Abstract
1,25-Dihydroxyvitamin D3 [1,25(OH)2D3] has many noncalcemic actions that rest on inhibition of proliferation and promotion of differentiation in malignant and normal cell types. 1,25(OH)2D3 stimulates osteoblast differentiation of human marrow stromal cells (hMSCs), but little is known about the effects of 25-hydroxyvitamin D3 [25(OH)D3] on these cells. Recent evidence shows that hMSCs participate in vitamin D metabolism and can activate 25(OH)D3 by CYP27B1/1α-hydroxylase. These studies test the hypothesis that antiproliferative and prodifferentiation effects of 25(OH)D3 in hMSCs depend on CYP27B1. We studied hMSCs that constitutively express high (hMSCshi-1α) or low (hMSCslo-1α) levels of CYP27B1 with equivalent expression of CYP24A1 and vitamin D receptor. In hMSCshi-1α, 25(OH)D3 reduced proliferation, downregulated proliferating cell nuclear antigen (PCNA), upregulated p21Waf1/Cip1, and decreased cyclin D1. Unlike 1,25(OH)2D3, the antiapoptotic effects of 25(OH)D3 on Bax and Bcl-2 were blocked by the P450 inhibitor ketoconazole. The antiproliferative effects of 25(OH)D3 in hMSCshi-1α and of 1,25(OH)2D3 in both samples of hMSCs were explained by cell cycle arrest, not by increased apoptosis. Stimulation of osteoblast differentiation in hMSCshi-1α by 25(OH)D3 was prevented by ketoconazole and upon transfection with CYP27B1 siRNA. These data indicate that CYP27B1 is required for 25(OH)D3's action in hMSCs. Three lines of evidence indicate that CYP27B1 is required for the antiproliferative and prodifferentiation effects of 25(OH)D3 on hMSCs: Those effects were not seen (1) in hMSCs with low constitutive expression of CYP27B1, (2) in hMSCs treated with ketoconazole, and (3) in hMSCs in which CYP27B1 expression was silenced. Osteoblast differentiation and skeletal homeostasis may be regulated by autocrine/paracrine actions of 25(OH)D3 in hMSCs. © 2011 American Society for Bone and Mineral Research.
Keywords: BONE MARROW STROMAL CELLS, VITAMIN D, PROLIFERATION, OSTEOBLAST DIFFERENTIATION, APOPTOSIS
Introduction
Vitamin D is an important regulator of mineral and bone metabolism, and it is now appreciated that its metabolites and analogues have many other actions. Calcitriol, or 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3], is the most active metabolite, with high affinity for the nuclear vitamin D receptor (VDR).(1) It is produced in the kidney by the 1α-hydroxylation of the precursor 25-hydroxyvitamin D3 [25(OH)D3] by the cytochrome P450 enzyme CYP27B1/1α-hydroxylase.(2) Hydroxylation of vitamin D metabolites at the carbon 24 position by 25-hydroxyvitamin D–24-hydroxylase (CYP24A1) is the first step in their inactivation and excretion. Basal expression of CYP24A1 is usually low but is highly induced by 1,25(OH)2D3.(1)
Calcitriol has major effects in inhibiting proliferation and promoting differentiation of many cell types, especially tumor cells such as human breast cancer cells,(3) colon sarcoma cells,(4) prostate cancer cells, colorectal adenoma, and carcinoma cells.(5) Epidemiologic and experimental studies also indicate that 1,25(OH)2D3 has antitumor effects(6); those effects are attributed to the inhibition of proliferation,(5,7,8) arrest of cell cycle,(3,9) increase in apoptosis,(4,10,11) and induction of differentiation.(12,13) The antiproliferative and prodifferentiation effects of 1,25(OH)2D3 also have been demonstrated for some nonmalignant cell types, such as human peripheral monocytes.(14,15) Little is known, however, about the effects of 25(OH)D3 on cell proliferation and differentiation.
