Abstract
Mesolimbic brain-derived neurotrophic factor (BDNF) is implicated in sustained behavioral changes following chronic social stress, and its depletion may reduce susceptibility to such behavioral alterations. Enhanced mesolimbic BDNF is proposed as pro-depressive and anhedonic, while depleting ventral tegmetal area (VTA) BDNF increases weight by enhancing hedonic eating. Here, we questioned whether depletion of VTA BDNF would alleviate social defeat stress-induced deficits in weight regulation, or affect social behavior in the presence or absence of social stress. Male Sprague-Dawley rats received bilateral intra-VTA infusions of adeno-associated virus (AAV) vectors containing shRNA against BDNF or a control virus. Three weeks later, rats underwent 4 episodes of social defeat stress involving exposure to an aggressive Long-Evans resident rat, or control handling every third day. Depleted VTA BDNF conferred resistance to the deficient weight regulation normally observed during intermittent social defeat stress, and enhanced long-term weight gain regardless of stress history. In addition, social approach and avoidance behavior towards a novel social target were measured 7 weeks after stress. Social defeat stress chronically reduced social behavior, whereas depletion of VTA BDNF chronically increased social behavior. Our results reveal that depletion of VTA BDNF alleviates some consequences of intermittent social defeat stress, enhances social behavior, and may contribute to weight gain. These data implicate VTA BDNF in protracted behavioral responses to stress, social stimuli, and weight regulation.
Keywords: ventral tegmental area, brain-derived neurotrophic factor, mesolimbic, feeding, dopamine, weight
Introduction
Social stress is an ethologically salient stressor which has played a significant role in the evolution of behavior [3]. Such stress exposure can be maladaptive, producing anxiety-like or depressive-like behavioral changes [1, 2, 13, 17, 18, 26].
We previously showed that intermittent social defeat stress chronically enhances VTA BDNF expression in rats [10], which may be important for persistent behavioral alterations. Increased mesolimbic BDNF has been proposed to be pro-depressive [21], and chronic social stress is often used to investigate mechanisms of depression [1, 13]. Indeed in a mouse model of chronic social stress, increased BDNF activity within the mesolimbic dopamine (DA) projection produced susceptibility to depressive-like symptoms, including social avoidance behavior, abnormal weight regulation, and reduced sucrose preference reflecting anhedonia, which was alleviated by reducing mesolimbic BDNF signaling [1, 13]. Likewise, VTA BDNF was recently implicated in mouse food reward in a study showing that reducing VTA BDNF selectively increased hedonic eating [6]. Because increased mesolimbic BDNF is linked to depressive-like or anhedonic behavior [13], reducing VTA BDNF may counteract this.
It should be noted that not all models of social stress produce depressive-like behavior; intermittent and continuous social stress exposure in rats produce opposite effects on VTA BDNF [10, 19], and on behaviors such as drug responsiveness [7, 11, 15, 20]. Specifically, continuous social stress reduces both VTA BDNF expression and responsiveness to psychostimulants, whereas both are enhanced by intermittent social stress exposure. Thus, continuous and intermittent social defeat stress may produce phenotypes that model different behavioral conditions and thus different underlying mechanisms.
Additionally, most studies investigating mesolimbic BDNF and social stress have utilized mouse models. Drawing similarities between laboratory rats and mice regarding conspecific aggression and defense warrants caution, as differences in behavioral responses to stress, such as defensive freezing, may exist across species [4]. Social aggression and defense are complex behaviors, and inter-species variation of these behaviors suggests the possibility that they are mediated by different mechanisms. Nevertheless, some evidence suggests a similar role for mesolimbic BDNF in rats and in mice, whereby increasing mesolimbic BDNF enhances learned helplessness, a depressive-like behavior, and decreasing BDNF signaling by tyrosine kinase receptor B (TrkB) inactivation in the nucleus accumbens has the opposite effect [9]. However, this was observed in the absence of stress, and may therefore not accurately reflect mechanisms underlying social stress. As a result, it is necessary to determine whether mesolimbic BDNF plays a similar role in the context of social stress.
In this study, we use local virus-mediated depletion of BDNF to investigate the role of VTA BDNF in the effects of intermittent social defeat stress on altered short-term weight regulation during stress and long-term effects on social behavior. An additional aim was to assess whether VTA BDNF depletion affects general, long-term weight regulation.
