Abstract
Objective
Angiotensin converting enzyme 2 (ACE2) is an endogenous counter-regulator of the renin-angiotensin system. The relationship between soluble ACE2 (sACE2), myocardial function, and clinical outcomes in patients with chronic systolic heart failure is not well established.
Methods
We measured sACE2 activity in 113 patients with chronic systolic heart failure (left ventricular ejection fraction [LVEF] ≤ 35%, NYHA class II-IV). Comprehensive echocardiography was performed at the time of blood sampling. We prospectively examined adverse clinical events (death, cardiac transplant, and heart failure hospitalizations) over 34 ± 17 months.
Results
Patients who had higher sACE2 plasma activity were more likely to have a lower LVEF (Spearman’s r= −0.36, p <0.001), greater RV systolic dysfunction (r=0.33, p<0.001), higher estimated pulmonary artery systolic pressure (r=0.35, p=0.002), larger LV end diastolic diameter (r=0.23, p=0.02), and higher plasma NT-proBNP levels (r=0.35, p<0.001). sACE2 was less associated with diastolic dysfunction (r=0.19, p=0.05), and was similar between patients with ischemic and non-ischemic cardiomyopathies. There was no relationship between sACE2 activity and markers of systemic inflammation. After adjusting for NT-proBNP and LVEF, sACE2 activity remained an independent predictor of adverse clinical events (HR=1.7 [95% CI: 1.1 – 2.6], p=0.018).
Conclusions
Elevated plasma sACE2 activity was associated with greater severity of myocardial dysfunction and was an independent predictor of adverse clinical events.
Keywords: Heart failure, ACE2, remodeling, angiotensin
Introduction
Exogenous pharmacological inhibition of the classic renin-angiotensin-aldosterone system (RAAS) has resulted in important clinical progress in the treatment of heart failure, and has highlighted the detrimental effect this pathway plays in heart failure physiology.1 A failure to adequately inhibit the RAAS is associated with increased morbidity and mortality in patients with heart failure.2 As a result, effort has been put forth to further understand the regulation of the RAAS. It was therefore with great interest that a new member of this pathway was recently discovered. Angiotensin converting enzyme 2 (ACE2) is an ACE homologue that acts as a novel endogenous inhibitor of the RAAS, and is thereby protective in heart failure pathogenesis.3
The deleterious effects of the RAAS are primarily related to increased production of angiotensin II (Ang II), which results in numerous pathophysiological sequelae that include promoting hypertension, cardiac hypertrophy and adrenergic activity (as reviewed in 4). ACE2 counteracts the effects of Ang II through two related mechanisms. ACE2 is an integral membrane carboxypeptidase that removes the terminal amino-acid from Ang II,5 the net effect being similar to the combination of ACE inhibitors and angiotensin receptor blocker (ARB) therapy. Interestingly, the degradation product itself is a biologically active peptide [Ang(1-7)] that binds to the Mas receptor, inducing vasodilation through the actions of nitric oxide.6–8 Infusion of Ang(1-7) or Ang(1-7) analogues into post-infarct animals prevents the development of heart failure, indicating activation of a cardioprotective ACE2/Ang(1-7)/Mas axis may possess therapeutic utility.9–11
Clinical studies were initially hampered by the requirement to obtain invasive cardiac tissue samples to measure ACE2. Our group has recently demonstrated that membrane ACE2 can be cleaved into a soluble form (sACE2), and sACE2 activity can be measured in human plasma and can be elevated in patients with heart failure.12 The primary objective of this study is to extend our previous work in demonstrating the clinical significance of plasma sACE2 activity in the failing human heart. In particular, we aim to determine the precise relationship between plasma sACE2 activity and cardiac structure, systolic and diastolic performance, and overall prognosis in patients with chronic systolic heart failure. We hypothesized that elevated sACE2 activity would be observed in patients with worsening cardiac function and may be predictive of long-term clinical outcomes.
Methods
Study design and population
The neurohormonal sub-study of the ADEPT (Assessment of Doppler Echocardiography in Prognosis and Therapy) study has been previously described 13, and was approved by the Cleveland Clinic Institutional Review Board. After informed consent, 113 ambulatory patients with stable, chronic systolic heart failure (left ventricular ejection fraction [LVEF] ≤ 35%, New York Heart Association functional class II to IV) underwent echocardiographic evaluation of systolic and diastolic performance as well as plasma sample collection. Clinical events (all-cause mortality, cardiac transplantation, or heart failure hospitalization) were prospectively tracked for 34 ± 17 by scheduled telephone follow-up and validated by chart review as previously described.12 Approximately 65% of the patients completed the 33 month follow-up. Estimated glomerular filtration rate (eGFR) was calculated using the Modified Diet in Renal Disease equation based on serum creatinine, age, gender, and ethnicity.
All samples were collected using EDTA-plasma tubes, processed and frozen at −80°C until analyzed. Cardiac and inflammatory biomarkers were measured by commercially available assays as previously described (aminoterminal [NT]-proBNP: Roche Elecsys, Indianapolis IN; high-sensitive C-reactive protein [hsCRP]: Dade Behring Inc, Deerfield IL 14; myeloperoxidase [MPO]: CardioMPO II test (PrognostiX Inc, Cleveland Ohio 15).