In addition to kidney tubule cells, other human cells, notably osteoblasts(16) and their progenitors in the bone marrow,(17) produce 1,25(OH)2D3. Bone cells participate in vitamin D metabolism and also are targets of 1,25(OH)2D3 action.(17) The differentiation of human marrow stromal cells (hMSCs, aka mesenchymal stem cells)(18) and rat osteogenic ROS 17/2 cells(19) to osteoblasts is stimulated by 1,25(OH)2D3. Less is known about the effects of 25(OH)D3 on bone cells. In recent studies with freshly isolated hMSCs from 19 subjects, 1,25(OH)2D3 stimulated osteoblast differentiation in all samples, and 25(OH)D3 did so in two-thirds of them.(17) The variability in response to 25(OH)D3 may be due to differences in expression of CYP27B1. The combined presence of CYP27B1 and VDR indicates possible autocrine/paracrine roles for 25(OH)D3 in hMSCs. This study tests the hypothesis that the antiproliferative and prodifferentiation effects of 25(OH)D3 in hMSCs depend on CYP27B1.
Materials and Methods
Cells and reagents
Bone marrow samples were obtained with institutional review board approval as femoral tissue discarded during primary hip arthroplasty for osteoarthritis. A series of samples from 22 subjects (average age is 58 ± 15 years) was prepared and screened. Low-density marrow mononuclear cells were isolated by centrifugation on Ficoll/Histopaque 1077 (Sigma, St Louis, MO, USA).(20) This procedure removes differentiated cells and enriches for undifferentiated low-density marrow mononuclear cells that include a population of nonadherent hematopoietic cells and a fraction capable of adherence and differentiation into musculoskeletal cells. The nonadherent hematopoietic stem cells were rinsed away 24 hours after seeding, and the adherent hMSCs were expanded in monolayer culture with standard growth medium, phenol red–free α modified essential medium α-MEM), 10% fetal bovine serum–heat inactivated (FBS-HI), 100 U/mL of penicillin, and 100 µg/mL of streptomycin (Invitrogen, Carlsbad, CA, USA). All samples were used at passages 2 through 4. Some experiments used standard osteogenic medium (ie, phenol red–free α-MEM, 10% FBS-HI, 100 U/mL of penicillin, 100 µg/mL of streptomycin, 10 nM dexamethasone, 5 mM β-glycerophosphate, and 50 µg/mL of ascorbate-2-phosphate) or osteogenic medium (ie, phenol red–free α-MEM, 1% FBS-HI, 100 U/mL of penicillin, 100 µg/mL of streptomycin, 10 nM dexamethasone, 5 mM β-glycerophosphate, and 50 µg/mL of ascorbate-2-phosphate). After transfection with siRNA, all media used were without 100 U/mL of penicillin and 100 µg/mL of streptomycin. Reagents such as 25(OH)D3, 1,25(OH)2D3, and ketoconazole were purchased from Sigma; each was prepared as a stock solution at 10−3 M in absolute ethanol and stored at −80°C. In preliminary dose-finding studies (data not shown) with Western immunoblotting, we found no responses to 1, 10, or 100 nM 25(OH)D3 and responses to 1000 nM 25(OH)D3; thus most experiments used 1000 nM 25(OH)D3. In all 3-day experiments, vitamin D metabolites were added daily to control for inactivation by 24-hydroxylation.
RNA isolation and RT-PCR
Total RNA was isolated from human MSCs with TRIZOL reagent (Invitrogen). For reverse-transcriptase polymerase chain reaction (RT-PCR), 2 µg of total RNA was reverse-transcribed into cDNA with SuperScript II (Invitrogen) following the manufacturer's instructions. Concentrations of cDNA and amplification conditions were optimized for each gene product to reflect the exponential phase of amplification. One-twentieth of the cDNA was used in each 50-µL PCR reaction (30 to 40 cycles of 94°C for 1 minute, 55 to 60°C for 1 minute, and 72°C for 2 minutes), as described previously.(20) Gene-specific primer pairs (Table 1) for CYP27B1,(17) CYP24A1,(17) VDR,(17) Cbfa1/Runx2 (Runx2),(21) AlkP,(21) bone sialoprotein (BSP),(21) Bax,(21) and Bcl-2(22) were used for amplification. PCR products were separated by agarose gel electrophoresis and were quantified by densitometry of captured gel images with a Kodak Gel Logic 200 Imaging System and Kodak Molecular Imaging Software following the manufacturer's instructions (Kodak Molecular Imaging Systems, Rochester, NY, USA). Data were expressed by normalizing the densitometric units to GAPDH (internal control).
Table 1.
