Abstract
Cord blood (CB) is used increasingly in transplant patients lacking sibling or unrelated donors. A major hurdle to the use of CB is low cell dose which is largely responsible for elevated risk of graft failure and significantly delayed neutrophil and platelet engraftment. Since a positive correlation has been demonstrated between the total nucleated cell (TNC) and CD34+ cell dose transplanted and time to neutrophil and platelet engraftment, strategies to increase these measures are under development. One strategy includes the ex vivo expansion of CB mononuclear cells (MNC) with mesenchymal stem cells (MSC) in a cytokine cocktail. We demonstrate that this strategy can be further improved if CD3+ and/or CD14+ cells are first depleted from the CB MNC prior to ex vivo expansion. Ready translation of this depletion strategy to improve ex vivo CB expansion in the clinic is feasible since clinical-grade devices and reagents are available. Ultimately the goal of improving TNC and CD34+ transplant doses is to further improve the rate of neutrophil and platelet engraftment in CB recipients.
Keywords: Cord blood, ex vivo expansion, inhibitory “accessory” cells, mononuclear cells
Introduction
Umbilical cord blood (CB) is rapidly becoming an important source of tissue for hematopoietic transplantation due largely to its ethnic diversity, relative ease of collection, ready availability from frozen banks, reduced incidence and severity of graft versus host disease and tolerance of higher degrees of HLA disparity between donor and recipient when compared to the use of bone marrow (BM) or cytokine-mobilized peripheral blood (PB).1-12
Outside pediatric transplantation, low cell dose remains a major drawback to the successful use of CB transplantation and is largely responsible for the significantly delayed neutrophil and platelet engraftment and elevated risk of graft failure observed for CB recipients when compared to BM and PB transplant recipients. A positive correlation between the total nucleated cell (TNC) dose7,11 and CD34+ cell dose12 transplanted and time to neutrophil and platelet engraftment has been identified for CB recipients. To this end, a number of strategies to overcome the low TNC and CD34+ doses associated with CB have been developed with the goal of improving the speed of neutrophil and platelet engraftment. Approaches currently include the transplantation of two unmanipulated CB units3,13,14, and the use of ex vivo expansion techniques.15-20
Initial ex vivo expansion studies revealed that the selection of CD34+ cells from CB mononuclear cell (MNC) was first required before successful ex vivo expansion in culture medium containing a cocktail of growth factors was achieved.21 These data suggested that the presence of non-CD34+ “accessory” cells in the CB MNC may negatively impact the efficacy of ex vivo expansion. While CD34+ cell selection from the CB MNC was required, it is not an efficient process and significant CD34+ cell losses occur. In an MDACC double CB expansion trial, the recovery of CD34+ cells from CB units following positive selection was only 35% (range: 4% to 70%) (personal communication EJS). As a direct consequence of the significant CD34+ cell losses, even if significant levels of ex vivo expansion are achieved, cell yields for transplant remain low. Ex vivo expansion strategies which do not require initial CD34+ selection were therefore investigated. It was subsequently shown that ex vivo expansion of primitive and more mature hematopoietic progenitors is achieved by the co-culture of non-selected CB MNC in culture medium containing a cocktail of growth factors together with cellular and extracellular components of the hematopoietic microenvironment provided by BM-derived mesenchymal stem cells (MSC).16,19 This ex vivo co-culture strategy lead to the development of a double CB clinical trial at the University of Texas M. D. Anderson Cancer Center (MDACC) with patients receiving one unmanipulated CB unit and one ex vivo expanded CB unit at transplant. The combination of an unmanipulated CB unit together with an ex vivo co-culture expanded CB unit allows significantly higher doses of TNC and CD34+ cells to be transplanted than have ever previously been achieved. An encouraging trend towards more rapid neutrophil and platelet engraftment has been observed in patients receiving these significantly elevated numbers of TNC and CD34+ cells and provides the impetus to further refine and improve the current ex vivo co-culture expansion strategy.
While the ex vivo expansion achieved with CB MNC/MSC co-culture is markedly better than that achieved with CD34+ selection and liquid culture,19 it was hypothesized that ex vivo expansion would be further improved if putative inhibitory “accessory” cells present in the CB MNC were removed prior to ex vivo CB MNC/MSC co-culture. Previous reports have cited instances where T-lymphocytes,22,23 natural killer (NK) cells,24,25 macrophages26,27 and other less well defined “accessory” cells28,29 have been shown to negatively impact hematopoiesis. Phenotypic analysis of CB revealed that the major cellular components are CD3+, CD14+, CD56+ and CD19+ cells and each was investigated (alone and in combination) with respect to their potential as inhibitory “accessory” cells influencing the magnitude of CD34+ ex vivo expansion achieved.
Initially, using an ‘add-back’ strategy we demonstrated that the combination of CD3+ and CD14+ cells had a marked inhibitory effect on ex vivo CD34+ expansion, suggesting that CD3+ and/or CD14+ cells were putative inhibitory ‘accessory’ cells. We subsequently confirmed these ‘add-back’ data by a more clinically-applicable depletion strategy and demonstrated that ex vivo CD34+ expansion was markedly improved following CD3+ and/or CD14+ cell depletion. It is hypothesized that improving the level of ex vivo expansion achieved will increase the TNC and CD34+ cell dose available for transplant and thereby improve platelet and neutrophil engraftment for CB recipients.
Materials and Methods
Cord blood units
Frozen CB units were released for research use under the University of Texas M. D. Anderson Cancer Center Internal Review Board approved Protocol LAB03-0796.