Transthoracic echocardiography
Comprehensive transthoracic echocardiography was performed using commercially available HDI 5000 (Phillips Medical Systems, Bothell WA) and Acuson Sequoia (Siemens Medical Solutions USA Inc., Malvern PA) machines. Two-dimensional and color Doppler imaging was performed in standard parasternal and apical views. Diastolic indexes (including pulse-wave Doppler, color M-mode, and tissue Doppler imaging) were acquired over 10 consecutive beats using sweep speeds of 50 cm/s and 100 cm/s using previously described techniques. Classification of diastolic stage was determined as follows: Stage I (impaired relaxation) consists of mitral E/A < 1, deceleration time > 220 ms, pulmonary vein S/D < 1, atrial reversal (AR) > 35 cm/s; Stage II (pseudonormal) shows mitral E/A = 1 to 2, pulmonary vein S/D < 1, deceleration time < 220 ms, AR > 35 cm/s; Stage III (restrictive) gives mitral E/A > 2, pulmonary vein S/D < 1, deceleration time < 150 ms, AR > 35 cm/s. Estimates for left atrial pressure were determined from pulmonary vein S/D, E/septal Ea and mitral E/Vp ratios. The LVEF and cardiac volumes were measured using the Simpson biplane method. Measurements were averaged over 3 cycles (5 cycles for atrial fibrillation), and 2 experienced individuals who were blinded from the neurohormonal data made all measurements.
sACE2 enzymatic assay
A specific fluorometric assay to measure sACE2 was performed based on a quenched fluorescent substrate (QFS) (7-methoxycoumarin-4-yl)-acetyl-Ala-Pro-Lys(2,4-dintirophenyl, R&D Systems, Minneapolis MN) protocol previously developed.16 Specificity was determined by pre-incubating plasma for 30 min with the specific human ACE2 inhibitor DX600 (Phoenix Pharmaceuticals, Burlingame CA).17 Patient’s plasma sACE2 activity was determined at 21 hours and expressed as the relative fluorescence units / hr (RFU/hr) of the sample minus the RFU/hr of that sample in the presence of 1 µM DX600 at 1 hour. Those values were normalized to a recombinant ACE2 standard curve (ng/mL, R&D Systems, Minneapolis MN). Intra-assay variability was 6.1 ± 1.7% and inter-assay variability was 20.6 ± 5.5%.
Statistical analysis
Plasma sACE2 activity was non-normally distributed (expressed as median and inter-quartile range [IQR], or as a value derived from receiver operating characteristic analysis [ROC]). Analysis of variance or the Kruskal-Wallis test (for non-normally distributed data) was used to assess differences in continuous clinical variables across sACE2 tertiles according to whether or not the distribution was normal, whereas contingency table analysis was performed to assess differences in clinical proportions across sACE2 tertiles. Normality was assessed by the Shapiro-Wilk W test. Systolic blood pressure, eGFR, NT-proBNP, LV end-diastolic volume index, and LVEF were non-normally distributed, and age and heart rate were normally distributed. The Spearman rank correlation method was used as a nonparametric measure of association for correlations between plasma sACE2 activity and all clinical variables. The odds ratios of having altered systolic or diastolic performances were calculated from multivariate logistic regression across 1st, 2nd, and 3rd tertiles of sACE2 activity with respect to the 1st tertile (odds ratio = 1.0). Kaplan-Meier survival plots were calculated from baseline to time of all-cause mortality, cardiac transplantation, or heart failure hospitalization over a mean follow-up of 33 months. All univariate and multivariate Cox proportional hazard analyses were also calculated with all-cause mortality, cardiac transplantation, or heart failure hospitalization as outcomes, and with plasma sACE2 activity treated as either a categorical variable (Table 3) or continuous variable (Table 3 and Table 4). When sACE2 was used as a categorical variable, we modeled differences in outcomes for patients with values above the ROC-derived sACE2 value relative to below the ROC value. ROC curve analysis was performed to determine the incremental prognostic value of sACE2 activity with NT-proBNP, LVEF and E/septal Ea ratio. A p value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.1 and JMP version 5.1 (SAS Institute Inc., Cary NC).
Table 3.
Prognostic Ability of sACE2 and Traditional Cardiovascular Risk Factors in Predicting Death, Transplantation or heart failure Hospitalizations
| Variable 1 alone | Variable 1 + sACE2† | |||||
|---|---|---|---|---|---|---|
| Variable 1 | AUC | Χ2 | P value | AUC | Χ2 | P value |
| Continuous values | ||||||
| sACE2 (ng/mL) | 0.71 | 15.0 | 0.0001 | - | - | - |
| NT-proBNP (pg/mL) | 0.71 | 5.9 | 0.05 | 0.76 | 17.2 | 0.0002 |
| LVEF(%) | 0.67 | 7.5 | 0.006 | 0.77 | 19.0 | <0.0001 |
| E/septal Ea ratio | 0.74 | 17.0 | <0.0001 | 0.81 | 26.9 | <0.0001 |
| ROC derived values* | ||||||
| sACE2 ≥ 28.3 ng/mL | 0.66 | 10.2 | 0.001 | - | - | - |
| NT-proBNP ≥ 2215 pg/mL | 0.71 | 16.0 | <0.0001 | 0.78 | 22.2 | <0.0001 |
| LVEF ≤ 24% | 0.60 | 3.5 | 0.06 | 0.71 | 13.3 | 0.001 |
| E/septal Ea ratio ≥ 19.5 | 0.72 | 17.2 | <0.0001 | 0.77 | 23.8 | <0.0001 |
Optimal cutoff values were identified by receiver operator characteristic (ROC) analysis for each variable
sACE2 was used as either a continuous variable or as a ROC-derived value (> 28.3 ng/mL) depending on the above grouping
AUC–area under the curve; Χ2–Chi-square
Table 4.