Primer Sets Used for RT-PCR
| Accession number | Primer name | Sequence (5′→3′) | Product size (bp) |
|---|---|---|---|
| NM_000785.3 | CYP27B1 | F = GCTACACGAGCTGCAGGTGCAGGGC | 252 |
| R = AGCGGGGCCAGGAGACTGCGGAGCC | |||
| NM_001128915.1 | CYP24A1 | F = GCAGCCTAGTGCAGATTT | 335 |
| R = ATTCACCCAGAACTGTTG | |||
| NM_001017535.1 | VDR | F = AGCCTCAATGAGGAG CACTCCAAG | 208 |
| R = ACGGGTGAGGAGGGCTGCTGAGTA | |||
| NM_004324.3 | Bax | F = GAGGATGATTGCCGCCGTGGAC | 279 |
| R = CGGTGGTGGGGGTGAGGAGG | |||
| NM_000633.2 | Bcl-2 | F = CTTTCCATGTTGTTGGCCGGATCA | 137 |
| R = CCCAGGGCAAAGAAATGCAAGTGA | |||
| NM_001015051.3 | Runx2 | F = GTTTGTTCTCTGACCGCCTC | 318 |
| R = CCAGTTCTGAGGCACCTGAAA | |||
| NM_000478.4 | AlkP | F = GCGAACGTATTTCTCCAGACCCAG | 369 |
| R = TTCCAAACAGGAGAGTCGCTTCA | |||
| NM_004967 | BSP | F = TCAGCATTTTGGGAATGGCC | 657 |
| R = GAGGTTGTTGTCTTCGAGGT | |||
| NM_002046.3 | GAPDH | F = TGATGACATCAAGAAGGTGGTGAAG | 240 |
| R = TCCTTGGAGGCCATGTGGGCCAT |
In vitro biosynthesis of 1,25(OH)2D3 by hMSCs
For comparing synthesis of 1,25(OH)2D3, hMSCs (three replicate wells) were cultivated in 12-well plates until confluence, and then the medium was changed to serum-free α-MEM supplemented with 1% insulin-transferrin-selenium plus linoleic-bovine serum albumin (ITS)+1, 10 µM 1,2-dianilinoethane (N,N'-diphenylethylene diamine; Sigma) and treated with or without 1000 nM 25(OH)D3 for 24 hours.(17) This concentration of substrate 25(OH)D3 is customary for in vitro biosynthesis studies.(17,23) 1,2-Dianilinoethane was added to the cultures as an antioxidant. Supernatants were harvested and stored at −20°C prior to analysis for 1,25(OH)2D3 content. The 1,25(OH)2D3 levels in the media were determined quantitatively with a 1,25(OH)2D3 EIA kit (Immunodiagnostic Systems, Ltd., Fountain Hills, AZ, USA) according to the manufacturer's instructions. The hMSCs were lysed with a buffer containing 150 mM NaCl, 3 mM NaHCO3, 0.1% Triton X-100, and a mixture of protease inhibitors (Roche Diagnostics, Mannheim, Germany). Protein concentration was determined with the BCA System (Thermo Fisher Scientific, Rockford, IL, USA). The CYP27B1 activity was expressed as biosynthesized 1,25(OH)2D3 in medium per milligram of protein per hour (femtomoles per milligram of protein per hour).
Proliferation
Human MSCs (hMSCshi-1α and hMSCslo-1α) at passage 2 were seeded at 3000/cm2 in 12-well plates. Cells were cultured in replicate (12 replicate wells) in standard growth medium (10% FBS-HI) in the absence or presence of 1, 10, or 100 nM 1,25(OH)2D3 or 25(OH)D3 for 3 days. Cells were suspended with 0.5 mL of 0.05% trypsin-ethylenediamine tetraacetic acid (Invitrogen), and cell number was determined by hemacytometer.
