Randomization may be achieved at the expense of relevance. L. J. Cronbach, Designing Evaluations of Educational and Social Problems1
The Randomized Controlled Trial “Gold Standard”
Medical education researchers are inevitably more familiar with the multisite randomized controlled trial (RCT) “gold standard” from the clinical research paradigm. In an RCT, trainees are randomly assigned to receive 1 of 2 or more educational interventions. Randomized controlled trials are quantitative, comparative, controlled experiments in which treatment effect sizes may be determined with less bias than observational trials.2 Randomization is considered the most powerful experimental design in clinical trials: with other variables equal between groups, on average, any differences in outcome can be attributed to the intervention.2
With trainees moving through educational processes in real time, the difficulties of randomization become immediately clear. Residents and fellows usually experience rotations at different times: those experiencing the intervention later may learn from intervening experiences and no longer be comparable to those experiencing the intervention at an earlier time. Also, trainees have the option to refuse randomization, yet they cannot miss critical educational experiences. Use of a “placebo” is often contraindicated. Multisite interventions, while providing more subjects (with greater power to detect differences) and more generalizability, present challenges due to training differences. Also, the cost of multisite studies can be high and industry support for education trials rarely exists.
While large numbers of medical undergraduates often receive nearly identical “treatments,” in which 1 or more variables may be altered, this is not true for graduate medical education. Residency and fellowship training are usually highly individualized, which makes the RCT model increasingly unsuitable as training advances.
In addition to feasibility considerations, experts question the applicability of research models, derived from clinical research, for education studies.3,4 The highly complex system of education may be a poor fit for the RCT model, which requires clear inclusion/exclusion criteria and interventions administered identically via multiple physicians (ie, teachers).3,5,6 Regehr asks whether simulating placebo-controlled efficacy clinical trials, in which 1 or a few variables may be tightly controlled, is a worthwhile goal for medical education research.3 In education studies, variables can rarely be controlled tightly and blinding of subjects and study personnel may be unethical or impossible.3,7 Finally, defining the therapeutic intervention in education research is much more difficult than in clinical trials. As Norman suggests, one cannot “apply curriculum daily” in the same way that one can prescribe a medication.5
RCTs in Education Research
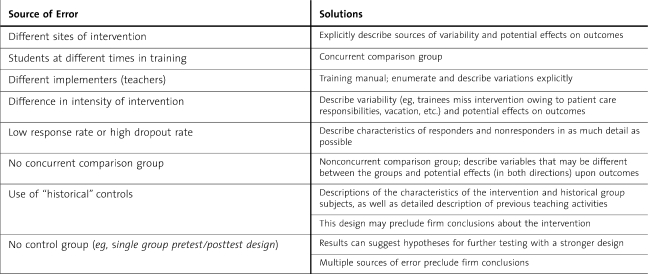
The primary advantage of randomization is that it reduces allocation bias, which derives from baseline variables that may influence outcome(s). Randomization ensures that baseline characteristics, not known to be related to the outcome of interest, are equally distributed among the groups. Although differences among participants are 1 source of error, randomization will not control for other sources of error, which are likely to occur in education studies (table 1). Variations in those implementing the intervention, settings, and other execution factors may have more impact than baseline variations in the subjects. Other common “confounders” that randomization may not control for are effects of pretests on learning (encouraging differential study or learning);8 Hawthorne effects (changes in participant motivation); effects of other, nonintervention training experiences occurring during the study intervention;5 and high participant dropout (eg, less than 75% response rates).9 Contextual factors may affect outcomes in ways that randomization cannot fix.10 Especially if the intervention is fairly dilute (eg, a workshop, short course, or online cases), it may not be apparent whether the intervention is causing the outcome effects versus contextual factors.
Table 1.
Sources of Error in Experimental Education Studies,8,14
In education studies it is often difficult to “blind” learners to their assigned group. Without blinding, residents can react to the knowledge that they are being studied or assigned to a particular group. Within training programs trainees interact to a great extent, resulting in contamination effects (ie, trainees sharing learning with each other) that further compromise randomization. Active interventions that are deemed critical to learning cannot be withheld, although crossover designs may be used in this situation. However, crossover designs may also involve contamination of learning between groups.