Use of magnetic activated cell sorting (MACS)
MACS was used to positively select (for preliminary ‘add-back’ experiments) or deplete (for subsequent depletion experiments) specific cell populations. Single frozen CB units were thawed and washed in CliniMACS wash buffer: CliniMACS buffer (Miltenyi Biotec, Auburn, CA) containing 0.5% human serum albumin (HSA) (Flexbumin, Baxter Healthcare Corp., Deerfield, IL). Small cell aggregates were removed using sterile 70 μm nylon cell strainers (BD Falcon, Bedford, MA). Equal fractions of CB MNC were incubated at +4°C for 5 minutes in a solution of Gammaguard (Baxter Healthcare Corp.) to block non-specific binding of MACS beads and at +4°C for 20 minutes in an excess of specific MACS reagents (beads) to positively select either CD34+, CD3+, CD14+, CD19+ or CD56+ cells (Miltenyi Biotec). In positively selecting a specific cell population from CB MNC (e.g. for use in ‘add-back’ experiments), a product depleted of that specific cell population was also generated (e.g. for use in depletion experiments). CD3 and CD14 depletion was achieved by combining CD3 and CD14 MACS beads with the CB MNC during the incubation. This simultaneous depletion was shown to be as effective as a two step, sequential depletion process. A fraction of CB MNC equivalent to that subjected to MACS (selection or depletion), was untreated and served as the unmanipulated CB MNC control.
After incubation, unbound MACS beads were removed by washing in CliniMACS wash buffer. Cells were passed through pre-separation filters (Miltenyi Biotec, Auburn, CA) and onto MACS LS separation columns according to manufacturer's instructions (Miltenyi Biotec, Auburn, CA). LS columns provided both the ‘selected’ product (positive fraction retained in the column and used in ‘add-back’ experiments) and ‘depleted’ product (negative fraction as ‘flow through’ used in depletion experiments). Positive and negative fractions were collected, centrifuged and cells resuspended in medium. Cell numbers were determined and flow cytometry (see below) performed to determine the efficiency of the selection/depletion procedure.
“Add-back” experiments
‘Add-back’ experiments were designed to expose selected CD34+ to numbers of ‘accessory’ cells' that would be present in the unmanipulated CB MNC. Since 5 cell populations were required (CD34+, CD3+, CD14+, CD19+ or CD56+ cells), 20% fractions of the total CB MNC were subjected to CD34+, CD3+, CD14+, CD19+ or CD56+ cell selection. CD34+, CD3+, CD14+, CD19+ or CD56+ cells derived from the 20% CB fractions were collected into 2 mls of Expansion Medium [CellGro SCGM (CellGenix, Antioch, IL) containing 10% fetal bovine serum (FBS, Hyclone, Logan, UT) antibiotics (100 U/ml Penicillin and 100 μg/ml Streptomycin (Gibco, Grand Island, NY) and 2 mM L-glutamine (HyClone, Logan, UT) and supplemented with 100 ng/ml each of Stem Cell Factor (SCF), Flt-3 Ligand (FL), Thombopoietin (TPO) from CellGenix (Antioch, IL) and Granulocyte Colony-Stimulating Factor (G-CSF, Neupogen Filgrastim, Amgen, Thousand Oaks, CA)]. Sixteen different culture combinations were identified: CD34+ cells were cultured alone and with various CD3+ ± CD14+ ± CD19+ ± CD56+ combinations (see Figure 1). Cultures were performed in 6 well, flat-bottomed tissue culture plates containing pre-established BM-derived MSC monolayers. MSC were provided as mesenchymal precursor cells (MPC) by Angioblast Systems, Inc., (New York, NY). Routinely, ex vivo expansion cultures are performed with 10% of an unmanipulated CB MNC in 50 mls of Expansion Medium in 150 cm2 tissue culture flasks containing pre-established monolayers of MSC. With the reduction in the size of the culture (culture area reduced from 150 cm2 to approximately 7 cm2), the number of cells plated (reduced from 10% to 0.5% per culture) and culture volume (reduced from 50 mls to 2.5 mls) were similarly reduced.
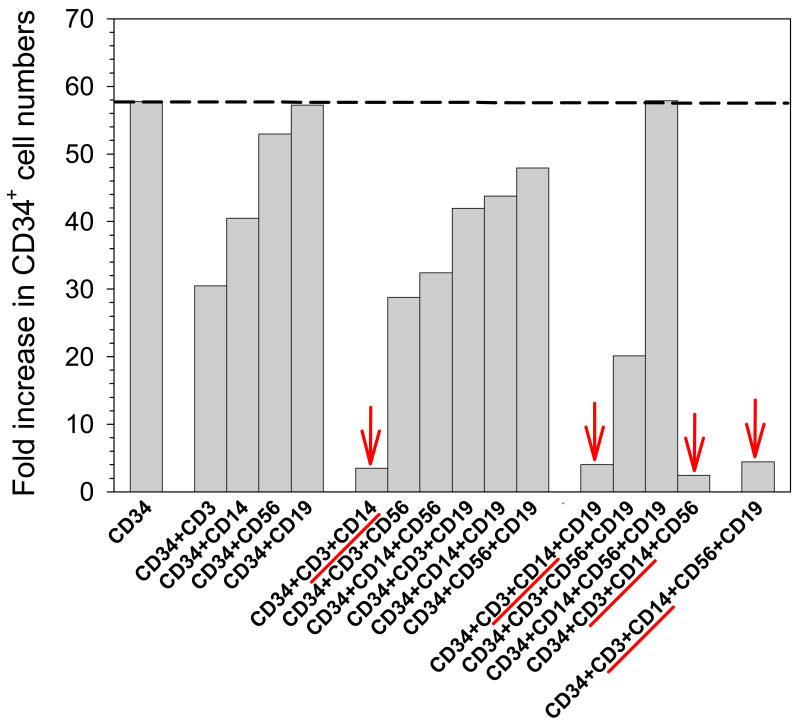
Figure 1. Representative ‘Add-back’ experiment.