Western immunoblot
Human MSCs were cultured in 100-mm dishes in standard growth medium (10% FBS-HI). At 50% confluence, the cells were treated with 1, 10, 100 nM 1,25(OH)2D3 or 1000 nM 25(OH)D3 for 3 days. Whole-cell lysates were prepared with lysis buffer (150 mM NaCl, 3 mM NaHCO3, 0.1% Triton X-100, and a mixture of protease inhibitors; Roche Diagnostics, Mannheim, Germany) and were homogenized with a pestle (Kontes, Vineland, NJ, USA) and centrifuged at 16,000g (Eppendorf centrifuge; Eppendorf, Hamburg, Germany). Protein concentration was determined (BCA system; Thermo Fisher Scientific). Western immunoblotting was performed as described previously.(20) In brief, proteins were resolved on 4% to 12% SDS-PAGE (NuPAGE Bis-Tris gel; Invitrogen) and transferred onto polyvinylidene fluoride membranes (PVDF; Amersham Biosciences, Piscataway, NJ, USA). The membranes were blocked with 5% nonfat milk in PBS buffer containing 0.1% Tween-20 (PBST) for 2 to 3 hours at room temperature and incubated at 4°C overnight with primary antibodies proliferating cell nuclear antigen (PCNA) (1:3000; Abcam, Cambridge, UK), CYP27B1 (H-90, 1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), β-actin (1:8000, Santa Cruz Biotechnology, Inc.), and Bax, Bcl-2, p21Waf1/Cip1, and cyclin D1 (each at 1:1000; Cell Signaling Technology, Beverly, MA, USA). After removal of the unbound primary antibodies by three 5-minute washes with PBST, the membranes were incubated with horseradish peroxidase–conjugated secondary antibodies (1:5000) for 1 hour at room temperature and washed three times for 5 minutes with PBST. The antibody-associated protein bands were revealed with the ECL-plus Western blotting system (Amersham Biosciences).
Alkaline phosphatase (AlkP) enzymatic activity assay
For AlkP enzymatic activity assay, the concentration of serum in standard osteogenic medium (10% FBS-HI) was reduced to 1% FBS-HI to minimize possible subsequent differences in proliferation that could confound interpretation of the effects of vitamin D metabolites on osteoblastogenesis. The medium was changed every 2 days. AlkP enzyme activity was measured spectrophotometrically, as described previously.(21) Protein concentration was determined with the BCA system (Thermo Fisher Scientific, Inc.). The AlkP enzyme activity was expressed as micromoles per minute per gram of protein, and some was calculated as the ratio of treated relative to control.
RNA interference with CYP27B1 siRNA
Transient transfection of siRNA into hMSCshi-1α was performed by electroporation with the Human MSC Nucleofector Kit (Lonza/Amaxa Biosystems, Walkersville, MD, USA) with either CYP27B1 siRNA, nonsilencing control siRNA (a nonhomologous, scrambled sequence equivalent; Santa Cruz Biotechnology, Inc.), or PBS according to the manufacturer's instructions and as described previously.(24) In brief, hMSCshi-1α were harvested by trypsinization and resuspended at 106 cells in 100 µL of Nucleofector Solution (Lonza/Amaxa Biosystems) with 10 or 100 pmol of CYP27B1 siRNA. Electroporation was performed in Nucleofector II device with Program U-23 (Lonza/Amaxa Biosystems). Immediately after electroporation, the cells were transferred to 60-mm dishes or 12-well plates in phenol red–free α-MEM and 10% FBS-HI. Some cells were collected at 80% confluence for RT-PCR or Western immunoblot analysis to determine the effect of CYP27B1 siRNA. Some cells that were cultured until confluent in the 12-well plates were treated with or without 1000 nM 25(OH)D3 in serum-free α-MEM supplemented with 1% ITS+1, 10 µM 1,2-dianilinoethane (N,N'-diphenylethylene diamine) for 24 hours to assess 1α-hydroxlyase activity. Cellular 1,25(OH)2D3 production was determined by EIA as described under “In vitro biosynthesis of 1,25(OH)2D3 by hMSCs.” At 24 hours after electroporation, some cells were treated with either 25(OH)D3 (1000 nM) or vehicle control (ethanol) daily in standard growth medium (10% FBS-HI) for another 72 hours for RT-PCR assays. When some cells in the 12-well plates were nearly 80% confluent, the medium was changed to the osteogenic medium with 1% FBS-HI ± 10 nM 25(OH)D3 for 7 days for assessment of alkP enzymatic activity as another index of osteoblast differentiation.
Statistical analysis
Experiments were performed at least in triplicate. Group data are presented as mean ± SEM unless otherwise indicated. Quantitative data were analyzed with nonparametric tools, either the Mann-Whitney test or Spearman correlation test. If data allowed, parametric tools were used, either t test for two group or one-way ANOVA for multiple group comparisons or Pearson correlation test. A value of p < .05 was considered significant.