Materials and Methods
Viral constructs
Vectors, packaged in plasmids providing AAV2 replicase and AAV9 capsid functions, and a third plasmid encoding Adenovirus helper functions (pHelper; Stratagene, La Jolla, CA), were co-transfected into AAV-293 cells (Stratagene), molar ratio 1:1:1. Vector plasmids, wherein shRNAs were flanked between a hybrid chicken beta-actin promoter with CMV-IE enhancers, and bGH polyA, were initially provided by Ronald Klein, LSUHSC. shRNA was under control of a Pol III murine U6 promoter. The cDNA sequence was ACGGTCACAGTCCTGGAGAAATTTGACGGTCACAGACCTGGAGAAAT TCAAGAGATTTCTCCAGGACTGTGACCGTTTTTTCTAGAAAAAACGGTCACAGTCCT GGAGAAATCTCTTGAATTTCTCCAGGTCTGTGACCGT. AAV Vectors containing shRNAs also included coding information for eGFP, controlled by a viral promoter. A similarly packaged AAV-eGFP plasmid was used as a control virus. Cells were harvested 48 hrs post-transfection, and cell pellets were re-suspended in DMEM. Intracellular virus particles were released by three consecutive rounds of freeze-thaw, followed by centrifugation at 13,000 rpm for 10 min. Vector stocks were stored at 80ºC, and titered by real-time PCR (ABI Prism 7700 Sequence Detection System; Perkin-Elmer Applied Biosystems, Foster City, CA). Titers were approximately 1012 DNase Resistant Particles/ml.
Animals
Subjects were male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA and Hollister, CA, USA), weighing 175–200 g upon arrival. Rats were group-housed under a reverse light/dark cycle (12h:12h, lights off at 9:00 h) with unlimited access to food (Purina Rodent Diet, Brentwood, MO, USA) and water, and habituated for one week in their home cages before procedures. On a separate rack in the same room, stimulus male Long-Evans rats (Charles River), termed “residents,” were pair-housed with females for social defeat procedures. Residents were screened for aggressive behavior as described previously [10]. All experimental procedures were approved by the Arizona State University Institutional Animal Care and Use Committee, and conducted in accord with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Surgery
Rats received bilateral intra-VTA infusions of AAV-eGFP-shRNA or AAV-eGFP vectors using stereotaxic surgery under isofluorane gas anesthesia. Viruses were infused with a Hamilton syringe; 0.25–0.5 μl/side for detection of in vivo BDNF depletion, and 0.5 μl each side for behavioral experiments at a rate of 0.05 μl/min; 10° angle, from bregma: AP: −5.1, DV:−8.8, ML:±2.15 [23]. Rats were allowed 3 weeks for full expression of viral products before behavioral procedures. Two additional rats received unilateral intra-VTA injections of AAV-shRNA for later processing with BDNF immunohistochemistry.
Confirmation of viral efficacy
To verify in vivo viral BDNF depletion, one group of rats was euthanized 3 weeks after surgery without behavioral testing. Rats participating in behavioral experiments were euthanized 2 weeks after completion of behavioral experiments (12 weeks after surgery) to avoid potential confounds of acute effects of social behavior testing on VTA BDNF measurements. Following rapid decapitation, brains were removed and quick frozen in 2-methylbutane. Bilateral VTA tissue punches (0.5 mm diameter) were taken in a cryostat at 20°C, then sonicated in 300 μl of lysis buffer (137 m M NaCl, 20 mM Tris, 1.0% NP-40, 10.0% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 0.5 m M sodium vanadate; Fisher Scientific, Hampton, NH); homogenates incubated at 4°C for 30 min and were centrifuged at 12,000 × g for 10 min. The protein concentrations of the supernatants were determined using the Micro-BCA assay kit (Pierce, Rockford, IL), and sandwich-style ELISAs were performed to determine BDNF content using the BDNF Emax ImmunoAssay System kit (Promega Corporation, Madison, WI). BDNF content was interpolated from a standard curve, which was divided by total protein in each sample to determine pg BDNF/μg total protein. Data are presented as percent VTA BDNF expression of control virus-infused rats.
