus ball (mycetoma) as a form of noninvasive fungal sinus disease, separate from and unrelated to invasive fungal sinus pathology. AFRS is a truly unique pathologic entity, defined largely by the presence of allergic fungal mucin, which is a thick, tenacious, eosinophilic secretion with characteristic histologic findings. This mucin is grossly and microscopically similar to that found in the lungs of patients with allergic bronchopulmonary aspergillosis (ABPA), and this pulmonary correlate helped guide the early understanding of the pathogenesis of AFRS.2 Since its initial characterization in the 1970s, AFRS has been the subject of much debate and controversy regarding its pathogenesis, diagnosis, classification, and optimal management.
DIAGNOSIS
Diagnosis begins with a thorough clinical history. Commonly, the patient will present with a history of sinus disease strongly recalcitrant to traditional medical and even surgical therapy aimed largely at bacterial rhinosinusitis.3 Several courses of antibiotics and topical nasal preparations may have been tried with little success. Unique features of AFRS that can alert the clinician to a possible diagnosis include a young (mean age is 22 years), immunocompetent patient with unilateral or asymmetric involvement of the paranasal sinuses, a history of atopy, nasal casts, and polyposis, and a lack of significant pain.4 Nasal casts are green to black rubbery formed elements made of allergic mucin. The presentation may be dramatic, with a significant number of patients presenting with proptosis, telecanthus, or gross facial dysmorphia.5 AFRS occurs throughout the United States, with increased prevalence in the Mississippi basin and southwestern states.6 The diagnostic dilemma is differentiating AFRS from other fungal entities involving the paranasal sinuses, including saprophytic fungal growth, mycetoma, eosinophilic mucin rhinosinusitis, and invasive fungal sinusitis.
In 1994, Bent and Kuhn published their diagnostic criteria centered on the histologic, radiographic, and immunologic characteristics of the disease.7 Others have proposed several sets of criteria that have served to further the discussion of and investigation into this unique disease; however, the Bent and Kuhn criteria (Table 1) are largely regarded as the standard for diagnosis today. Patients must meet all the major criteria for diagnosis, while the minor criteria serve to support the diagnosis and describe individual patients but are not used to make a diagnosis. The major criteria include a history of type I hypersensitivity by history, skin testing, or in vitro testing; nasal polyposis; characteristic computed tomography (CT) scan findings; the presence of eosinophilic mucin without invasion; and a positive fungal stain of sinus contents removed at the time of surgery. The minor criteria include a history of asthma, unilateral predominance of disease, radiographic evidence of bone erosion, fungal cultures, presence of Charcot-Leyden crystals in surgical specimens, and serum eosinophilia.
Table 1. .
Bent and Kuhn Diagnostic Criteria
The histopathologic findings in AFRS are critical to the diagnosis. Microscopic review of mucosal specimens on hematoxylin-eosin (H&E) staining will show typical inflammatory infiltrate composed of eosinophils, lymphocytes, and plasma cells.6 The mucosa will be hypertrophic and hyperplastic but should not have evidence of necrosis, giant cells, granulomas, or invasion into surrounding structures. Such findings would lend support to a diagnosis of a fungal process other than AFRS.
It is important to note that examination of the unique allergic fungal mucin itself, and not the surrounding mucosa, is the most reliable indicator of disease. Grossly, this thick, highly viscous, variably colored mucin has been described as being similar to peanut butter or axle grease.6 Microscopically, the mucin often takes on a chondroid appearance with sheets of eosinophils, frequently with the presence of eosinophilic breakdown products or Charcot-Leyden crystals6 that can easily be seen with H&E staining. Fungi themselves do not stain with H&E staining; however, their negative image can sometimes be appreciated. Special stains containing silver are usually needed to appreciate the branching, noninvasive fungal hyphae.
Fungal cultures should be interpreted with caution. They are best used as supportive evidence because of their variable yield (64%–100%). A diagnosis of AFRS is possible in the context of negative cultures, and saprophytic fungal growth does not diagnose AFRS in the context of positive cultures alone.