Univariate and Multivariate Cox proportional hazard analyses of adverse clinical events based on plasma sACE2 activity
| Variable | HR (95% CI) | P value |
|---|---|---|
| Log2 sACE2 (ng/mL) (31 events)*,† | 1.92 (1.36 – 2.69) | <0.001 |
| Adjusted for Age (yrs) | 1.86 (1.33 – 2.60) | <0.001 |
| Adjusted for Gender (% Male) | 1.88 (1.32 – 2.65) | <0.001 |
| Adjusted for LVEF (%) | 1.77 (1.21 – 2.55) | 0.003 |
| Adjusted for Log2 NT-proBNP | 1.77 (1.17 – 2.67) | 0.007 |
| Adjusted for eGFR (mL/min) | 1.86 (1.32 – 2.61) | <0.001 |
| Adjusted for Diastolic stage | 1.60 (1.14 – 2.24) | 0.007 |
| Adjusted for E/septal Ea | 1.79 (1.28 – 2.51) | <0.0001 |
| Adjusted for RV dysfunction class | 1.59 (1.10 – 2.29) | 0.014 |
| Adjusted for Tricuspid regurgitation area (cm2) | 1.66 (1.18 – 2.37) | 0.004 |
| Multivariable model (28 events) *,† | ||
| Log2 sACE2 (ng/mL) | 1.67 (1.07 – 2.58) | 0.025 |
| Log2 NT-proBNP | 1.55 (1.01 – 2.33) | 0.04 |
| LVEF (%) | 0.76 (0.50 – 1.14) | NS |
Adverse event rate: Combined end-point of all-cause mortality, transplant or heart failure hospitalizations
Hazard ratios (HR) per 1-SD increments (1-SD for Log2 sACE2=0.94; 1-SD for Log2 NT proBNP=1.94; 1-SD for LVEF=6.01%; 1-SD for Age=13.2 years)
Results
In our study cohort, the mean and median sACE2 plasma activity were 27.8 ± 20.8 ng/mL and 21.7 ng/mL (IQR = 15.8–33.0 ng/mL), respectively. Table 1 demonstrates the baseline clinical and biochemical characteristics of the cohort stratified by tertiles of sACE2 activity, comparing the 3rd to the 1st tertile. Increased sACE2 correlated significantly with LV systolic dysfunction, LV dilatation, and also right ventricular (RV) systolic dysfunction and increased estimated pulmonary artery systolic pressure (Table 1). Plasma sACE2 activity correlated with indices of diastolic dysfunction and elevated estimated LV filling pressures, although this correlation was less robust (Table 2). Utilizing the variables associated with sACE2 activity, both RV systolic dysfunction (β = 0.28, p = 0.008) and LV systolic dysfunction (β = −0.22, p=0.047) remained independently associated with plasma sACE2 levels after multivariate regression analysis (probability to enter or leave p = 0.20). There was no relationship between sACE2 activity and plasma hsCRP or MPO levels (Table 2).
Table 1.