Additionally, 20 μm cryosections just rostral and caudal to tissue punches were taken from rats in behavioral experiments and were visually inspected for GFP fluorescence to confirm continued viral expression throughout behavioral procedures as well as histological accuracy of viral injections.
BDNF Immunohistochemistry
Three weeks after surgery, unilaterally injected rats were anesthetized and perfused transcardially with 10 ml of 10% heparin in 0.1 M phosphate buffered saline (pH 7.4) followed by 200 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Brains were removed and post-fixed for 1.5 h at 4 °C, then placed in graded concentrations of sucrose in 0.1 M PB at 4°C. Twenty μm coronal sections (−5.0 to −5.2 from bregma [23]) were collected in 0.1 M PB, mounted onto glass slides (Superfrost Plus; Fisher Scientific), and stored at −35°C.
During processing, sections were thawed, then washed in 0.05 M potassium phosphate-buffered saline (KPBS), blocked for 1 h in 5% normal goat serum (NGS) and 0.4% Triton X-100 in KPBS, then incubated with anti-BDNF polyclonal antibody (1779 SP; 1:300; Chemicon/Millipore; Temecula, CA) for 48 hours at 4°C. This was followed by 1 h incubation in biotin-conjugated goat anti-rabbit serum (1:40 dilution in NGS, Vectastain ABC kit; Vector Laboratories, Burlingame, CA), washing, 45 min incubation with an avidin–biotin–peroxidase complex, then processing with nickel-intensified DAB using substrate kit (Vector).
Behavioral Procedures
We previously showed that repeated social defeat stress produces molecular (specific to VTA) and behavioral effects apparent between 28 and 60 days after stress termination [10, 22]. In the current study, we examined social behavior 50–51 days after stress (presented as time after surgery in Fig. 1E), which is within this time frame. Weight analyses for stress-induced weight changes were undertaken for only the 10-day stress or handling period, as stress-induced weight changes do no persist after stress termination using this protocol [10]. Long-term effects of depleted VTA BDNF on general weight regulation were assessed 20, 30, and 50 days after surgery in handled rats only to avoid the confound of stress-induced changes in weight regulation in this measure. See Figure 1E for timeline of all behavioral procedures.
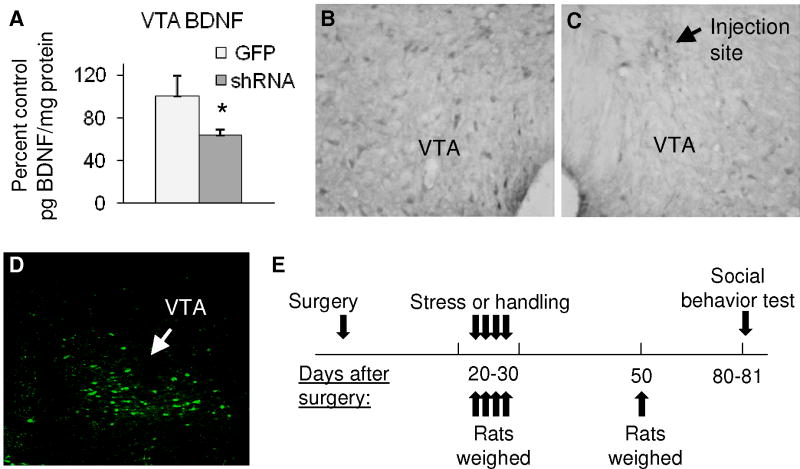
Figure 1. AAV-mediated depletion of BDNF in the VTA and timeline of experiments.
A - Percent control BDNF expression (pg BDNF/mg protein). Infusion of AAV-GFP-shRNA into the VTA reduced BDNF by approximately 36% (*p < 0.05; n = 6–10) as measured by BDNF ELISA in animals euthanized 3 weeks after viral infusion. B - BDNF immunolabeling in the intact VTA and C - near the injection site of AAV-GFP-shRNA 3 weeks earlier (contralateral side of the same section as shown in B); magnification 200X. D - Fluorescence image of GFP expression in rat VTA 3 weeks after viral infusion; magnification 100X. E -Timeline of behavioral experiments.
