Abstract
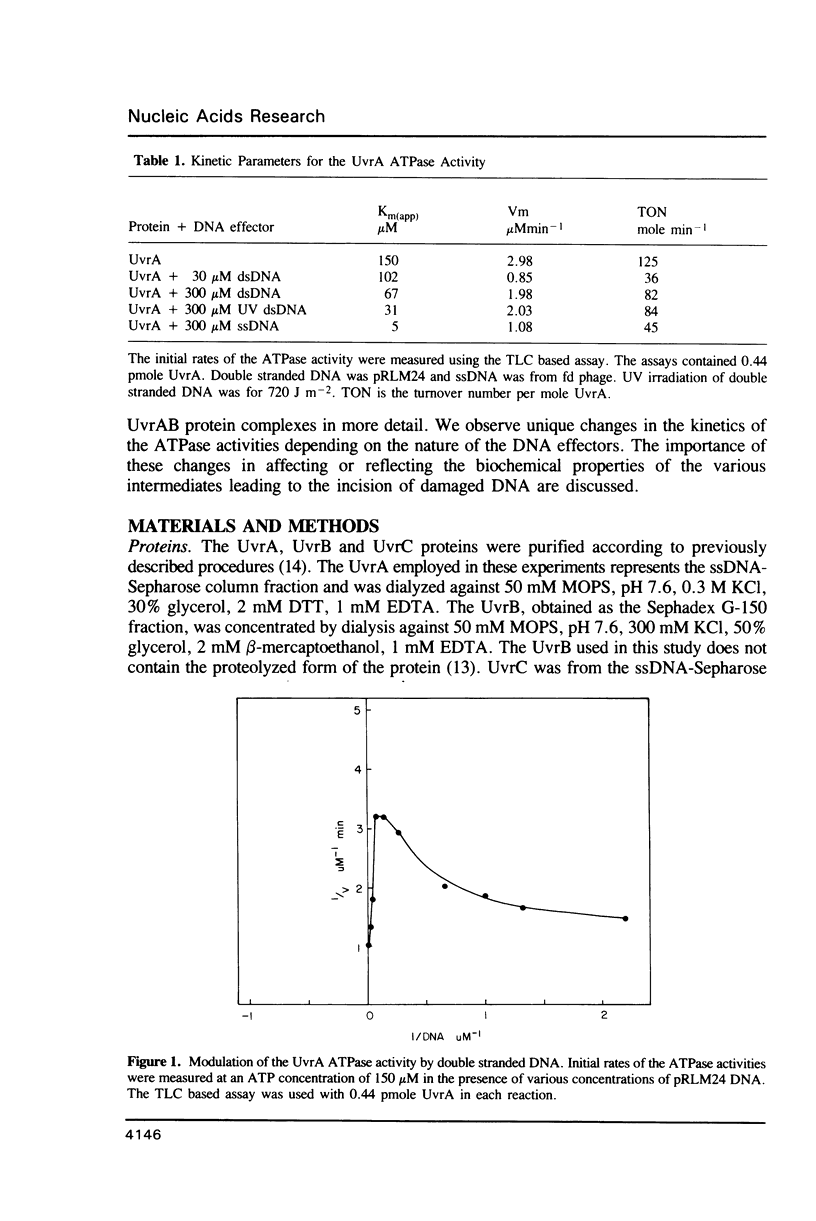
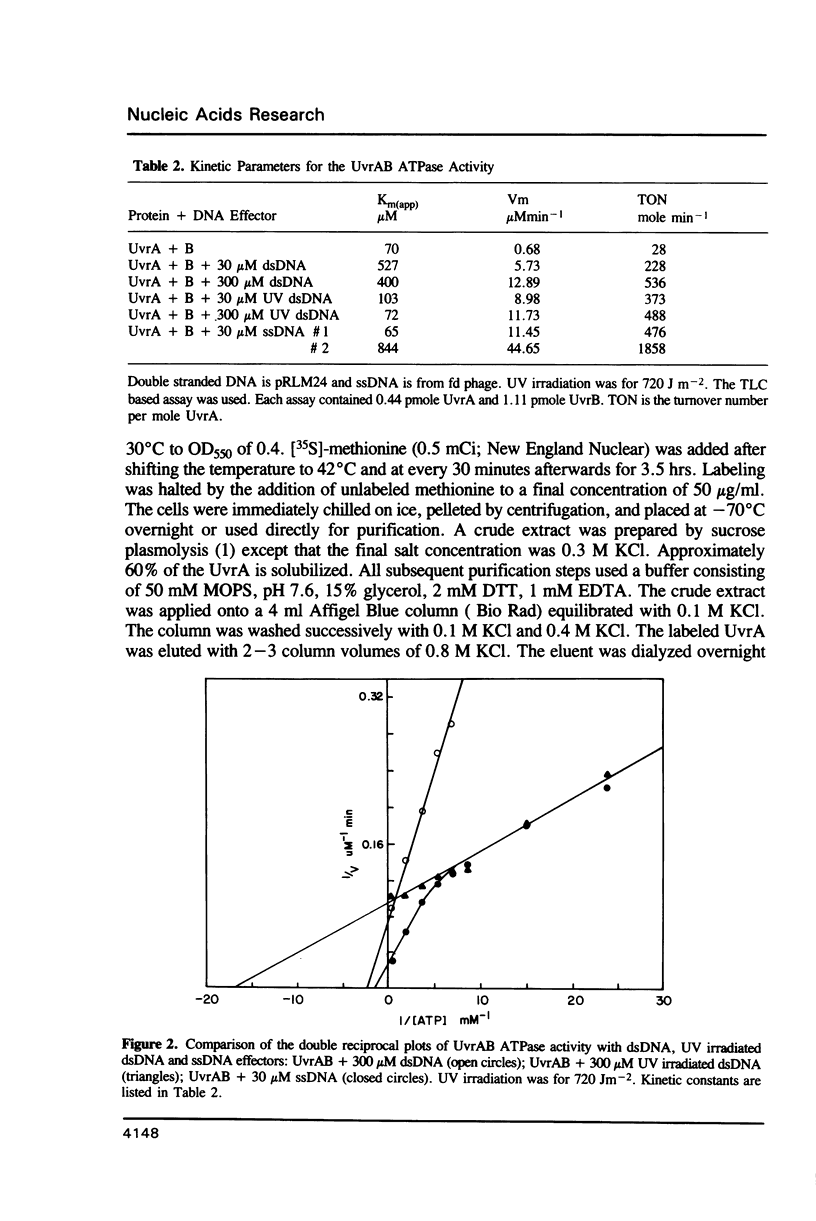
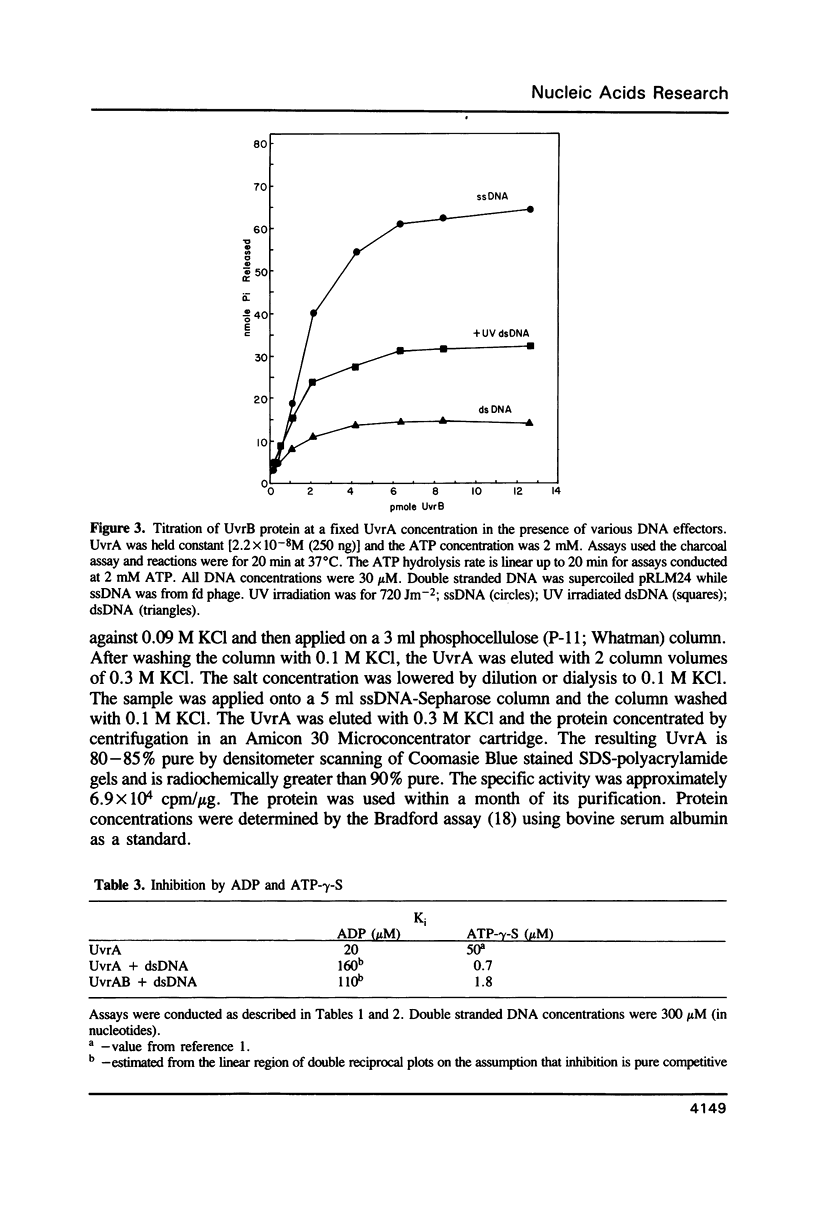
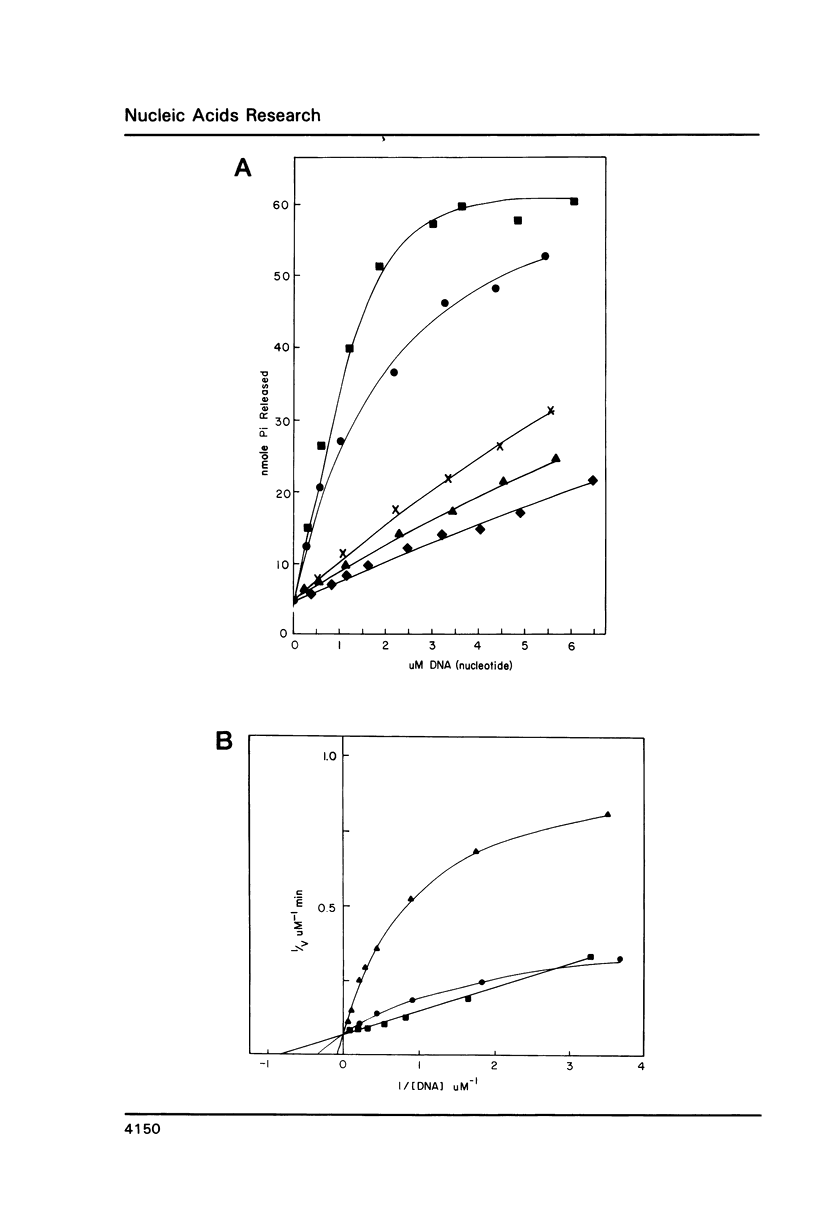
We have analyzed the ATPase activity exhibited by the UvrABC DNA repair complex. The UvrA protein is an ATPase whose lack of DNA dependence may be related to the ATP induced monomer-dimer transitions. ATP induced dimerization may be responsible for the enhanced DNA binding activity observed in the presence of ATP. Although the UvrA ATPase is not stimulated by dsDNA, such DNA can modulate the UvrA ATPase activity by decreases in Km and Vm and alterations in the Ki for ADP and ATP-gamma-S. The induction of such changes upon binding to DNA may be necessary for cooperative interactions of UvrA with UvrB that result in a DNA stimulated ATPase for the UvrAB protein complex. The UvrAB ATPase displays unique kinetic profiles that are dependent on the structure of the DNA effector. These kinetic changes correlate with changes in footprinting patterns, the stabilization of protein complexes on DNA damage and with the expression of helicase activity.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arikan E., Kulkarni M. S., Thomas D. C., Sancar A. Sequences of the E. coli uvrB gene and protein. Nucleic Acids Res. 1986 Mar 25;14(6):2637–2650. doi: 10.1093/nar/14.