CD34+, CD3+, CD14+, CD56+ and CD19+ cells were isolated from CB MNC by MACS. CD34+ cells were then cultured alone or in the presence of CD3+ ± CD14+ ± CD56+ ± CD19+ cells at levels comparable to those present in the initial CB MNC. While CD34+ ex vivo expansion was reduced in the presence of CD3+ or CD14+ cells, maximal reduction was observed when CD3+ and CD14+ cells were present (indicated by arrows).
Positively selected CD34+ cells derived from 0.5% CB MNC, corresponded to approximately 100 μl of the 2 mls containing the selected product from 20% of CB MNC, were added to each culture well. For the addition of ‘accessory’ cells, approximately 100 μl of the 2 mls of cell suspension containing the selected product from 20% of CB MNC (CD3+, CD14+, CD19+ or CD56+ cells) was added to yield the 16 different conditions assayed (CD34+ ± CD3+ ± CD14+ ± CD19+ ± CD56+).
Expansion Medium was added to a final volume of approximately 2.5 mls. ‘Add back’ culture was performed at 37°C in a 5%CO2 in air fully humidified atmosphere. After 7 days, the 2.5 mls of medium containing non-adherent cells was removed and replaced with 2.5 mls of fresh Expansion Medium and culture continued for an additional 7 days. In addition, a known volume of the medium containing non-adherent cells was diluted in fresh Expansion Medium and culture continued for 7 days in a tissue culture flask at 37°C in a 5%CO2 in air fully humidified atmosphere (‘liquid’ culture). After 14 days, all hematopoietic cells (from the ‘liquid’ culture fraction and from the non-adherent and adherent fractions of the co-cultures) were analyzed for TNC number and number of CD34+ cells present for each condition. The number of hematopoietic cells in the adherent fraction (adhering to the MSC) consistently constituted only a small fraction of the hematopoietic cells in the culture well at day 14.
TNC number was determined by hemacytometer and the proportion of CD34+ cells present was determined by flow cytometry. Total output TNC and CD34+ numbers were calculated for each condition (CD34+ ± CD3+ ± CD14+ ± CD19+ ± CD56+). Total output TNC and CD34+ numbers were calculated for each condition and the fold increase in TNC and CD34+ cell numbers from those input (day 0) to those output following 14 days of ex vivo expansion was calculated.
“Depletion” experiments
Negative fractions (cells not retained in the MACS column) were collected into 2mls of Expansion medium as: (1) CD3, (2) CD14 or (3) CD3 and CD14 ‘depleted’ products. Since equal fractions of CB MNC were used for selection, all the negative fractions contained similar numbers of the non-depleted populations i.e. CD34+, CD56+ and CD19+ cells. When present they also contained similar numbers of CD3+ cells (CD14 depleted sample) or CD14+ cells (CD3 depleted sample), or neither (CD3 and CD14 depleted sample). This was confirmed by flow cytometry (data not shown). Cultures were performed in 75 cm2 tissue culture flasks (BD Falcon) containing prepared BM-derived MSC monolayers for co-culture, as previously described.19 As the size of the culture was reduced (culture area reduced from 150 cm2 to 75 cm2), the number of cells cultured and volume of incubation medium was similarly reduced.
The depletion product (CD3 ± CD14 depleted) from approximately 5% CB MNC was cultured in 25 ml of Expansion Medium. Cells were cultured at 37°C in a 5%CO2 in air, fully humidified atmosphere for 7 days. After 7 days, the 25 mls of medium containing non-adherent cells was removed and replaced with 25 mls of fresh Expansion Medium and culture continued for an additional 7 days. In addition, a known volume of the medium containing non-adherent cells was diluted in fresh Expansion Medium and culture of this fraction continued for 7 days in a tissue culture flask at 37°C in a 5%CO2 in air fully humidified atmosphere (‘liquid’ culture). At day 14, all hematopoietic cells (from the ‘liquid’ culture fraction and from the non-adherent and adherent fractions of the co-cultures) were analyzed for TNC number and number of CD34+ cells present for each condition. TNC number was determined by hemacytometer and the proportion of CD34+ cells present was determined by flow cytometry. Total output TNC and CD34+ numbers were calculated for each condition (MNC or CD3+ and/or CD14+ depleted). Total output TNC and CD34+ numbers were calculated for each condition and the fold increase in TNC and CD34+ cell numbers from those input (day 0) to those output following 14 days of ex vivo expansion was calculated.
Flow cytometric analysis
The cellular composition of the CB MNC prior to, and following MACS selection/depletion, and post-culture was confirmed by flow cytometry using fluorescently-conjugated detection antibodies (CD34, CD3 CD14, CD19, CD56 and CD45 - all BD Biosciences, San Jose, CA). Briefly, cells were washed in Flow Stain Buffer (FSB): Dulbecco's Phosphate Buffered Saline (Gibco, Grand Island, NY) containing 1% (v/v) Donor Goat Serum (JRH Biosciences, Lenexa, KS) and incubated with appropriate fluorescently-labeled antibodies for 20 minutes with occasional mixing. Unbound antibodies were removed by washing in FSB and cells fixed using a solution of 1.6% (v/v) paraformaldehyde in FBS. Fluorescence staining was revealed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and analysis performed using CELLQuest Pro software (Becton Dickinson, San Jose, CA). The absolute number of each cell type revealed by flow cytometry (CD34+, CD3+, CD14+, CD19+ or CD56+) was calculated by reference to the appropriate TNC count performed by hemacytometer.
Results
“Add-back” experiments
Although representative data from a single ‘add-back’ experiment is shown,(Figure 1) a similar pattern of ex vivo expansion was observed in other ‘add-back’ experiments. In this representative experiment, CD34+ ex vivo expansion under 16 different conditions is shown. Culture conditions range from CD34+ alone; to a combination of cells that was essentially that of CB MNC prior to any manipulation.