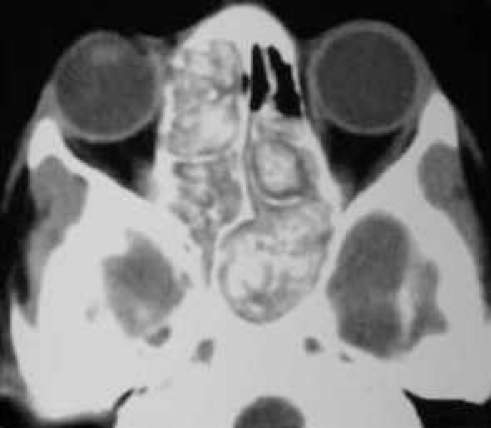
Characteristic imaging findings are a critical component of the AFRS diagnosis. CT findings will often demonstrate unilateral or asymmetric involvement of the sinuses.6,7 Allergic mucin provides the well-recognized heterogeneous signal intensity that is characteristic of but not specific to AFRS (Figure 1). This heterogeneity was initially thought to be related to hemosiderin accumulation in the mucin, but more recent theories center on the deposition of heavy metals such as iron and manganese.6,7 The ethmoid sinus is the most commonly involved sinus. Bony erosion and expansion of the fungal mucin are often seen on CT scan, are related to the expansive nature of the mucin and local inflammatory milieu, and are not caused by true fungal invasion.8 The remodeling and thinning of bony walls are seen in up to 56% of cases, most frequently in the orbit, followed by the anterior, middle, and posterior cranial fossae.9 Local bony involvement is 10 times more common in AFRS than in other forms of chronic rhinosinusitis (CRS).7 Several authors have documented the high rate of proptosis seen in the pediatric AFRS population, with approximately 50% of children having orbital erosion and proptosis.10,11 Fortunately, significantly decreased orbital volumes (to approximately 70% of normal) have been noted to return to 90% of normal after successful management.10,11
Figure 1. .
Computed tomography demonstrating the heterogeneous signal intensity that is characteristic of allergic fungal rhinosinusitis.
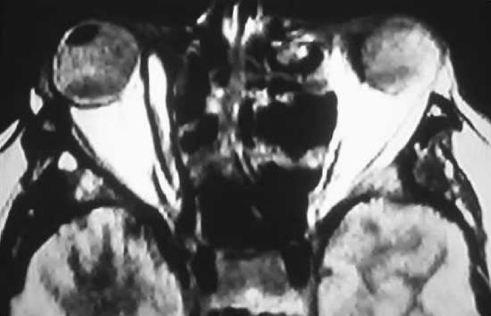
Magnetic resonance imaging has been shown to demonstrate a high specificity for AFRS, especially when combined with CT.12 The high protein concentration of allergic mucin (greater than 28%) results in crosslinking and slows macromolecular motion, giving rise to T1 central hypointensity and T2 central signal void (Figure 2). Both T1 and T2 series demonstrate peripheral enhancement.
Figure 2. .
Magnetic resonance imaging that shows T1 central hypointensity and T2 central signal void.
Finally, laboratory findings are also helpful in the diagnosis of AFRS. Total immunoglobulin E (IgE) levels are generally elevated, often to more than 1,000 U/mL. Mabry and colleagues13–15 demonstrated broad sensitivity to both fungal and nonfungal antigens, emphasizing that AFRS patients are generally atopic. Interestingly, the reactions were not fungal specific, although typically only one fungus was isolated from the culture. This finding could represent a common fungal epitope to explain the broad reactivity, or possibly—as Schubert described—the presence of a superantigen that could contribute to the nonspecific reactivity of these patients.16
PATHOGENESIS
The pathogenesis of AFRS is not yet fully understood and is a subject of controversy. Early reports described a similar mechanism to ABPA, namely Gell and Coombs types I and III hypersensitivity responses to inhaled fungal antigens.17 This immunologic theory is supported by the work of Manning and Holman,4 who proposed a cycle of initial antigenic stimulus, followed by hypersensitivity reactions and a resultant self-perpetuating cycle of inflammation, obstruction, and antigenic exposure. In their first experiment, Manning and Holman4 prospectively compared 8 patients with culture-positive Bipolaris AFRS to 10 nonsinusitis control subjects and found Bipolaris-specific IgE and immunoglobulin G (IgG) antibodies by radioallergosorbent test (82%) and enzyme-linked immunosorbent assay (94%).4 These patients also demonstrated positive skin testing to Bipolaris These results implicated the importance of allergy to fungal antigens in the pathophysiology of AFRS. A complementary experiment within the same study compared 14 mucosal specimens from AFRS patients to those from 10 CRS patients. This study showed that eosinophilic mediators predominated over neutrophil-derived mediators in the AFRS specimens, whereas in the control group eosinophil and neutrophil mediators were equal, findings that lend further support for a noninfectious, immunologically mediated process.