Clinical characteristics based on tertiles sACE2 activity
| Variable | sACE2 Tertile 1 (n=38) |
sACE2 Tertile 2 (n=37) |
sACE2 Tertile 3 (n=38) |
P value |
|---|---|---|---|---|
| sACE2 (ng/mL) | < 17.4 | 17.4 – 28.8 | > 28.8 | - |
| Age (yrs) | 56±12 | 57±14 | 58±14 | NS |
| Gender (% Male) | 68% | 73% | 89% | 0.04 |
| NYHA III/IV (%) | 24% | 35% | 38% | NS |
| Ischemic etiology (%) | 37% | 41% | 49% | NS |
| Heart Rate | 71.1 | 74.0 | 79.6 | 0.007 |
| Systolic Blood Pressure (mmHg) | 112.6 | 113.1 | 108.5 | NS |
| Body Mass Index (kg/m2) | 30±5 | 28±4 | 28±6 | NS |
| Medications (%) | ||||
| Aldosterone Antagonist | 29% | 22% | 35% | NS |
| Loop Diuretic | 68% | 76% | 92% | 0.02 |
| Beta-Blocker | 61% | 53% | 59% | NS |
| ACE-Inhibitor | 82% | 86% | 68% | NS |
| ARB | 15% | 9% | 27% | NS |
| ACE-Inhibitor / ARB | 95% | 97% | 92% | NS |
| Digoxin | 53% | 66% | 59% | NS |
| Echocardiographic indices | ||||
| LVEF (%) | 28±5 | 26±6 | 23±6 | 0.001 |
| LVED volume index (mL/m2) | 101±32 | 114±37 | 119±36 | 0.04 |
| LVES volume index (mL/m2) | 74±27 | 85±27 | 93±33 | 0.02 |
| Diastolic stage = 3 (%) | 29% | 32% | 51% | 0.08 |
| E/Septal Ea ratio | 16±6 | 18±14 | 21±10 | 0.02 |
| RV systolic dysfunction ≥ 2+ (%) | 16% | 11% | 51% | 0.001 |
| PA systolic pressure (mmHg) | 33±12 | 37±14 | 43±16 | 0.01 |
| Mitral regurgitation ≥ 3+ (%) | 8% | 8% | 11% | NS |
| Median NT-proBNP (ng/mL) [IQR] | 652 [275, 2189] | 1549 [484, 2522] | 2004 [689, 4989] | 0.001 |
| eGFR (mL/min/1.73 m2) | 94±46 | 83±34 | 86±38 | NS |
| Co-morbid conditions | ||||
| Diabetes Type II (%) | 32 | 22 | 32 | NS |
| Hypertension (%) | 61 | 46 | 65 | NS |
sACE2=soluble angiotensin converting enzyme 2; NYHA=New York Heart Association. LVEF=left ventricular ejection fraction; LVED=left ventricular end diastolic; LVES=left ventricular end systolic; RV=Right ventricle; PA=pulmonary artery
Table 2.
Correlation between plasma sACE2 activity, biochemical and echocardiographic characteristics for the study population.
| Overall cohort | ||
|---|---|---|
| Variable | Spearman’s correlation |
p-value |
| Age (years) | 0.09 | NS |
| Biochemical Measurements | ||
| NT-proBNP (pg/mL) | 0.35 | <0.001 |
| hsCRP (mg/L) | 0.04 | NS |
| MPO (pM) | 0.09 | NS |
| eGFR (mL/min/1.73m2) | 0.02 | NS |
| Echocardiographic Measurements | ||
| LV ejection fraction (%) | −0.36 | <0.001 |
| LVED diameter | 0.23 | 0.017 |
| LVES diameter | 0.31 | 0.001 |
| Diastolic stage | 0.23 | 0.022 |
| Transmitral DT (ms) | −0.10 | NS |
| Transmitral E (cm/s) | −0.05 | NS |
| Transmitral A (cm/s) | −0.15 | NS |
| E/Septal Ea | 0.19 | 0.047 |
| TDI septal Aa (cm/s) | −0.30 | 0.002 |
| TDI septal Ea (cm/s) | −0.25 | 0.008 |
| TDI septal S (cm/s) | −0.26 | 0.006 |
| Pulmonary vein S/D ratio | −0.10 | NS |
| RV dysfunction class | 0.33 | <0.001 |
| TR velocity (cm/s) | 0.23 | 0.022 |
| PA systolic pressure (mmHg) | 0.35 | 0.002 |