Maximal CD34+ ex vivo expansion (approximately 58-fold) was observed when CD34+ cells were cultured with BM-derived MSC in Expansion Medium in the absence of any CB MNC ‘accessory’ cells. This level of CD34+ ex vivo expansion is not markedly different from that observed when CD34+ cells are incubated with CD56+ or CD19+, suggesting that neither of these cell types acting singly, inhibit CD34+ ex vivo expansion.
However, in the presence of CD3+ or CD14+ cells, CD34+ ex vivo expansion is reduced (by approximately 50% and 30%, respectively). These data suggest that both of these cell types acting singly inhibit CD34+ ex vivo expansion. Consistent with these data, ‘add-back’ combinations that contain CD3+ or CD14+ cells show a similar trend toward inhibition of CD34+ ex vivo expansion.
However, marked inhibition (>90%) of CD34+ ex vivo expansion was most evident when both CD3+ and CD14+ cells are present (as indicated by arrows in Figure 1). The presence of both CD3+ and CD14+ cells was associated with a profound inhibition of CD34+ ex vivo expansion, be it as part of a 2, 3 or 4 component ‘add-back’ combination.
While these data were focused on identifying inhibitory ‘accessory’ cells, no clear evidence for the existence of stimulatory ‘accessory’ cells (acting singly, or in combination) was found. CD34+ ex vivo expansion only matched, or was less than that observed with CD34+ cells alone. Evidence of stimulatory ‘accessory’ cells would have been observed as levels of CD34+ ex vivo expansion that exceeded those obtained following culture of CD34+ cells alone.
Efficacy of MACS depletion
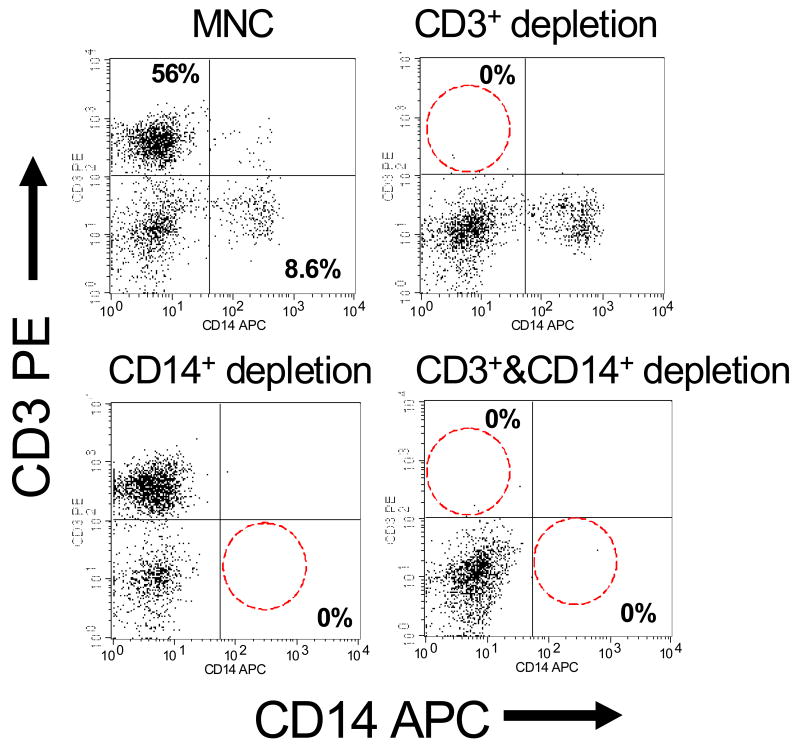
Representative flow cytometric data demonstrating the efficacy of depletion using MACS is shown.(Figure 2) Consistently high levels of CD3 and/or CD14 depletion were achieved. These data demonstrate the specificity and efficacy of the CD3 and/or CD14 depletion process. In this representative sample, the CB MNC contained approximately 56% CD3+ cells and approximately 9% CD14+ cells. Following CD3+ and/or CD14+ cell depletion procedures, each population was reduced to 0%. Further, analysis confirmed that the depletion of CD3+ cells did not impact the numbers of CD14+ cells and visa versa, nor did the depletion of CD3+ and/or CD14+ cells impact the numbers of CD34+ cells. Similarly, no impact on CD19+ and CD56+ cells was observed (data not shown).
Figure 2. Efficacy of CD3+ and/or CD14+ depletion by MACS.
Representative flow cytometric analyses of depleted and non-depleted CB-MNC products are shown. The efficacy of MACS depletion is demonstrated by the depletion of CD3+ (upper right panel), CD14+ (lower left panel) and CD3+ & CD14+ (lower right panel).
To investigate whether ‘processing’ of the CB MNC for MACS had any impact on the initial input numbers of CD34+ cells and the level of ex vivo CD34+ and TNC expansion achieved, (i) untreated MNC (not exposed to Miltenyi reagents) were passed through pre-separation filters and MACS LS separation columns, and (ii) CB MNC were treated with either CD3 and/or CD14 Reagents and passed through pre-separation filters and MACS LS separation columns that were outside of the magnetic separation device. No evidence that such MACS ‘processing’ had any impact on ex vivo expansion was observed (data not shown).
Ex vivo expansion of TNC and CD34+ following CD3+ and/or CD14+ depletion by MACS
Fold expansion of TNC and CD34+ numbers with no depletion (MNC) and following CD3 and/or CD14 depletion [MNC-CD3, MNC-CD14 and MNC-(CD3&CD14)] is shown in Figures 3 and 4, respectively. (Data is presented as mean fold increase ± SEM (n=4-6 replicate experiments). Control data from CD56 depleted samples is shown for comparison.