An important observation led to further questioning and theories attempting to explain the pathogenesis of AFRS. It was noted that some patients with the clinical picture of AFRS do not have allergies. Is it possible for nonatopic patients to have AFRS? An alternative theory was proposed by Ponikau et al,18 who demonstrated the ubiquitous presence of fungi within the nose and paranasal sinuses in 93% of patients undergoing surgery for any form of CRS. This study also showed that fungal-specific allergy was uncommon in these patients and concluded that most CRS is a T-cell mediated response to fungi, resulting in eosinophilic chemotaxis and activation. However, the question remains: If fungi are indeed ubiquitous, what determines why some patients develop AFRS while others do not?
Collins et al19 proposed a theory that AFRS is the result of a local, not systemic, hypersensitivity reaction. This study, which is based on finding fungus-specific IgE in the mucin of AFRS as well as non-AFRS patients, proposed evidence for a local type I response. This response may be entirely localized to the nose and paranasal sinuses without signs of systemic involvement. This idea may provide a compromise between the 2 previously described theories, providing evidence for the role of hypersensitivity reactions yet allowing an explanation for the observation that not all AFRS patients have signs of systemic allergy.
Lastly, Pant et al20 demonstrated the significance of humoral immunity in the pathogenesis of AFRS. This study looked at patients with eosinophilic mucin CRS (EMCRS), a broad clinical entity characterized by polypoid rhinosinusitis and eosinophilic mucin with or without fungal elements. Elevated levels of fungal-specific IgG3, rather than IgE, separated EMCRS and AFRS patients from those with other forms of CRS. In addition, fungal-specific IgE responses in fungal-allergic EMCRS patients were no different than those in fungal-allergic controls, raising the question of the role of fungal allergy in AFRS.
TREATMENT
Just as the understanding of the pathogenesis of this disease is evolving, so is the treatment protocol (Table 2). Early therapies were aimed at the eradication of Aspergillus species, largely because of the similarity between AFRS and ABPA, as well as early cultures and serological testing incorrectly implicating this fungus. Manning's work21 has identified the dematiaceous fungi, namely Bipolaris, as the culprit in the vast majority of cases. Correct identification of the causative organism has been accompanied by the development of multimodality treatment algorithms, with surgical therapy remaining the cornerstone for this recidivistic disease.
Table 2. .
Allergic Fungal Rhinosinusitis Treatment Options
Traditional surgery has been aggressive, with radical removal of mucosa and frequent external approaches. The strategy has evolved to incorporate almost exclusively endoscopic tissue-sparing techniques described as conservative but complete.22,23 Designed to remove obstruction and allow for natural drainage patterns, the goal is complete removal of all mucin and debris to eliminate the antigenic-inciting factor. In addition, postoperative access must be kept in mind to allow for examination in the clinic and diagnosis of recurrence. The physical characteristics of AFRS have significant implications for the surgeon.6 The slow accumulation of allergic fungal mucin and associated bony decalcification may mimic invasion and result in the loss of normal bony landmarks, placing vital structures at risk. In addition, the inherent nasal polyposis contributes to the distorted anatomy and increases bleeding in the surgical field.
The use of an oral corticosteroid (OCS) and other medical therapies in the treatment of AFRS arose directly from the success of such treatments in ABPA. The efficacy of OCS therapy is well documented in the literature; benefits include increased cure rates and clinically milder disease among those in whom disease recurred, increased time to revision surgery, reduction in mucosal stage of disease, and reduced systemic IgE levels.2,5 No optimal OCS dosing regimen has been established at this time. Preoperative institution of therapy is generally encouraged to improve surgical exposure and decrease blood loss, with a taper over a period of months after surgery. Intermittent burst therapy can be used for upper respiratory infections as needed.