hsCRP–highly sensitive C reactive protein; LVED–Left ventricular end diastolic; LVES–Left ventricular end systolic; TDI–tissue Doppler imaging; RV–right ventricle; TR–tricuspid regurgitation; PA–pulmonary artery; DT–deceleration time; hsCRP-high-sensitivity C-reactive protein; MPO-myeloperoxidase.
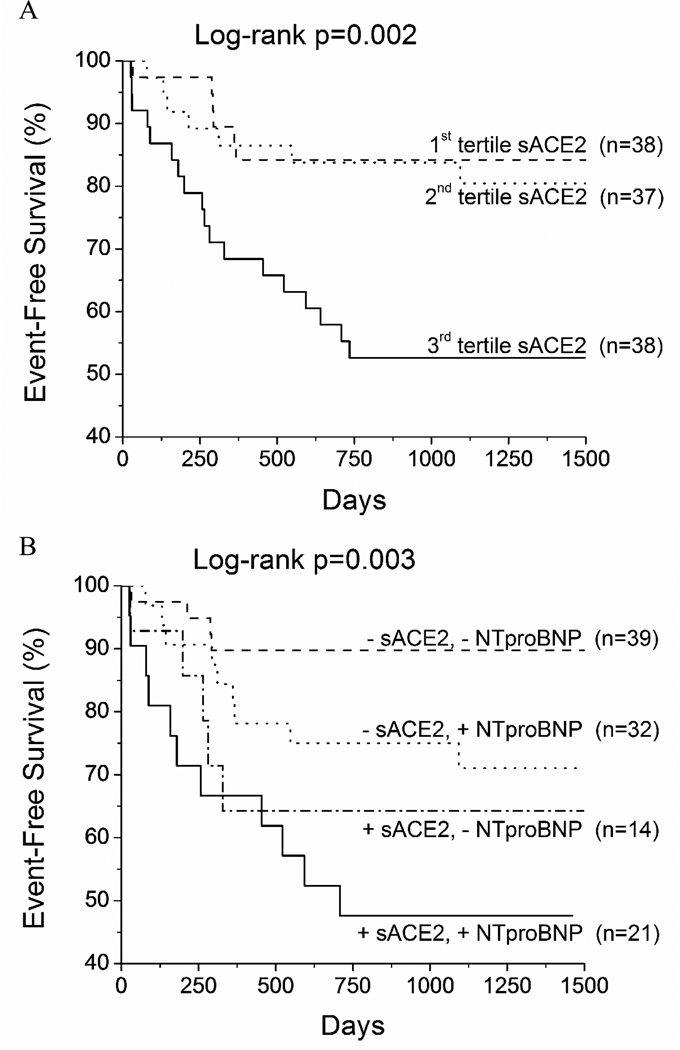
After a mean follow-up time of 34 ± 17 months, 23% of patients experienced death or cardiac transplantation, and 29% experienced the combined end-point of death, transplantation or heart failure hospitalization. Figure 1a demonstrates the long-term adverse clinical event rate for patients with chronic systolic heart failure stratified by tertiles of plasma sACE2 activity. Patients in the highest tertile fared significantly worse than those within the lowest two tertiles. Using ROC curve analysis, a value of 28.3 ng/mL gave an area under the curve = 0.662 (chisquared = 10.2, p = 0.0001). Patients with sACE2 > 28.3 ng/mL had a hazard ratio (HR) = 3.17 (1.56–6.62), p = 0.0015 compared to those below for the combined end-point, and therefore, the ROC-derived value was used for subsequent analysis. Figure 1b compares adverse events rates in patients stratified by plasma sACE2 activity (28.3 ng/mL) and then further stratified by median NT-proBNP levels (1240 pg/mL). Patients with increased sACE2 and NT-proBNP levels had significantly worse clinical outcomes than those with low sACE2 and NT-proBNP levels. Compared with patients with a low likelihood of adverse events (NT-proBNP below the median), those with increased sACE2 activity (>28.3 ng/mL) had a significantly increased event rate [HR = 4.0 (1.1–16.3), p <0.05]. Addition of sACE2 to NT-proBNP, LVEF and E/septal Ea significantly augmented the prognostic accuracy of these traditional prognostic factors, as either continuous or ROC-derived variables (Table 3).
Figure 1. Kaplan-Meier analysis of all-cause mortality, cardiac transplantation, or heart failure hospitalization.
A) Patients were stratified based on tertiles of plasma sACE2 activity (tertile 1: sACE2 < 17.9 ng/mL, tertile 2: 17.9–28.8 ng/mL, tertile 3: >28.8 ng/mL). B) −/+ NT-proBNP = below / above median NT-proBNP (1,240 pg/mL); −/+ sACE2 = below / above ROC-derived value for sACE2 (28.3 ng/mL). sACE2=soluble angiotensin converting enzyme 2. NT-proBNP = Nterminal pro B-type natriuretic peptide.
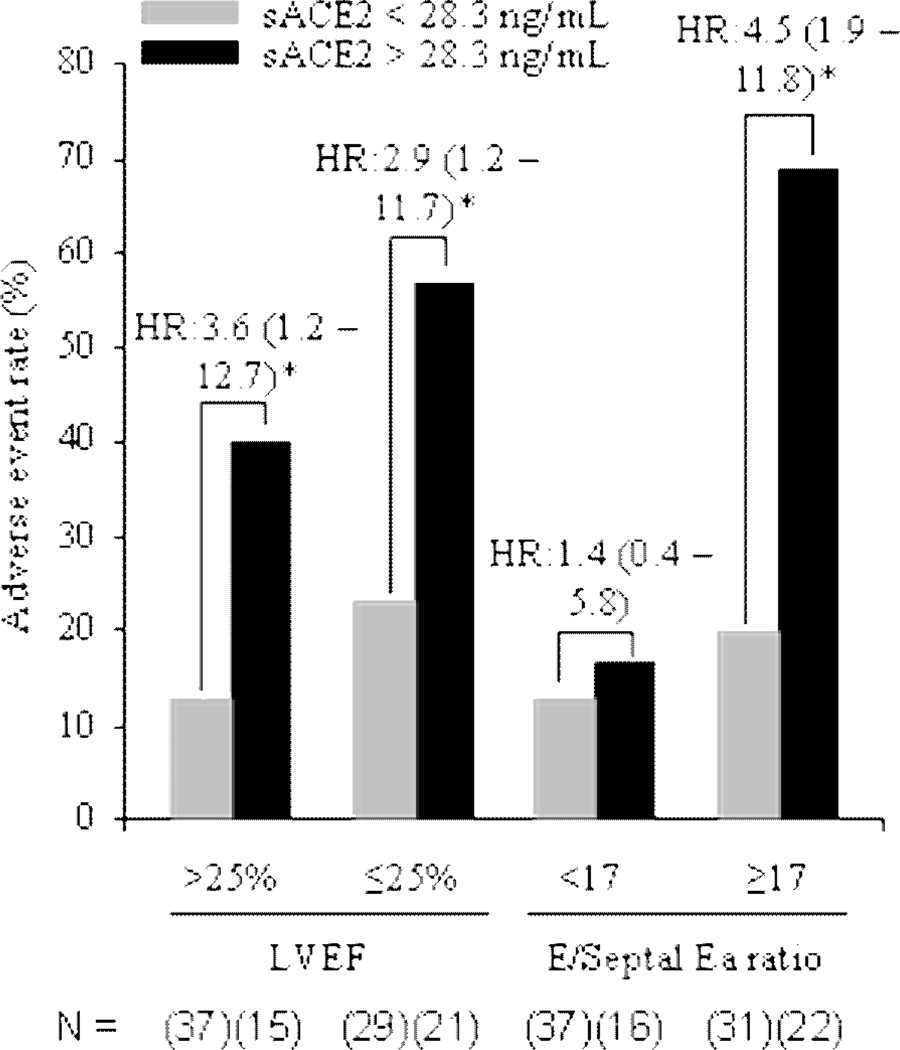
Plasma sACE2 activity continued to predict long-term clinical events even after adjustment for traditional risk factors (Table 4). The prognostic ability of sACE2 was not limited to the combined end-point. All-cause mortality alone was increased in patients with elevated sACE2 activity (HR = 2.17 (1.30–3.59), p=0.003). sACE2 was predictive of adverse events even in patients with both severe systolic dysfunction and a restrictive diastolic filling pattern (Figure 2).