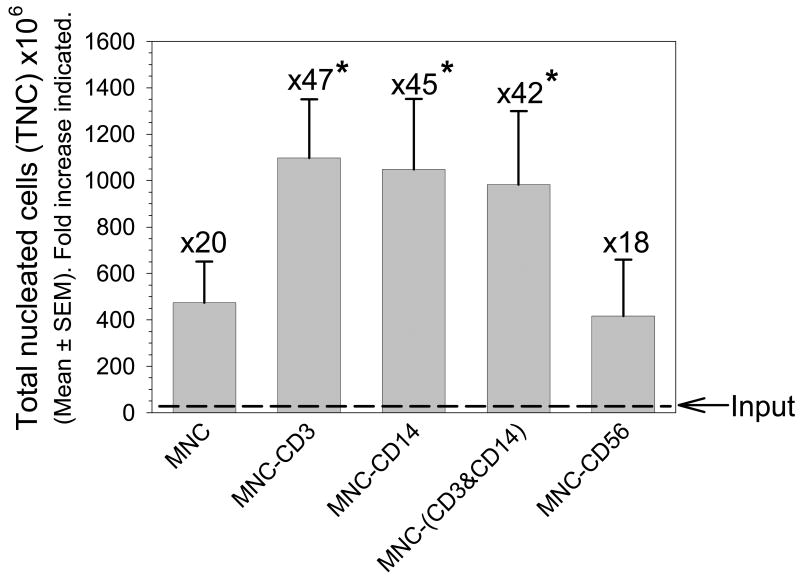
Figure 3. Impact of CD3+ and/or CD14+ depletion on TNC expansion.
TNC numbers are shown for each condition following expansion (mean±SEM, n=4-6 replicate experiments. *Statistically-significant from MNC, P≤0.05). Fold increase from input (dashed line) to output is also shown. TNC expansion was approximately 2.3, 2.2 and 2.1-fold greater than MNC following CD3, CD14, and CD3 & CD14 depletion, respectively.
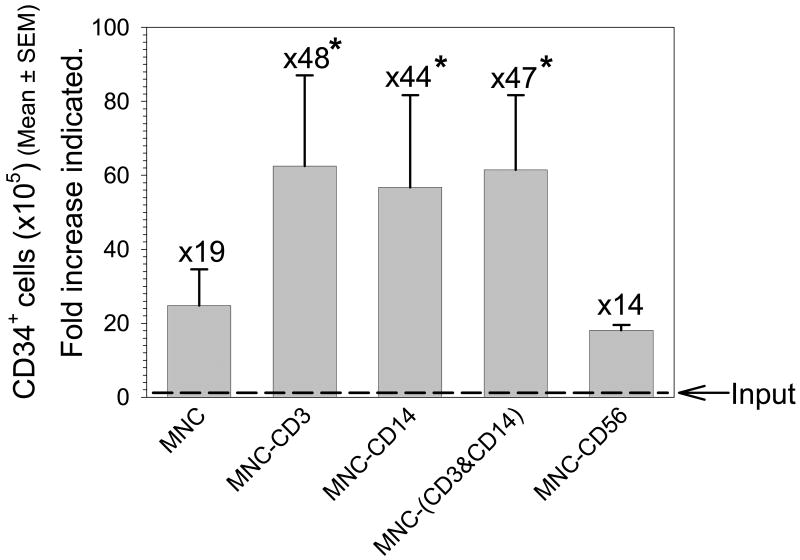
Figure 4. Impact of CD3+ and/or CD14+ depletion on CD34+ cell expansion.
CD34+ cell numbers are shown for each condition following expansion (mean±SEM, n=4-6 replicate experiments. *Statistically-significant from MNC, P≤0.05). Fold increase from input (dashed line) to output is also shown. CD34+ cell expansion was approximately 2.5, 2.3 and 2.5-fold greater than MNC following CD3, CD14, and CD3 & CD14 depletion, respectively.
(i) No depletion
With no depletion (MNC) an input TNC number of 23.5±2.6×106 cells contained approximately 1.3±0.4×105 CD34+ cells (approximately 0.6% of pre-expansion TNC). After 14 days of ex vivo expansion culture, TNC and CD34+ cell numbers were increased by approximately 20- and 19-fold, respectively to 473.5±164.8×106 TNC and 24.8±9.8 ×105 CD34+ cells (approximately 0.5% of post-expansion product).
(ii) CD3 depletion
CD3 depletion (MNC-CD3) reduced the input TNC number from that seen in the non-depleted CB MNC by approximately 42%, from 23.5±2.6×106 cells to 13.6±3.3 ×106 cells. The 1.4±0.5×105 CD34+ cells contained in the CD3 depleted fraction, was similar to the 1.3±0.5×105 CD34+ cells contained in the MNC (no depletion). These data demonstrate that the depletion of CD3+ cells did not impact the CD34+ population. After 14 days of ex vivo expansion culture, TNC numbers were increased approximately 47-fold from the initial input of 23.5±2.6×106 TNC to 1097.3±287.6×106 TNC.(Figure 3) It should be noted that this expansion actually represents a >80-fold increase over the post-depletion value of 13.6±3.3 ×106 cells.
After 14 days of ex vivo expansion culture, CD34+ cell numbers were increased approximately 48-fold from the initial input of 1.3±0.5×105 CD34+ cells to 62.5±24.2 ×105 CD34+ cells. (Figure 4) This represents approximately 0.6% of the post-expansion product.
Ex vivo expansion following CD3 depletion delivered significantly greater TNC numbers (2.3-fold, P≤0.05) and significantly greater CD34+ cell numbers (2.5-fold, P≤0.05) than did ex vivo expansion of unmanipulated CB MNC (no depletion). The increases in TNC and CD34+ cell numbers following CD3 depletion are not significantly different from those obtained following CD14, or CD3 and CD14 depletion.
(iii) CD14 depletion
CD14 depletion (MNC-CD14) reduced the input TNC number from that seen in the non-depleted CB MNC by approximately 22%, from 23.5±2.6×106 TNC to 18.2±1.4 ×106 TNC. The 1.3±0.5×105 CD34+ cells contained in the CD14 depleted fraction, was identical to that contained in the unmanipulated MNC (no depletion) demonstrating that the depletion of CD14+ cells did not impact CD34+ numbers.