Immunotherapy (IT) has also been shown to be very efficacious in the treatment of AFRS since it was initiated in 1993. Mabry and colleagues13–15 have published results on the use of IT in the treatment of AFRS, showing that treating reactivity to both fungal and nonfungal antigens resulted in elimination of nasal crusting and mucin deposits. Interestingly, these patients also had no need for OCS and a limited need for topical corticosteroids. The protocol includes preoperative and postoperative testing for common molds, the selection of which is not limited by culture results, as fungal cultures have notoriously low yields. In addition, testing is performed for nonfungal antigens. Therapy is initiated 4–6 weeks after surgery and is predicated on the removal of all allergic mucin at the time of surgery to reduce the antigenic load and prevent worsening of disease. The optimal length of treatment has not yet been determined.
Antifungal therapy initially started because of high rates of recurrence following surgical therapy alone but has largely fallen out of favor with the advent of OCS and IT. Kennedy and colleagues24 showed no improvement in the radiographic appearance of the disease or in symptoms in patients treated with oral terbinafine for 6 weeks. Several investigators have evaluated intranasal antifungals with mixed results.25 These findings emphasize the need for further work in this area and underlie the reason why antifungal therapy is not widely employed in the treatment of AFRS.
In conclusion, AFRS is a relatively new clinical entity; many questions surround its diagnosis, pathogenesis, and optimal treatment. The Bent and Kuhn criteria are generally the most widely accepted diagnostic criteria in use today. Theories on pathogenesis include hypersensitivity and T-cell mediated reactions as well as a humoral immune response. Treatment is largely surgical, with a strong role for oral corticosteroids and an emerging role for IT. Antifungals, both systemic and topical, currently have a limited role in treatment, although this area needs further study.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education competencies for Patient Care, Medical Knowledge, and Systems-Based Practice.
REFERENCES
- 1.Safirstein B. Allergic bronchopulmonary aspergillosis with obstruction of the upper respiratory tract. Chest. 1976;70:788–790. doi: 10.1378/chest.70.6.788. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn FA, Javer AR. Allergic fungal rhinosinusitis: our experience. Arch Otolaryngol Head Neck Surg. 1998;124(10):1179–1180. doi: 10.1001/archotol.124.10.1179. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn FA, Javer AR. Allergic fungal rhinosinusitis: perioperative management, prevention of recurrence, and role of steroids and antifungal agents. Otolaryngol Clin North Am. 2000;33(2):419–433. doi: 10.1016/s0030-6665(00)80016-x. [DOI] [PubMed] [Google Scholar]
- 4.Manning SC, Holman M. Further evidence for allergic pathophysiology in allergic fungal sinusitis. Laryngoscope. 1998;108(10):1485–1496. doi: 10.1097/00005537-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Ryan MW, Marple BF. Allergic fungal rhinosinusitis: diagnosis and management. Curr Opin Otolaryngol Head Neck Surg. 2007;15(1):18–22. doi: 10.1097/MOO.0b013e328013dbd9. [DOI] [PubMed] [Google Scholar]
- 6.Marple BF. Allergic fungal rhinosinusitis: current theories and management strategies. Laryngoscope. 2001;111(6):1006–1019. doi: 10.1097/00005537-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Bent JP, 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111(5):580–588. doi: 10.1177/019459989411100508. [DOI] [PubMed] [Google Scholar]