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D. J., Popoff S., Sancar A., Rupp W. D. Reactions of the UVRABC excision nuclease with DNA damaged by diamminedichloroplatinum(II). Nucleic Acids Res. 1985 Oct 25;13(20):7395–7412. doi: 10.1093/nar/13.20.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caron P. R., Grossman L. Involvement of a cryptic ATPase activity of UvrB and its proteolysis product, UvrB* in DNA repair. Nucleic Acids Res. 1988 Oct 25;16(20):9651–9662. doi: 10.1093/nar/16.20.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Sancar A. Sequences of Escherichia coli uvrA gene and protein reveal two potential ATP binding sites. J Biol Chem. 1986 Apr 15;261(11):4895–4901. [PubMed] [Google Scholar]
- Kacinski B. M., Sancar A., Rupp W. D. A general approach for purifying proteins encoded by cloned genes without using a functional assay: isolation of the uvrA gene product from radiolabeled maxicells. Nucleic Acids Res. 1981 Sep 25;9(18):4495–4508. doi: 10.1093/nar/9.18.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. C., Alberts B. M. Characterization of the DNA-dependent GTPase activity of T4 gene 41 protein, an essential component of the T4 bacteriophage DNA replication apparatus. J Biol Chem. 1981 Mar 25;256(6):2813–2820. [PubMed] [Google Scholar]