After 14 days of ex vivo expansion culture, TNC numbers were increased approximately 45-fold from the initial input of 23.5±2.6×106 TNC to an output of 1097.3±287.6×106 TNC.(Figure 3) It should be noted that this expansion actually represents a >50-fold increase over the post-depletion value of 18.2±1.4×106 TNC. CD34+ cell numbers were increased approximately 44-fold from the initial input to 56.7±25.0 ×105 CD34+ cells.(Figure 4) This represents approximately 0.5% of the post-expansion product.
Ex vivo expansion following CD14 depletion delivered significantly greater TNC (2.2-fold, P≤0.05), and CD34+ cell numbers (2.3-fold, P≤0.05), respectively, than did ex vivo expansion of unmanipulated MNC (no depletion). The increases in TNC and CD34+ cell numbers following CD14 depletion are not significantly different from those obtained following CD3, or CD3 and CD14 depletion.
(iv) CD3 and CD14 depletion
CD3 and CD14 depletion [MNC-(CD3&CD14)] reduced the input TNC number from that seen in the non-depleted CB MNC by approximately 58%, from 23.5±2.6×106 cells to 9.8±1.4 ×106 cells. These data are consistent with the value that would be predicted following the individual depletion of CD3+ (approximately 42%) and CD14+ cells (approximately 22%). The 1.3±0.5×105 CD34+ cells contained in the CD3 and CD14-depleted fraction, was similar to that present in the unmanipulated MNC (no depletion) and demonstrated that the simultaneous depletion of CD3+ and CD14+ cells did not impact CD34+ numbers.
After 14 days of ex vivo expansion culture, TNC numbers were increased approximately 42-fold from the initial input of 23.5±2.6×106 TNC to an output of 982.1±302.7×106 TNC.(Figure 3) It should be noted that this expansion actually represents a >100-fold increase from the post-depletion value of 9.8±1.4×106 TNC.
CD34+ cell numbers were increased approximately 47-fold from the initial input of 1.3±0.5×105 CD34+ cells to 61.5±24.2 ×105 CD34+ cells.(Figure 4) This represents approximately 0.6% of post-expansion product.
Ex vivo expansion following CD3 and CD14 depletion delivered significantly greater TNC (2.1-fold, P≤0.05) and CD34+ cell numbers (2.5-fold, P≤0.05), respectively, than did ex vivo expansion of unmanipulated MNC (no depletion) when initial (pre-depletion) inputs were compared. The increases in TNC and CD34+ cell numbers following CD3 and CD14 depletion are not significantly different from those obtained following CD3 or CD14 depletion.
(v) CD56+ depletion
In a limited number of experiments CD56 depletion was performed as a control. ‘Add-back’ experiments suggested that the presence of CD56+ cells did not inhibit CD34+ ex vivo expansion.(Figure 1) CD56 depletion (MNC-CD56) reduced the input TNC number from that seen in the non-depleted CB MNC by approximately 14% from 23.5±2.6×106 cells to 20.2±4.0×106 cells. The number of CD34+ cells contained in the CD56 depleted fraction was similar to the 1.3±0.4×105 CD34+ cells present in the unmanipulated MNC (no depletion) and suggest that the depletion of CD56+ did not impact CD34+ numbers.
After 14 days of ex vivo expansion culture, TNC numbers were increased approximately 18-fold from the initial input of 23.5±2.6×106 TNC to an output of 416.7±187.7×106 TNC.(Figure 3) It should be noted that this increase in TNC numbers actually represents a >20-fold increase from the post-depletion value of 20.2±4.0×106 TNC. CD34+ cell numbers were increased approximately 14-fold from the initial input of 1.3±0.5×105 CD34+ cells to 18.1±1.4×105 CD34+ cells.(Figure 4) This represents approximately 0.4% of post-expansion product.
The increase in TNC and CD34+ cell numbers following CD56 depletion is significantly less than that obtained following CD3 and/or CD14 depletion (P≤0.05), however, it is not significantly different (P>0.05) from that obtained following the ex vivo expansion of unmanipulated (non-depleted) MNC.
Statistical analysis
Where appropriate data were compared using the Student's t-test (Excel, Microsoft) with significance assumed at P≤0.05.
Discussion
Strategies to overcome the low TNC and CD34+ doses associated with CB transplantation have been developed with the goal of improving the speed of neutrophil and platelet engraftment and reducing the risk of graft failure and include the transplantation of two unmanipulated CB units3,13,14, and the ex vivo expansion of one of the two CB units prior to transplant.15-20 Here we focus specifically on the contribution of ex vivo CB expansion and demonstrate that a relatively simple refinement to a current ex vivo CB MNC/MSC co-culture expansion technique, might further improve the magnitude of ex vivo expansion achieved and therefore possibly positively impact the rate of neutrophil and platelet engraftment and reduce the risk of graft failure.
Ex vivo expansion using a liquid culture technique was shown to be effective only if CD34+ or CD133+ CB progenitors were first positively selected from the CB MNC cell milieu21 (albeit an inefficient process) suggesting that the removal of the putative negative influences of non-CD34+ or non-CD133+ “accessory” cells was required. Here we present evidence for the presence of negative “accessory” cells in CB MNC and demonstrate that the depletion of these inhibitory “accessory” cells and/or the factors they produce, improves ex vivo expansion in a CB MNC/MSC co-culture system.