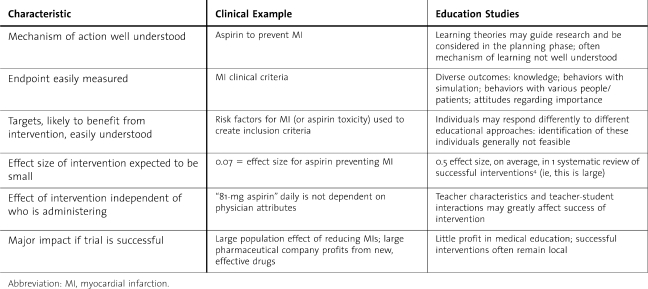
When should an RCT be used in education experiments? According to Norman,6 randomization is most useful in examining relatively standardized interventions, such as web-based learning and, possibly, clinical simulation. He recommends that randomization be considered when (1) prior observational studies support the hypothesis; (2) the mechanism of learning is understood; (3) the outcome of the intervention is easily measured and accepted as related to the intervention; (4) the subgroups likely to benefit from the intervention are also easily identified; (5) the effect size of the intervention is small; and (6) the results from the trial may have a large impact, to justify the costs of an RCT6 (table 2). These criteria are not often satisfied in medical education studies.
Table 2.
Randomized Controlled Trials in Clinical Research Versus Education Research6
Even in clinical research, RCTs are most helpful for therapeutic trials, rather than for risk factor identification or prognosis.6 Likewise in education research, there are research questions for which randomization will be inappropriate: residents cannot be randomly assigned to whether they are from rural versus urban areas, married, or female. Studies of predictive factors and career choices will need cohort, case-control, and case-series designs. In summary, randomization in medical education research is not a cure and not the best method for many research hypotheses.
Alternatives to Randomization
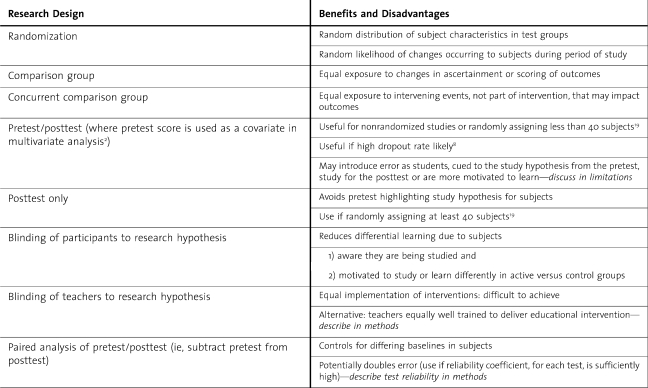
While qualitative research is an obvious starting point for many educational questions, this valuable method will be discussed in a future editorial, while we focus here on quantitative methods (table 3). Nonrandomized methods are common in education research and considered by experts as not inferior to RCTs. In systematic reviews, Best Evidence in Medical Education groups grade the strength of articles on several factors, but not whether the study was randomized.11
Table 3.
Choosing a Quantitative Education Research Design
Perhaps a more relevant clinical research model for educators is the “pragmatic trial.”12 In a pragmatic trial, 2 or more medical interventions are compared in real-world practice. Patients are heterogeneous from a wide variety of practice settings, nonblinded, and may choose to switch treatments. Of note, nonadherence, a major threat to useful findings from pragmatic clinical trials, may not be as problematic in educational studies. However, a much greater number of subjects are usually needed to determine true differences (or equivalence) among interventions, which will present a challenge for education researchers, as the structure and funds for large multi-institution studies are at present scarce. The pragmatic trial paradigm has been suggested for patient safety research, in which context is also a critical variable.13 As considerable overlap exists, examining successful research designs in patient safety research may yield insights for education research.
Borrowing from epidemiology research methods, observational designs can be cross-sectional or longitudinal. Longitudinal studies may use ongoing surveillance or repeated cross-sectional methods to measure change over time.14 To strengthen these research designs, one must include a comparison group. The comparison group may be a concurrent cohort or matched “case-control” format. These studies may be prospective (assembled, described, and followed forward) or retrospective, if sufficient past data on key variables are available.