Resident-intruder social defeat stress
Rats were housed individually throughout stress or handling exposure, which commenced three weeks after surgery. Social defeat stress occurred intermittently, every third day for 10 days as previously described [10]; control rats were handled and weighed on days their counterparts were stressed and weighed [28]. Briefly, the intruder Sprague-Dawley rat was placed into the home cage of the resident Long-Evans rat. The intruder remained under a stainless steel protective cage (25 × 15 × 15 cm) for 5 min, after which the cage was removed, and the resident then displayed aggressive behavior; “defeat” occurred when the intruder exhibited a supine posture for at least 4 sec. Aggressive interactions were 2–5 min in duration. The protective cage was then re-placed over the intruder for an additional 20 min, after which the intruder rat was returned to its home cage.
Body weight analyses
Rats had unlimited access to rat chow and were weighed on each day they were exposed to stress or handling (between days 20 and 30 after surgery), and 50 days after surgery.
Social behavior assay
Seven weeks after social defeat stress (80–81 days after surgery), we assessed social approach/avoidance behavior in a subgroup of rats using a procedure adapted from Berton and colleagues [1]. Testing consisted of exposure to a non-aggressive, unfamiliar rat which was placed under a wire mesh cage (25 × 15 × 15 cm) against the short wall of a clear plastic open field (48.3 × 37.5 × 21.0 cm). Each experimental rat was introduced into the arena and its trajectory was tracked for two 5 min consecutive sessions. During the first session, the arena contained an empty wire mesh cage; conditions were identical in the second session except a novel stimulus male Sprague-Dawley rat was placed into the wire mesh cage. Videotracking data (Clever Sys., Inc., Reston, VA) reflected time spent by the experimental rat in the “interaction zone” (a region between 6.25 and 9 cm around the wire mesh cage) and the “avoidance zone” (16.1 × 37.5 cm on opposite wall from wire mesh cage). The remaining area was considered a neutral zone. Each zone comprised approximately a third of the total arena area. Distance traveled and percentage of time spent, entries, and latency to enter in each zone were recorded.
Statistical Analyses
BDNF ELISA data were subject to t-test for equal or unequal variance where appropriate. For social behavior and weight tracking during stress, two-way ANOVAs were used to analyze data (stress condition × viral condition). Long-term weight tracking data were analyzed in handled rats only using mixed two-way ANOVA, with viral condition as the between subjects factor and time period after surgery as the within-subjects factor. Pair-wise comparisons were used to determine individual group differences where appropriate. All data are presented as mean ± SEM.
Results
Effect of virus on VTA BDNF expression
Viral knockdown significantly reduced VTA BDNF expression by 36 ± 5.2 % (t(12) = 2.46, p < 0.05) 3 weeks later (Fig. 1A). This was qualitatively confirmed by visual observation of reduced VTA BDNF immunolabeling and presence of GFP (Fig. 1B–D). We also confirmed that viral expression continued throughout behavioral experiments by visualization of GFP in the VTA (data not shown). When assessed 14 weeks after surgery (two weeks after completion of all behavioral procedures), shRNA expression lowered BDNF levels by 12.2 ± 6.6 %, although this effect did not reach statistical significance (p = 0.11). VTA BDNF expression in individual groups was as follows: GFP handled: 10.04 ± 0.81 pg BDNF/mg total protein; GFP stressed: 11.16 ± 0.61; shRNA handled: 9.20 ± 1.28; shRNA stressed: 9.41 ± 0.88.