Results
Expression of CYP27B1 and CYP24A1 genes and 1α-hydroxylase activity in hMSCs
Gene expression analysis with hMSCs from 22 subjects showed a wide range of constitutive expression of CYP27B1 (Fig. 1A, showing 7 representative samples). Two samples of hMSCs were selected for detailed studies, having either high (hMSCshi-1α, from a 42-year-old man) or low (hMSCslo-1α, from a 46-year-old man) levels of CYP27B1, with equivalent expression of CYP24A1 and VDR (Fig. 1B). Their activity for 1α-hydroxylation was compared by measuring production of 1,25(OH)2D3. Biosynthesis of 1,25(OH)2D3 in the hMSCshi-1α was 2.98-fold greater that in the hMSCslo-1α (4433.4 versus 1487.9 fmol/mg protein per hour, p < .0001; Fig. 1C). Upregulation of CYP24A1 by 1,25(OH)2D3 (1, 10, and 100 nM) or 25(OH)D3 (1000 nM) treatment was equivalent in both specimens of hMSCs (Fig. 1D).
Fig. 1.
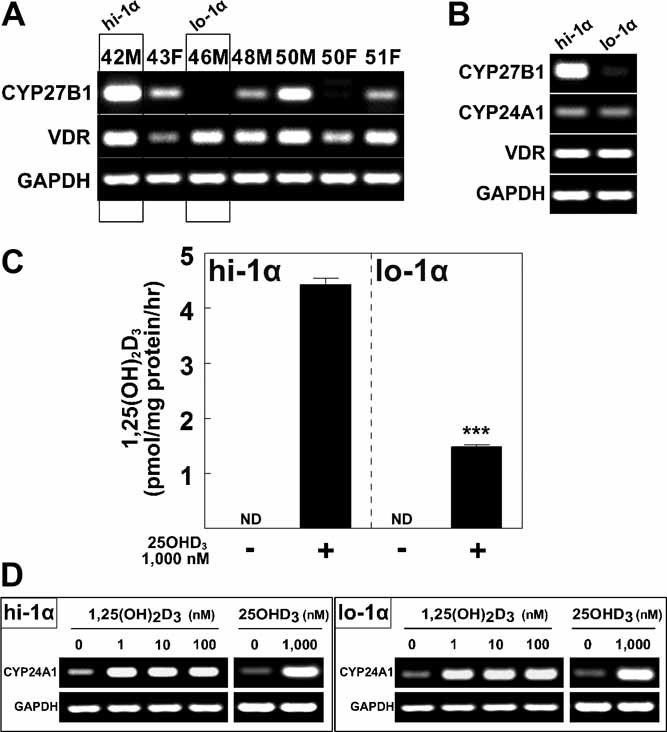
Expression of CYP27B1 and CYP24A1 genes and 1α-hydroxylase activity in hMSCs. (A) Gel electrophoretogram shows RT-PCR products CYP27B1, VDR, and GAPDH in 7 representative specimens of hMSCs. Labels for lanes indicate age and gender. (B) Gel electrophoretogram shows RT-PCR products for CYP27B1, CYP24A1, VDR, and GAPDH in selected hMSCshi-1α (from a 42-year-old man) and hMSCslo-1α (from a 46-year-old man). (C) 1,25(OH)2D3 synthesis was measured in hMSCshi-1α and hMSCslo-1α. Cultures were treated with or without 1000 nM 25(OH)D3 in serum-free α-MEM supplemented with 1% ITS+1, 10 µM 1,2-dianilinoethane (N,N'-diphenylethylene diamine) for 24 hours. Cellular 1,25(OH)2D3 production (three replicate wells) was determined by EIA. There was no detectable (ND) 1,25(OH)2D3 in cultures without 25(OH)D3 exogenous substrate. ***p < .001. (D) Gel electrophoretogram shows RT-PCR products for CYP24A1 and GAPDH in hMSChi-1α and hMSClo-1α cultures after 3 days in standard growth medium with 10% FBS-HI in the absence or presence of 1,25(OH)2D3 or 25(OH)D3.