One of the most common designs noted in original research submissions to the Journal of Graduate Medical Education is the single group design. Without a comparison group, this design may suggest hypotheses for future study, but will not generate firm conclusions. Potential alternatives include the use of historical controls—with inherent concerns about significantly different cohorts—and crossover alternate rotation designs. Residents may be assigned to the intervention on alternate rotations, with full discussion of potential bias in the assignments.15 A repeated measures crossover design is valuable in many nonrotation-based interventions as well.
BOX Suggestions to Strengthen Quantitative Research Methods
Adequate sample size
More than 1 iteration of the intervention
Multiple sites
Low dropout rates/high participation rates
Comparison group—describe thoroughly
Crossover
Historical
Different site with usual teaching, without new intervention
Comparison group receives an active intervention
Residents not cued to “new” approach
No pretest unless already part of rotation/experience
Ensure equal application of intervention
Training manual or other method, for teachers
Rich discussion of potential bias in study
Use limitations to fully explore alternatives to original hypothesis
Next steps: more rigorous methods to confirm findings
Validity Concerns With RCT and Non-RCT Research
When the priority is to find and publish positive results, less consideration may be given to the causes of the differences observed.3 The key question is whether the differences are due to the intervention or to potential bias. Experts assert that often it is confounders that cause the positive results in education studies, rather than the intervention itself.3,16 If these confounding factors are discussed thoroughly, important insights may result and actually provide more enlightenment than the “positive findings.” When nonrandomized, noncontrolled designs are used, Colliver and McGaghie emphasize that the potential “threats to validity” thus introduced must be discussed thoroughly in “a central place in the study” rather than as a perfunctory list in the limitations section.16 Without this meticulous analysis of potential confounding variables, important research questions are missed and overinterpretation of results is common.
Researchers are often faced with the problem of small sample sizes. Several iterations of the intervention and data collection may be necessary to obtain sufficient numbers of subjects, for firm conclusions. In research submissions to the Journal of Graduate Medical Education, this potential solution is often overlooked. Some researchers, faced with a small number of subjects to study, may forgo a control group: all subjects receive the intervention. These are often termed “show and tell” studies, reports of a single iteration that happens to find a positive result.
While randomization is not necessary, a comparison group is essential in education research. Sometimes teachers initiate curriculum improvement or new mandated requirements first and later decide to publish as a research study.17 If the intervention involved all available subjects, a delayed search for a comparison group may be difficult, yet not impossible. Any comparison group is better than none, but dissimilar comparison groups may introduce a large degree of bias. While these descriptive studies may generate useful hypotheses and stimulate key discussions, more rigorous methods will be needed to build evidence in favor of the intervention and should be the “next step” for alert researchers.
Summary
While useful in some situations, randomization is not the “gold standard” for medical education research. More important is that decisions regarding methodology precede the intervention, that adequate numbers of subjects and iterations are used, that a comparison group is included, and that limitations are addressed in a thoughtful, thorough manner.
In addition, the literature demonstrates quite definitively that medical learners will learn whatever we teach and also may supplement any teaching deficits to meet certification requirements.18 Thus, to increase our understanding of effective educational interventions, a new educational intervention should be compared to another effective intervention. Unlike clinical research, a placebo arm is rarely helpful. Comparing the new educational intervention to “usual” practices is productive as long as students are not “cued” to the novelty of the research arm—which may enhance (or negatively bias) their learning—and the usual practices are well described.
Whether randomized or nonrandomized, medical education studies must carefully analyze sources of bias—unforeseen confounding variables—to explain the observed results and ensure that subsequent researchers will find these results reproducible. Rather than an obligatory listing of these potential sources of error in the discussion, researchers will enhance existing knowledge through a careful and detailed analysis of sources of bias that may have affected the results.3 Equally important, research designs must include a control group, concurrent if possible, to ensure equal likelihood of exposure to nonintervention events that could bias the results.
If educators begin to work together, more collaborative, multi-institutional projects, perhaps akin to “pragmatic trials,”12 may be produced in the future. This is likely to add substantially to our understanding of effective resident education.