Effect of VTA BDNF depletion on body weight regulation
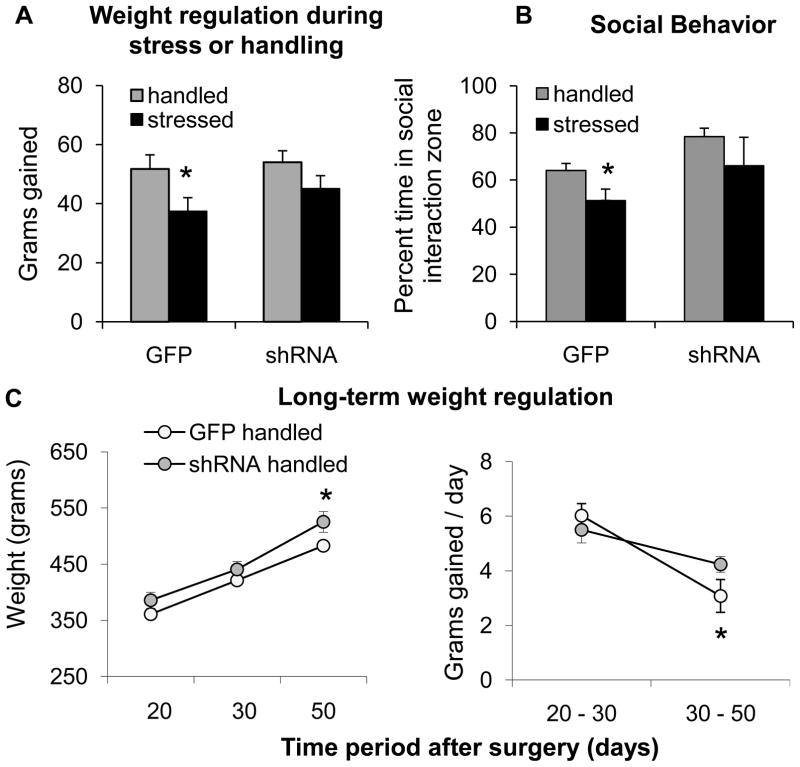
Two-way ANOVA revealed a main effect of stress on weight gained over the 10-day stress period (F(1,45) = 5.89, p < 0.05, Fig. 2A). Pair-wise comparisons showed that in rats receiving the control virus, stress exposure significantly decreased the amount of weight gained between the first and last day of stress (p < 0.05). This was not the case in rats receiving shRNA virus, in which handled rats gained a similar amount of weight as rats exposed to stress (p = 0.22). ANOVA showed no significant interaction between viral and stress condition; and no main effect of viral infusion alone during the 10-day stress or handling period.
Figure 2. Effect of VTA BDNF depletion on stress-related regulation of weight gain, social behavior, and overall weight regulation.
A – Grams gained between first and last day of stress or handling (10 days) in rats receiving either control (GFP) or shRNA viral infusion into the VTA followed by stress or handling (*p < 0.05 vs. GFP handled; n = 10–14 per group). B – Percentage of time spent in social interaction zone (*p < 0.05 vs. GFP handled; n = 5–14 per group). C - Long-term body weight regulation of handled rats receiving either control (GFP) or shRNA virus intra-VTA infusion. Data represent overall weight in grams (left; *p < 0.05 vs. GFP handled) and rate of weight change over time (grams gained/day) after surgery (right; *p < 0.05 vs. 20–30 days after surgery; n = 10 per group).
For long-term weight tracking, data reflect both the total weight in grams of rats and rate of weight gain in grams gained/day in handled rats only in order to determine effect of VTA BDNF depletion on general long-term weight regulation. For total weight, two-way ANOVA showed a main effect of time on weight gain (F(1,18) = 405.5, p < 0.05; Fig. 2C, left). ANOVA also revealed a main effect of virus approaching significance (F(1,18) = 3.56, p = 0.075). There was no significant interaction between time and viral factors. Pair-wise comparisons showed that rats receiving shRNA were heavier than those receiving the control virus at 50 days (p < 0.05), though not 20 or 30 days after surgery. These data indicate that rats receiving intra-VTA shRNA gained more weight at longer time-points after surgery. Data for rate of weight gain was consistent with this finding; two-way ANOVA showed an interaction between viral condition and rate of weight gain approaching significance (F(1,18) = 3.02, p = 0.1; Fig. 2C right), and a main effect of time on weight gain (F(1,18) = 19.03, p < 0.05), with no main effect of virus. Pairwise comparisons revealed that rats receiving the control virus gained significantly less weight per day over time (p < 0.05), whereas rats receiving the shRNA virus maintained their initial rate of weight gain. Additionally, the rate of weight gain of rats receiving shRNA tended to be higher than those receiving the control virus 30–50 days after surgery (p = 0.08).