Based on “add-back” experiments, CD3+ and CD14+ cells appeared to be candidate inhibitory “accessory” cells. Subsequently, ex vivo expansion of CD34+ in CB MNC/MSC co-culture was performed where CD3+ and/or CD14+ cells were first depleted from the CB MNC. We demonstrate that the depletion of CD3+ and/or CD14+ cells improves the ex vivo expansion of TNC and CD34+ cells obtained with CB MNC/MSC co-culture.19
‘Add-back’ experiments demonstrated that both CD3+ and CD14+ cells were required before a maximal negative impact on CD34+ ex vivo expansion was observed. These data were confirmed by the observation that CD34+ expansion was improved (>2-fold) with the depletion of both CD3+ and CD14+ cells from CB MNC prior to CB MNC/MSC co-culture. However, a similar improvement in CD34+ expansion was also observed when either CD3+ or CD14+ cells were depleted from CB MNC prior to MSC co-culture. These data suggest that the removal of one, or other, or both populations, has a similar positive impact on CD34+ ex vivo expansion. This observation might be explained by the possible existence of an inhibitory ‘cross-talk’ between the CD3+ cells and CD14+ cell populations. One population may stimulate the production of an inhibitory activity by the other to ultimately regulate CD34+ proliferation. Alternatively, each population may produce unique factors that may synergize to inhibit CD34+ ex vivo expansion. In such models, the removal of one, or other, or both populations could therefore be explained to similarly improve CD34+ ex vivo expansion.
Targeting the depletion of one inhibitory “accessory” cell population (CD3+ or CD14+ cells) rather than both (CD3+ and CD14+ cells) to improve ex vivo CB expansion reduces both the complexity and cost of the procedure. In addition, there may be advantages to the targeted depletion of only one population. For example, the CD3+ cell population collected after CD3 depletion could be used to further develop immunologic and/or anti-tumor strategies to potentially supplement hematopoietic reconstitution of the transplant recipient.30-32
Further, the observation that inhibitory activity may be associated with CD14+ cells present in the CB MNC might not be surprising since macrophages have previously been shown to produce stem cell-specific proliferation regulators.33-36 Whether the putative CD3+/CD14+ interaction requires cell-cell contact, and/or is mediated through humoral factors, is under investigation. Further, the specific subsets of the CD3+ population and/or the CD14+ population, which may be responsible for the inhibitory activity, remain to be identified.
The prospect of identifying possible ‘inhibitory factors’ lends itself to the possible clinical application of activity-neutralizing antibodies and/or function-blocking agents to optimize ex vivo CD34+ expansion. Such an approach may replace the need for depletion to remove specific ‘accessory’ cells.
The inhibition of CD34+ ex vivo expansion observed in the presence of both CD3+ and CD14+ cells during CB MNC/MSC co-culture, might only be a relatively short-lived, early event, since the current ex vivo culture conditions (SCF, TPO, G-CSF and Flt-3L) do not promote CD3+ cell survival. CD3+ cells are therefore ‘lost’ from the culture relatively early in the ex vivo expansion culture, presumably releasing their contribution to any putative inhibition of CD34+ ex vivo expansion. This would restrict the impact of any CD3+/CD14+ inhibitory “cross-talk” to the earliest parts of the CB MNC/MSC co-culture. This might, at least in apart, explain the initial ‘lag’ observed in ex vivo expansion cultures. It is possible that the >2-fold improvement in TNC and CD34+ ex vivo expansion observed with CD3+ and/or CD14+ depletion is a consequence of reducing this initial ‘lag’ in TNC and CD34+ ex vivo expansion.
The attraction of a CD3+ or CD14+ depletion strategy to remove inhibitory “accessory” cells and/or the factors they produce from the CB MNC/MSC co-culture and improve CD34+ expansion is that it does not adversely impact the input CD34+ population, unlike the use of a positive CD34+ selection strategy where significant CD34+ losses are incurred. Further, the relative ease, rapidity and availability of clinical-grade Food and Drug Administration (FDA)-compliant devices and MACS Reagents (Miltenyi Biotec, Antioch, IL), give this approach a significant advantage over other selection/depletion techniques. Since clinical-grade devices and reagents are available, the use of an “accessory” cell depletion strategy and its beneficial impact on ex vivo CB expansion could be readily translated to the clinic. Ultimately, increased TNC and CD34+ cell doses may contribute to improve neutrophil and platelet engraftment for CB recipients thereby improving the efficacy and ultimately the cost-effectiveness of CB transplantation as a treatment rationale.37
Acknowledgments
This work was supported by NCI R01 CA061508-16. The authors gratefully acknowledge the provision of MPC by Angioblast Systems, Inc., New York, NY.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
References
- 1.Barker JN, Davies SM, Defor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97:2957–2961. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- 2.Barker JN, Weisdorf DJ, Defor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 3.Barker JN, Weisdorf DJ, Defor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 4.Brunstein CG, Barker JN, Weisdorf DJ, Defor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman E, Rocha V, Chevret S. Results of unrelated umbilical cord blood hematopoietic stem cell transplantation. Rev Clin Exp Hematol. 2001;5:87–99. doi: 10.1046/j.1468-0734.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32:397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 9.Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 10.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 11.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 12.Wagner JE, Barker JN, Defor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 13.Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344:1870–1871. doi: 10.1056/NEJM200106143442417. [DOI] [PubMed] [Google Scholar]