References
- 1.Cronbach LJ. Designing Evaluations of Educational and Social Problems. San Francisco, CA: Jossey-Bass; 1982. [Google Scholar]
- 2.Stolberg HO, Norman G, Trop I. Fundamentals of clinical research for radiologists: randomized controlled trials. Am J Roentgenol. 2004;183:1539–1544. doi: 10.2214/ajr.183.6.01831539. [DOI] [PubMed] [Google Scholar]
- 3.Regehr G. It's NOT rocket science: rethinking our metaphors for research in health professions education. Med Educ. 2010;44:31–39. doi: 10.1111/j.1365-2923.2009.03418.x. [DOI] [PubMed] [Google Scholar]
- 4.Eva KW. Broadening the debate about quality in medical education research. Med Educ. 2009;43:294–296. doi: 10.1111/j.1365-2923.2009.03342.x. [DOI] [PubMed] [Google Scholar]
- 5.Norman G. RCT = results confounded and trivial: the perils of grand educational experiments. Med Educ. 2003;37:582–584. doi: 10.1046/j.1365-2923.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- 6.Norman G. Is experimental research passé. Adv Health Sci Educ Theory Pract. 2010;15(3):297–301. doi: 10.1007/s10459-010-9243-6. [DOI] [PubMed] [Google Scholar]
- 7.Prideaux D. Researching the outcomes of educational interventions: a matter of design. BMJ. 324:126–127. doi: 10.1136/bmj.324.7330.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook DA, Beckman TJ. Reflections on experimental research in medical education. Adv Health Sci Educ Theory Pract. 2010;15(3):455–464. doi: 10.1007/s10459-008-9117-3. [DOI] [PubMed] [Google Scholar]
- 9.Reed DA, Beckman TJ, Wright SM, Levine RB, Kern DE, Cook DA. Predictive validity evidence for medical education research study quality instrument scores: quality of submissions to JGIM's medical education special issue. J Gen Intern Med. 2008;23:903–907. doi: 10.1007/s11606-008-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berliner DC. Educational research: the hardest science of all. Educ Researcher. 2002;31:18–20. [Google Scholar]
- 11.Harden RM, Grant J, Buckley G, Hart IR. BEME guide No. 1: best evidence medical education. Med Teach. 1999;21:553–562. doi: 10.1080/01421599978960. [DOI] [PubMed] [Google Scholar]
- 12.Ware JH, Hamel MB. Pragmatic trials—guides to better patient care. New Engl J Med. 2011;364:1685–1687. doi: 10.1056/NEJMp1103502. [DOI] [PubMed] [Google Scholar]
- 13.Clancy CM, Berwick DM. The science of safety improvement: learning while doing. Ann Intern Med. 2011;154:699–701. doi: 10.7326/0003-4819-154-10-201105170-00013. [DOI] [PubMed] [Google Scholar]
- 14.Carney PA, Nierenberg DW, Pipas CF, et al. Educational epidemiology: applying population-based design and analytic approaches to study medical education. JAMA. 2004;292:1044–1050. doi: 10.1001/jama.292.9.1044. [DOI] [PubMed] [Google Scholar]
- 15.Shea JA, Arnold L, Mann KV. RIME perspective on the quality and relevance of current and future medical education research. Acad Med. 2004;79:931–938. doi: 10.1097/00001888-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Colliver JA, McGaghie AC. The reputation of medical education research: quasi-experimentation and unresolved threats to validity. Teach Learn Med. 2008;20:101–103. doi: 10.1080/10401330801989497. [DOI] [PubMed] [Google Scholar]
- 17.Gruppen LD. Improving medical education research. Teach Learn Med. 2007;19:331–335. doi: 10.1080/10401330701542370. [DOI] [PubMed] [Google Scholar]
- 18.ten Cate O. What happens to the student: the neglected variable in educational outcome research. Adv Health Sci Educ Theory Pract. 2001;6(1):81–88. doi: 10.1023/a:1009874100973. [DOI] [PubMed] [Google Scholar]
- 19.Fraenkel JR, Wallen NE. How to Design and Evaluate Research in Education. New York, NY: McGraw-Hill; 2003. [Google Scholar]