Effect of VTA BDNF depletion on social behavior
Opposing main effects of both stress exposure and VTA BDNF depletion on social behavior were revealed by two-way ANOVA (Fig. 2B). Stress exposure reduced (F(1,34) = 4.67, p < 0.05), whereas BDNF depletion increased, time spent in the social approach zone (F(1,34) = 6.26, p < 0.05). Two-way ANOVA showed no significant interaction, though pair-wise comparisons showed that stress exposure reduced social approach behavior (p < 0.05) in rats receiving control virus, whereas social approach did not differ significantly between handled and stressed rats after BDNF depletion (p = 0.21). Pair-wise comparisons also showed that in both handled and stressed rats, shRNA tended to increase time spent in the interaction zone (p = 0.08 and 0.09, respectively). No differences were observed in any other measure of social behavior or locomotion, although rats previously exposed to stress showed reduced locomotor activity regardless of viral condition (p < 0.05, data not shown) during the initial 5 min period in the absence of a novel rat. Nevertheless, locomotor activity was similar across groups during the social behavior test.
Discussion
The results reveal that depletion of BDNF in the VTA partially attenuated weight deficits accompanying repeated social defeat stress exposure, increased social behavior, and enhanced long-term weight gain.
The present findings demonstrate that repeated social defeat stress rapidly produces transient deficits in weight gain, an effect which was partially normalized by depletion of VTA BDNF. Because reduced VTA BDNF from the viral depletion was already present at the onset of stress exposure, our data suggest that lower VTA BDNF level before or during social defeat stress exposure may augment stress resilience. This is similar to observations from a mouse model of continuous social defeat stress [13]. The results could be clinically relevant, because enhanced BDNF might increase stress responsiveness in humans. The single nucleotide polymorphism resulting in a val66met substitution in the BDNF peptide sequence elevates BDNF release [8], and men with the 66val allele have lower salivary cortisol response to social stress exposure [27]. Thus, possessing the val66met polymorphism that results in less BDNF release may confer stress resilience.
We found that all handled rats with depleted VTA BDNF gained more weight at longer time-points after surgery. This is consistent with the hyperphagia and obesity observed when BDNF is globally depleted [12, 16, 25], and attenuated weight gain seen when BDNF is infused intracranially [14, 24]. Food restriction partially alleviates obesity in BDNF knockout mice [5, 12], suggesting that food intake and not physiological dysfunction mediates this effect. Although hypothalamic BDNF dysregulation underlies most of these weight effects [25, 29], recent work implicates VTA BDNF in hedonic food reward. Specifically, VTA BDNF depletion in mice increases selective consumption of high fat food and produced weight gain [6]. Thus, our findings are in line with a role of VTA BDNF in regulation of hedonic eating.
We also noted reduced social approach behavior persisting for at least 7 weeks in rats exposed to repeated social defeat stress. Reduced social interaction has been demonstrated after continuous social defeat stress in mice [1], suggesting that social behavior is affected similarly by mild and severe stress and in both rats and mice. Our data show that reducing VTA BDNF enhanced social behavior regardless of stress history, which is consistent with the relationship between mesolimbic BDNF and social behavior demonstrated for continuously defeated mice [1]. From our work alone, we cannot determine whether reduced VTA BDNF before or during social behavior was the crucial modulating factor. However, together, these findings indicate that stress-induced changes in social behavior may be particularly sensitive to prior stress and utilize similar underlying mechanisms across rodent species. In addition, it should be noted that the present paradigm of intermittent social defeat stress produced persisting effects on social behavior. We have shown that the same social stress procedure also enhances drug responsiveness after a similar time period [22], which suggests that even relatively minor exposure to social stress can have protracted behavioral consequences.
A final point is our observation that VTA BDNF depletion increased social behavior in all rats, regardless of prior stress exposure. Other depressive-like behaviors such as learned helplessness are alleviated by reduced mesolimbic BDNF signaling in non-stressed animals. For example, disruption of BDNF signaling by TrkB inactivation in the NAc reduces depressive-like behavior in rats with no stress history [9]. Together with our data, this suggests that depressive – like behavior, which may include social behavior, exists on a continuum which may be positively or negatively affected by fluctuations in mesolimbic BDNF, regardless of stress history.
Overall, our data implicate VTA BDNF in weight regulation during stress, social behavior, and general weight regulation, which is consistent with the proposed role of BDNF in the VTA-NAc projection plays in depressive-like behavior [21]. The persistence of stress-induced effects on reducing social behavior is particularly noteworthy. Furthermore, our findings suggest that VTA BDNF may play a broader role in the motivational value of socially relevant stimuli. It is not surprising that, given the role of BDNF in neuroplasticity, dynamic changes of BDNF in the mesolimbic system are relevant to vulnerability to stress responsiveness across rodent species, whether the stressor is mild or severe.