Figure 2. Adverse Event Rate and Corresponding Hazard Ratios for Subgroups.
Adverse event rate is calculated on the combined endpoint of death, heart transplantation and heart failure hospitalizations. Hazard ratios are calculated relative to the group with sACE2 levels below 28.3 ng/mL. Median values are used for left ventricular ejection fraction = (LVEF) and E/Septal Ea ratio; sACE2=soluble angiotensin converting enzyme 2. * P<0.05. n = (15–37).
Discussion
The RAAS plays a crucial role in both regulating normal cardiac function and perpetuating the ultimately detrimental pathways mediating the progression of heart failure. Targeted inhibition at nearly every level has resulted in important clinical progress in the treatment of heart failure, in particular, blockade of Ang II activity. As is common in other essential biological systems, ACE2 likely evolved as a natural counter-regulatory mechanism to limit Ang II over-activation. Our mechanistic demonstration of the potential role of sACE2 in patients with chronic systolic heart failure has three major findings. 1) Increased sACE2 activity is associated with worsening LV and RV systolic function; 2) sACE2 activity has no direct relationship with markers of systemic inflammation; and 3) increased sACE2 activity is an independent predictor of the combined endpoint of death, cardiac transplant, and heart failure hospitalizations. Taken together, our data directly linked the severity of heart failure and impairment of cardiac performance to levels of sACE2 enzymatic activity, reinforcing the potential importance of this counter-regulatory pathway in the development and progression of heart failure.
We have previously demonstrated that plasma sACE2 activity is elevated specifically in patients with a diagnosis of heart failure.12 In our current study, we have expanded our investigation and found the correlation between increased sACE2 activity and LV ejection fraction to be one of the most robust. Similarly, animal studies have demonstrated that increased ACE2 levels are found within the failing LV, and deletion of ACE2 results in a dilated cardiomyopathy.18,19 We also found sACE2 correlated strongly with RV systolic dysfunction. Increased ACE2 activity can be found in the RV, and not in the LV of patients with primary pulmonary hypertension, suggesting compartmentalization of ACE2 production in response to stress.20 Pulmonary hypertension-related RV systolic dysfunction is dependent on pressure overload.21 ACE2 has been shown to be an important cardioprotective factor in response to cardiac dysfunction precipitated by pressure-overload,22 as illustrated by the association between sACE2 activity and estimated pulmonary artery systolic pressure in our study.
Echocardiographic analysis of patients with ACE2 single nucleotide polymorphisms have demonstrated increased septal-wall thickness and cardiac hypertrophy with some variants, although there was no change in diastolic indices noted.23 It is presumed that these polymorphisms either alter expression or enzymatic activity. Diastolic dysfunction begins with the development of cardiac hypertrophy and increased deposition of collagen. Infusion of Ang II produces similar biochemical changes, and delivery of either ACE2 or Ang(1-7) can prevent both hypertrophy and collagen deposition.8,24 Deletion of the Mas receptor results in the development of cardiac hypertrophy, suggesting a direct involvement of ACE2 diastolic function.25 As with systolic heart failure, the ACE2/Ang(1-7)/Mas axis may be the primary regulatory step that limits Ang II-induced diastolic dysfunction. It is therefore interesting to note that in contrast with many other cardiac and inflammatory biomarkers, elevated plasma sACE2 activity only correlated modestly with worsening diastolic function in our study cohort.