- 14.de Lima M, St John L, Wieder ED, Lee MS, McMannis J, Karandish S, et al. Double-chimaerism after transplantation of two human leucocyte antigen mismatched, unrelated cord blood units. Br J Haematol. 2002;119:773–776. doi: 10.1046/j.1365-2141.2002.03893.x. [DOI] [PubMed] [Google Scholar]
- 15.McNiece IK, meida-Porada G, Shpall EJ, Zanjani E. Ex vivo expanded cord blood cells provide rapid engraftment in fetal sheep but lack long-term engrafting potential. Exp Hematol. 2002;30:612–616. doi: 10.1016/s0301-472x(02)00805-6. [DOI] [PubMed] [Google Scholar]
- 16.McNiece I, Harrington J, Turney J, Kellner J, Shpall EJ. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6:311–317. doi: 10.1080/14653240410004871. [DOI] [PubMed] [Google Scholar]
- 17.McNiece I, Kubegov D, Kerzic P, Shpall EJ, Gross S. Increased expansion and differentiation of cord blood products using a two-step expansion culture. Exp Hematol. 2000;28:1181–1186. doi: 10.1016/s0301-472x(00)00520-8. [DOI] [PubMed] [Google Scholar]
- 18.Shpall EJ, Quinones R, Giller R, Zeng C, Baron AE, Jones RB, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 19.Robinson SN, Ng J, Niu T, Yang H, McMannis JD, Karandish S, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lima M, McMannis J, Gee A, Komanduri K, Couriel D, Andersson BS, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briddell RA, Kern BP, Zilm KL, Stoney GB, McNiece IK. Purification of CD34+ cells is essential for optimal ex vivo expansion of umbilical cord blood cells. J Hematother. 1997;6:145–150. doi: 10.1089/scd.1.1997.6.145. [DOI] [PubMed] [Google Scholar]
- 22.Harada M, Odaka K, Kondo K, Nakao S, Ueda M, Matsue K, et al. Effect of activated lymphocytes on the regulation of hematopoiesis: suppression of in vitro granulopoiesis and erythropoiesis by OKT8+ Ia- T cells induced by concanavalin-A stimulation. Exp Hematol. 1985;13:963–967. [PubMed] [Google Scholar]
- 23.Harada M, Nakao S, Kondo K, Odaka K, Ueda M, Shiobara S, et al. Effect of activated lymphocytes on the regulation of hematopoiesis: enhancement and suppression of in vitro BFU-E growth by T cells stimulated by autologous non-T cells. Blood. 1986;67:1143–1147. [PubMed] [Google Scholar]
- 24.Degliantoni G, Perussia B, Mangoni L, Trinchieri G. Inhibition of bone marrow colony formation by human natural killer cells and by natural killer cell-derived colony-inhibiting activity. J Exp Med. 1985;161:1152–1168. doi: 10.1084/jem.161.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degliantoni G, Murphy M, Kobayashi M, Francis MK, Perussia B, Trinchieri G. Natural killer (NK) cell-derived hematopoietic colony-inhibiting activity and NK cytotoxic factor. Relationship with tumor necrosis factor and synergism with immune interferon. J Exp Med. 1985;162:1512–1530. doi: 10.1084/jem.162.5.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landoni VI, Vermeulen M, van RN, Gomez S, Palermo M, Isturiz MA, et al. Macrophage derived signalling regulates negatively the megakaryocyte compartment. Cell Mol Biol. 2004;50:667–675. [PubMed] [Google Scholar]
- 27.Zuckerman KS. Human erythroid burst-forming units. rowth in vitro is dependent on monocytes, but not T lymphocytes. G J Clin Invest. 1981;67:702–709. doi: 10.1172/JCI110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cicuttini FM, Loudovaris M, Boyd AW. Interactions between purified human cord blood haemopoietic progenitor cells and accessory cells. Br J Haematol. 1993;84:365–373. doi: 10.1111/j.1365-2141.1993.tb03088.x. [DOI] [PubMed] [Google Scholar]
- 29.Flores-Guzman P, Martinez-Jaramillo G, Montesinos JJ, Valencia I, Mayani H. Growth kinetics of progenitor cell-enriched hematopoietic cell populations in long-term liquid cultures under continuous removal of mature cells. Cytotherapy. 2006;8:299–307. doi: 10.1080/14653240600735776. [DOI] [PubMed] [Google Scholar]
- 30.Parmar S, Robinson SN, Komanduri K, St JL, Decker W, Xing D, et al. Ex vivo expanded umbilical cord blood T cells maintain naive phenotype and TCR diversity. Cytotherapy. 2006;8:149–157. doi: 10.1080/14653240600620812. [DOI] [PubMed] [Google Scholar]
- 31.Robinson KL, Ayello J, Hughes R, van d V, Issitt L, Kurtzberg J, et al. Ex vivo expansion, maturation, and activation of umbilical cord blood-derived T lymphocytes with IL-2, IL-12, anti-CD3, and IL-7. Potential for adoptive cellular immunotherapy post-umbilical cord blood transplantation. Exp Hematol. 2002;30:245–251. doi: 10.1016/s0301-472x(01)00781-0. [DOI] [PubMed] [Google Scholar]
- 32.Mazur MA, Davis CC, Szabolcs P. Ex vivo expansion and Th1/Tc1 maturation of umbilical cord blood T cells by CD3/CD28 costimulation. Biol Blood Marrow Transplant. 2008;14:1190–1196. doi: 10.1016/j.bbmt.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham GJ, Wright EG, Hewick R, Wolpe SD, Wilkie NM, Donaldson D, et al. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature. 1990;344:442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- 34.Graham GJ, Freshney MG, Donaldson D, Pragnell IB. Purification and biochemical characterisation of human and murine stem cell inhibitors (SCI) Growth Factors. 1992;7:151–160. doi: 10.3109/08977199209046404. [DOI] [PubMed] [Google Scholar]
- 35.Wright EG, Garland JM, Lord BI. Specific inhibition of haemopoietic stem cell proliferation: characteristics of the inhibitor producing cells. Leuk Res. 1980;4:537–545. doi: 10.1016/0145-2126(80)90065-x. [DOI] [PubMed] [Google Scholar]
- 36.Lord BI, Wright EG. Sources of haemopoietic stem cell proliferation: stimulators and inhibitors. Blood Cells. 1980;6:581–593. [PubMed] [Google Scholar]
- 37.Majhail NS, Mothukuri JM, Brunstein CG, Weisdorf DJ. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biol Blood Marrow Transplant. 2009;15:564–573. doi: 10.1016/j.bbmt.2009.01.011. [DOI] [PubMed] [Google Scholar]