- 8.Manning SC, Merkel M, Kriesel K, Vuitch F, Marple B. Computed tomography and magnetic resonance diagnosis of allergic fungal sinusitis. Laryngoscope. 1997;107(2):170–176. doi: 10.1097/00005537-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Ghegan MD, Lee FS, Schlosser RJ. Incidence of skull base and orbital erosion in allergic fungal rhinosinusitis (AFRS) and non-AFRS. Otolaryngol Head Neck Surg. 2006;134(4):592–595. doi: 10.1016/j.otohns.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Campbell JM, Graham M, Gray HC, Bower C, Blaiss MS, Jones SM. Allergic fungal sinusitis in children. Ann Allergy Asthma Immunol. 2006;96(2):286–290. doi: 10.1016/S1081-1206(10)61237-9. [DOI] [PubMed] [Google Scholar]
- 11.McClay JE, Marple B, Kapadia L, et al. Clinical presentation of allergic fungal sinusitis in children. Laryngoscope. 2002;112(3):565–569. doi: 10.1097/00005537-200203000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Zinreich SJ, Kennedy DW, Malat J, et al. Fungal sinusitis: diagnosis with CT and MR imaging. Radiology. 1988;169(2):439–444. doi: 10.1148/radiology.169.2.3174990. [DOI] [PubMed] [Google Scholar]
- 13.Mabry RL, Manning SC, Mabry CS. Immunotherapy in the treatment of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1997;116(1):31–35. doi: 10.1016/S0194-59989770348-6. [DOI] [PubMed] [Google Scholar]
- 14.Mabry RL, Marple BF, Folker RJ, Mabry CS. Immunotherapy for allergic fungal sinusitis: three years' experience. Otolaryngol Head Neck Surg. 1998;119(6):648–651. doi: 10.1016/S0194-5998(98)70027-0. [DOI] [PubMed] [Google Scholar]
- 15.Mabry RL, Mabry CS. Allergic fungal sinusitis: the role of immunotherapy. Otolaryngol Clin North Am. 2000;33(2):433–440. doi: 10.1016/s0030-6665(00)80017-1. [DOI] [PubMed] [Google Scholar]
- 16.Schubert MS. A superantigen hypothesis for the pathogenesis of chronic hypertrophic rhinosinusitis, allergic fungal sinusitis, and related disorders. Ann Allergy Asthma Immunol. 2001;87(3):181–188. doi: 10.1016/S1081-1206(10)62222-3. [DOI] [PubMed] [Google Scholar]
- 17.Safirstein BH. Allergic bronchopulmonary aspergillosis with obstruction of the upper respiratory tract. Chest. 1976;70(6):788–790. doi: 10.1378/chest.70.6.788. [DOI] [PubMed] [Google Scholar]
- 18.Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74(9):877–884. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 19.Collins M, Nair S, Smith W, Kette F, Gillis D, Wormald PJ. Role of local immunoglobulin E production in the pathophysiology of noninvasive fungal sinusitis. Laryngoscope. 2004;114(7):1242–1246. doi: 10.1097/00005537-200407000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Pant H, Kette FE, Smith WB, Wormald PJ, Macardle PJ. Fungal-specific humoral response in eosinophilic mucus chronic rhinosinusitis. Laryngoscope. 2005;115(4):601–606. doi: 10.1097/01.mlg.0000161341.00258.54. [DOI] [PubMed] [Google Scholar]
- 21.Manning SC, Schaefer SD, Close LG, Vuitch F. Culture-positive allergic fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1991;117(2):174–178. doi: 10.1001/archotol.1991.01870140062007. [DOI] [PubMed] [Google Scholar]
- 22.Schubert MS, Goetz DW. Evaluation and treatment of allergic fungal sinusitis. I. Demographics and diagnosis. J Allergy Clin Immunol. 1998;102(3):387–394. doi: 10.1016/s0091-6749(98)70125-3. [DOI] [PubMed] [Google Scholar]
- 23.Schubert MS. Allergic fungal sinusitis: pathogenesis and management strategies. Drugs. 2004;64(4):363–374. doi: 10.2165/00003495-200464040-00002. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy DW, Kuhn FA, Hamilos DL, et al. Treatment of chronic rhinosinusitis with high-dose oral terbinafine: a double blind, placebo-controlled study. Laryngoscope. 2005;115(10):1793–1799. doi: 10.1097/01.mlg.0000175683.81260.26. [DOI] [PubMed] [Google Scholar]
- 25.Stankiewicz JA, Musgrave BK, Scianna JM. Nasal amphotericin irrigation in chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2008;16(1):44–46. doi: 10.1097/MOO.0b013e3282f1c7ba. [DOI] [PubMed] [Google Scholar]