Highlights.
Social defeat stress chronically reduced social behavior, while depletion of brain-derived neurotrophic factor (BDNF) in the ventral tegmental area (VTA) chronically increased social behavior
Depleted VTA BDNF conferred resistance to deficient weight regulation in rats exposed to intermittent social defeat stress
Depleted VTA BDNF increased weight regardless of stress history
Acknowledgments
We would like to acknowledge Dr. Caroline Bass and Xianghui Ren from the Harvard Institutes of Medicine for assistance in constructing viral vectors. This work was supported by USPHS awards: F31DA022830 (SF), DA024817 (EMN), DA026451 (EMN) and MH073930 (RPH).
Abbreviations
- AAV
adeno-associated virus
- BDNF
brain-derived neurotrophic factor
- DA
dopamine
- GFP
green fluorescent protein
- KPBS
potassium phosphate-buffered saline
- PB
phosphate buffer
- TrkB
Tropomyosin-related kinase B
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard R, Blanchard D. Anti-predator defensive behaviors in a Visible Burrow System. J Comp Psychol. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard R, McKittrick C, Blanchard D. Animal models of social stress: Effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Horm Behav. 2003;44:161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 5.Coppola V, Tessarollo L. Control of hyperphagia prevents obesity in BDNF heterozygous mice. Neuroreport. 2004;15:2665–2668. doi: 10.1097/00001756-200412030-00022. [DOI] [PubMed] [Google Scholar]
- 6.Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci. 2010;30:2533–2541. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covington HE, Miczek KA. Repeated social defeat stress, cocaine, or morphine: Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- 8.Egan M, Kojima M, Callicott J, Goldberg T, Kolachana B, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger D. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell. 2003;24:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 9.Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Fanous S, Hammer RP, Jr, Nikulina EM. Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience. 2010;167:598–607. doi: 10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horger B, Iyasere C, Berhow M, Messer C, Nestler E, Taylor J. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kernie S, Liebl D, Parada L. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1299–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan V, Han M, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Lapchak P, Hefti F. BDNF and NGF treatment in lesioned rats: effects on cholinergic function and weight gain. Neuroreport. 1992;3:405–408. doi: 10.1097/00001756-199205000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Lu L, Dempsey J, Liu S, Bossert J, Shaham Y. A single infusion of BDNF into the ventral tegmental area induces long-lasting potentiation of cocaine-seeking after withdrawal. J Neuro. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyons W, Mamounas L, Ricaurte G, Coppola V, Reid S, Bora S, Wihler C, Koliatsos V, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meerlo P, Overkamp G, Daan S, Koolhaas VDHRHJ. Changes in behaviour and body weight following a single or double social defeat in rats. Stress. 1996;1:21–32. doi: 10.3109/10253899609001093. [DOI] [PubMed] [Google Scholar]
- 18.Menzaghi F, Howard R, Heinrichs S, Vale W, Rivier J, Koob G. Characterization of a novel and potent corticotropin-releasing factor antagonist in rats. J Pharmacol Exp Ther. 1994;2:564–572. [PubMed] [Google Scholar]
- 19.Miczek KA, Nikulina EM, Shimamoto A, Covington HE., 3rd Escalated or Suppressed Cocaine Reward, Tegmental BDNF, and Accumbal Dopamine Caused by Episodic versus Continuous Social Stress in Rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Nikulina EM, Covington HE, Ganschow L, Hammer RP, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos A, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier; Amsterdam: 2007. [Google Scholar]
- 24.Pelleymounter M, Cullen M, Wellman C. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131:229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 25.Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan R, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 26.Ruis M, te Brake J, Buwalda B, De Boer S, Meerlo P, Korte S, Blokhuis H, Koolhaas J. Housing familiar male wild type rats together reduces the long-term adverse behavioral and physiological effects of social defeat. Psychoneuroendocrino. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- 27.Shalev I, Lerer E, Israel S, Uzefovsky F, Gritsenko I, Mankuta D, Ebstein RP, Kaitz M. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrino. 2009;34:382–388. doi: 10.1016/j.psyneuen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol and Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- 29.Unger T, Calderon G, Bradley L, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]