ACE2 has known cardioprotective properties and is up-regulated in patients with heart failure, although the significance of sACE2 shedding is not yet understood.18,24,26 Plasma sACE2 release may directly parallel membrane expression, with a relatively constant rate of conversion between the membrane and soluble forms. The elevation in sACE2 activity we see in patients with poor outcomes would then be related to an ongoing counter-regulatory response, albeit insufficient, to elevated Ang II levels and heart failure progression. This would be analogous to increased BNP levels seen in patients with worsening HF. sACE2 is ubiquitously present in virtually every sample we have tested, whether patient or healthy control,12 indicating the presence of plasma sACE2 is part of normal physiology. Alternatively, the presence of increased plasma sACE2 activity may be related to the up-regulation of a protease, which cleaves ACE2 from the membrane, and therefore, decreases membrane ACE2 levels within the myocardium. As a result, there would be a further worsening in the myocardial Ang II/Ang(1-7) balance, which is itself associated with cardiac dysfunction.8 It is likely that multiple enzymes cleave ACE2 from the membrane, indicating multiple levels of regulation. The enzyme responsible for basal cleavage has yet to be identified, and ADAM17 is only partially responsible for the inducible cleavage of ACE2.27
Up-regulation of pro-inflammatory cytokines and activation of proteases during heart failure development has long been implicated as a pathologic mechanism of cardiac remodeling.28,29 Increased oxidative stress and neutrophil infiltration are seen in ACE2-deficient mice, identifying a possible link between inflammation and ACE2 cleavage.30 However, we did not find any association between plasma sACE2 activity and markers of systemic inflammation such as hsCRP or MPO, suggesting that if inflammation is involved in the cleavage of ACE2, then its mechanisms differ than those that promote C-reactive protein or MPO secretion.
Our outcomes data should be put into context of its relatively small event rate, but there is a promising association between elevated plasma sACE2 activity (especially above the third tertile) and the development of adverse clinical events. This is particularly apparent within a group of patients with below-median NT-proBNP levels (<1,240 pg/mL), the presence of elevated sACE2 activity signified a subset of patients with increased risk. Larger studies will be needed to validate these hypothesis-generating results and to validate the utility of measuring sACE2 as a prognostic marker.
Study limitations
Despite extensive echocardiographic evaluation, the association between sACE2 activity and echocardiographic indices only implied their potential relationships, and cannot illustrate cause-and-effect. Similarly with systemic inflammatory biomarkers, their lack of relationship with sACE2 activity does not imply a lack of regional or local inflammatory or oxidative stress mechanism that results from cleavage of sACE2 from the cell membrane. In fact, we cannot identify the source or the mechanisms responsible for the cleavage of ACE2 from the cell membranes in this study. As mentioned, our outcomes data were limited by small event rate. Nevertheless, as approaches of pharmacological ACE2 modulators are currently in development, our data provide promising support in targeting ACE2 for the management of human heart failure. However, mechanistic studies will determine the pathophysiological implication of ACE2 shedding, and whether to focus future therapies on strategies that promote increased ACE2 production or that prevent ACE2 cleavage.
Conclusion
We report that sACE2, a key cardioprotective counter-regulatory member of the RAAS, is elevated in patients with worsening left and right ventricular performance that is independent of systemic inflammation, and is a prognostic indicator of adverse clinical events.
Acknowledgments
FUNDING SOURCES:
The original ADEPT study was supported by the 2003 American Society of Echocardiography Outcomes Research Award (Drs. Troughton and Klein), GlaxoSmithKline Pharmaceuticals, and Roche Diagnostics Inc.
Abbreviations
- ACE2
angiotensin converting enzyme 2
- Ang II
angiotensin II
- AUC
area under the Curve
- CI
confidence interval
- LVEF
left ventricular ejection fraction
- NT-proBNP
amino-terminal pro brain natriuretic peptide
- sACE2
soluble angiotensin converting enzyme 2
- RAAS
renin-angiotensin-aldosterone system
- ROC
receiver operating characteristic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parmley WW. Evolution of angiotensin-converting enzyme inhibition in hypertension, heart failure, and vascular protection. Am J Med. 1998;105:27S–31S. doi: 10.1016/s0002-9343(98)00208-3. [DOI] [PubMed] [Google Scholar]
- 2.Packer M, Poole-Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Ryden L, Thygesen K, Uretsky BF. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999;100:2312–2318. doi: 10.1161/01.cir.100.23.2312. [DOI] [PubMed] [Google Scholar]