- Maxwell A., Craigie R., Mizuuchi K. B protein of bacteriophage mu is an ATPase that preferentially stimulates intermolecular DNA strand transfer. Proc Natl Acad Sci U S A. 1987 Feb;84(3):699–703. doi: 10.1073/pnas.84.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Milman G. Expression plasmid containing the lambda PL promoter and cI857 repressor. Methods Enzymol. 1987;153:482–491. doi: 10.1016/0076-6879(87)53073-7. [DOI] [PubMed] [Google Scholar]
- Oh E. Y., Grossman L. Helicase properties of the Escherichia coli UvrAB protein complex. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3638–3642. doi: 10.1073/pnas.84.11.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E. Y., Grossman L. The effect of Escherichia coli Uvr protein binding on the topology of supercoiled DNA. Nucleic Acids Res. 1986 Nov 11;14(21):8557–8571. doi: 10.1093/nar/14.21.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman D. A., Holbrook S. R., Pirkle D. H., Kim S. H. Molecular models for DNA damaged by photoreaction. Science. 1985 Mar 15;227(4692):1304–1308. doi: 10.1126/science.3975615. [DOI] [PubMed] [Google Scholar]
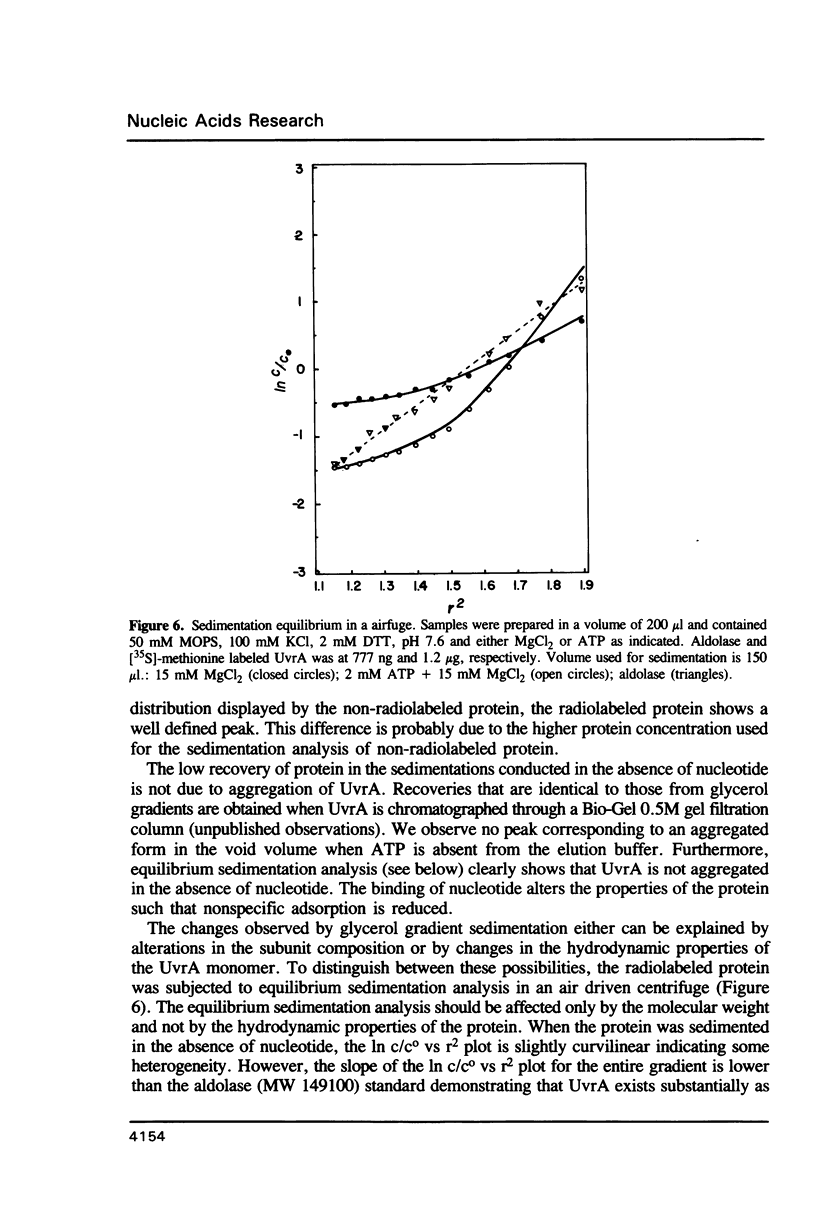
- Pollet R. J., Haase B. A., Standaert M. L. Macromolecular characterization by sedimentation equilibrium in the preparative ultracentrifuge. J Biol Chem. 1979 Jan 10;254(1):30–33. [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L., Nordenskjöld M., Söderhäll S., Jernström B. Strand-break formation in DNA modified by benzo[alpha]pyrene diolepoxide. Quantitative cleavage by Escherichia coli uvrABC endonuclease. Mutat Res. 1983 Jun;112(3):139–145. doi: 10.1016/0167-8817(83)90036-6. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L. Purification and properties of the uvrA protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):988–992. doi: 10.1073/pnas.79.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Kunkel T. A., Casna N. J., Ford J. P., Sancar A. Activities and incision patterns of ABC excinuclease on modified DNA containing single-base mismatches and extrahelical bases. J Biol Chem. 1986 Nov 5;261(31):14496–14505. [PubMed] [Google Scholar]
- Thomas D. C., Levy M., Sancar A. Amplification and purification of UvrA, UvrB, and UvrC proteins of Escherichia coli. J Biol Chem. 1985 Aug 15;260(17):9875–9883. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Hearst J. E., Sancar A. Construction of DNA substrates modified with psoralen at a unique site and study of the action mechanism of ABC excinuclease on these uniformly modified substrates. J Biol Chem. 1986 Oct 25;261(30):14135–14141. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Sancar A., Hearst J. E. DNase I footprint of ABC excinuclease. J Biol Chem. 1987 Sep 25;262(27):13180–13187. [PubMed] [Google Scholar]
- Washabaugh M. W., Collins K. D. Dihydroorotase from Escherichia coli. Purification and characterization. J Biol Chem. 1984 Mar 10;259(5):3293–3298. [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Grossman L. Protein complexes formed during the incision reaction catalyzed by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1986 Mar 25;14(6):2567–2582. doi: 10.1093/nar/14.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Grossman L. Enzymatic properties of purified Escherichia coli uvrABC proteins. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6157–6161. doi: 10.1073/pnas.80.20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Yoakum G. H., Grossman L. The purification of the Escherichia coli UvrABC incision system. Nucleic Acids Res. 1986 Nov 11;14(21):8535–8556. doi: 10.1093/nar/14.21.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]