- 3.Der Sarkissian S, Huentelman MJ, Stewart J, Katovich MJ, Raizada MK. ACE2: A novel therapeutic target for cardiovascular diseases. Prog Biophys Mol Biol. 2006;91:163–198. doi: 10.1016/j.pbiomolbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 5.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de BI, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 8.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, Speth RC, Raizada MK, Katovich M. Prevention of Angiotensin II-Induced Cardiac Remodeling by Angiotensin-(1-7) Am J Physiol Heart Circ Physiol. 2007;292:736–742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 9.Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AJ, Boomsma F, van Gilst WH. Angiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg B. An ACE in the hole alternative pathways of the renin angiotensin system and their potential role in cardiac remodeling. J Am Coll Cardiol. 2008;52:755–757. doi: 10.1016/j.jacc.2008.04.059. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira AJ, Jacoby BA, Araujo CA, Macedo FA, Silva GA, Almeida AP, Caliari MV, Santos RA. The nonpeptide angiotensin-(1-7) receptor Mas agonist AVE 0991 attenuates heart failure induced by myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292:1113–1119. doi: 10.1152/ajpheart.00828.2006. [DOI] [PubMed] [Google Scholar]
- 12.Epelman S, Tang WH, Chen SY, Francis GS, Sen S. Detection of Soluble Angiotensin Converting Enzyme 2 in Heart Failure: Insights into the Endogenous Counter-Regulatory Pathway of the Renin-Angiotensin-Aldosterone System. J Am Coll Cardiol. 2008;52:750–754. doi: 10.1016/j.jacc.2008.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troughton RW, Prior DL, Frampton CM, Nash PJ, Pereira JJ, Martin M, Fogarty A, Morehead AJ, Starling RC, Young JB, Thomas JD, Lauer MS, Klein AL. Usefulness of tissue doppler and color M-mode indexes of left ventricular diastolic function in predicting outcomes in systolic left ventricular heart failure (from the ADEPT study) Am J Cardiol. 2005;96:257–262. doi: 10.1016/j.amjcard.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 14.Tang WH, Shrestha K, Van Lente F, Troughton RW, Martin MG, Borowski AG, Jasper S, Klein AL. Usefulness of C-reactive protein and left ventricular diastolic performance for prognosis in patients with left ventricular systolic heart failure. Am J Cardiol. 2008;101:370–373. doi: 10.1016/j.amjcard.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Tang WH, Tong W, Troughton RW, Martin MG, Shrestha K, Borowski A, Jasper S, Hazen SL, Klein AL. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–2370. doi: 10.1016/j.jacc.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 16.Rice GI, Jones AL, Grant PJ, Carter AM, Turner AJ, Hooper NM. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48:914–920. doi: 10.1161/01.HYP.0000244543.91937.79. [DOI] [PubMed] [Google Scholar]
- 17.Huang L, Sexton DJ, Skogerson K, Devlin M, Smith R, Sanyal I, Parry T, Kent R, Enright J, Wu QL, Conley G, DeOliveira D, Morganelli L, Ducar M, Wescott CR, Ladner RC. Novel peptide inhibitors of angiotensin-converting enzyme 2. J Biol Chem. 2003;278:15532–15540. doi: 10.1074/jbc.M212934200. [DOI] [PubMed] [Google Scholar]
- 18.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- 19.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 20.Zisman LS. ACE and ACE2: a tale of two enzymes. Eur Heart J. 2005;26:322–324. doi: 10.1093/eurheartj/ehi043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcus JT, Vonk NA, Roeleveld RJ, Postmus PE, Heethaar RM, Van Rossum AC, Boonstra A. Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: noninvasive monitoring using MRI. Chest. 2001;119:1761–1765. doi: 10.1378/chest.119.6.1761. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto K, Ohishi M, Katsuya T, Ito N, Ikushima M, Kaibe M, Tatara Y, Shiota A, Sugano S, Takeda S, Rakugi H, Ogihara T. Deletion of Angiotensin-Converting Enzyme 2 Accelerates Pressure Overload-Induced Cardiac Dysfunction by Increasing Local Angiotensin II. Hypertension. 2006;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 23.Lieb W, Graf J, Gotz A, Konig IR, Mayer B, Fischer M, Stritzke J, Hengstenberg C, Holmer SR, Doring A, Lowel H, Schunkert H, Erdmann J. Association of angiotensin-converting enzyme 2 (ACE2) gene polymorphisms with parameters of left ventricular hypertrophy in men Results of the MONICA Augsburg echocardiographic substudy. J Mol Med. 2006;84:88–96. doi: 10.1007/s00109-005-0718-5. [DOI] [PubMed] [Google Scholar]
- 24.Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from Angiotensin II-induced Cardiac Hypertrophy and Fibrosis by Systemic Lentiviral Delivery of ACE2 in Rats. Exp Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 25.Santos RA, Castro CH, Gava E, Pinheiro SV, Almeida AP, Paula RD, Cruz JS, Ramos AS, Rosa KT, Irigoyen MC, Bader M, Alenina N, Kitten GT, Ferreira AJ. Impairment of in vitro and in vivo heart function in angiotensin-(1-7) receptor MAS knockout mice. Hypertension. 2006;47:996–1002. doi: 10.1161/01.HYP.0000215289.51180.5c. [DOI] [PubMed] [Google Scholar]
- 26.Goulter AB, Goddard MJ, Allen JC, Clark KL. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004;2:19–26. doi: 10.1186/1741-7015-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor Necrosis Factor-{alpha} Convertase (ADAM17) Mediates Regulated Ectodomain Shedding of the Severe-acute Respiratory Syndrome-Coronavirus (SARSCoV) Receptor, Angiotensin-converting Enzyme-2 (ACE2) J Biol Chem. 2008;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fedak PW, Moravec CS, McCarthy PM, Altamentova SM, Wong AP, Skrtic M, Verma S, Weisel RD, Li RK. Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation. 2006;113:238–245. doi: 10.1161/CIRCULATIONAHA.105.571414. [DOI] [PubMed] [Google Scholar]
- 29.Rutschow S, Li J, Schultheiss HP, Pauschinger M. Myocardial proteases and matrix remodeling in inflammatory heart disease. Cardiovasc Res. 2006;69:646–656. doi: 10.1016/j.cardiores.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, Tsushima RG, Scholey JW, Khokha R, Penninger JM. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 2007;75:29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]