Relative antiproliferative effects of 25(OH)D3 and 1,25(OH)2D3 on hMSCs
Two samples of hMSCs were cultured for 3 days after seeding in standard growth medium (10% FBS-HI). There was less cellularity in cultures of both hMSCshi-1α and hMSCslo-1α treated with 100 nM 1,25(OH)2D3 compared with vehicle control. In contrast, only the hMSCshi-1α were inhibited by 25(OH)D3 (Fig. 2A). There was a dose-dependent inhibition of proliferation with 1,25(OH)2D3 for both cell preparations (Fig. 2B). Both 25(OH)D3 and 1,25(OH)2D3 inhibited proliferation of hMSCshi-1α; there was a significant inhibition of proliferation of hMSCshi-1α at 100 nM of 25(OH)D3 (56% of control cell number, p < .001) and 1,25(OH)2D3 (50%; p < .001). In contrast, hMSCslo-1α were resistant to 25(OH)D3 (96%) yet were inhibited by 1,25(OH)2D3 (17%, p< .001; Fig. 2B). Consistent with the effects on cell numbers, PCNA was downregulated by 1,25(OH)2D3 in hMSCshi-1α and in hMSCslo-1α (Fig. 2C). With 25(OH)D3 treatment, PCNA in hMSCshi-1α was 64.9% of control, but for hMSCslo-1α, PCNA was equivalent to control. With hMSCshi-1α, both 25(OH)D3 and 1,25(OH)2D3 downregulated cyclin D1 and upregulated the negative regulator p21Waf1/Cip1 (Fig. 2D). In hMSCslo-1α, the effects of 1,25(OH)2D3 on cell cycle regulators were similar to those for hMSCshi-1α, but there were no effects by 25(OH)D3.
Fig. 2.
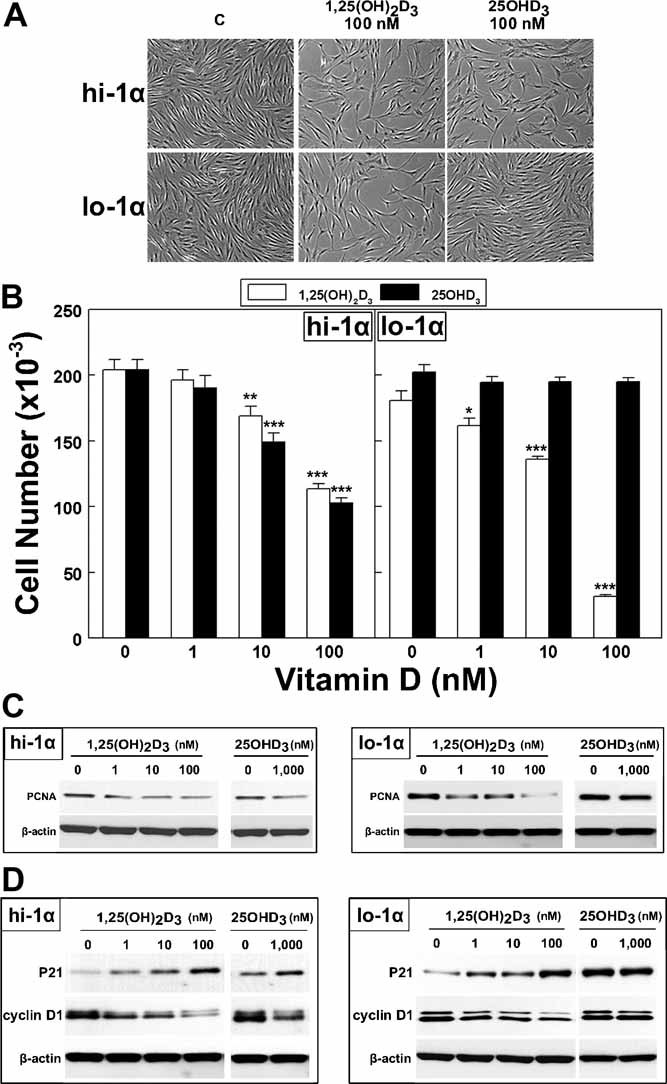
Relative effects of 25(OH)D3 and 1,25(OH)2D3 on proliferation of hMSCs. (A) Photomicrographs show hMSChi-1α and hMSClo-1α cultures after 3 days in the absence or presence of 100 nM 1,25(OH)2D3 or 25(OH)D3 (×200 magnification). (B) Cell number was determined in hMSChi-1α and hMSClo-1α cultures after 3 days in the absence or presence of 1, 10, 100 nM 1,25(OH)2D3 or 25(OH)D3. Results are expressed as mean ± SEM (12 replicate wells). *p < .05; **p < .01; ***p < .001. (C) Western immunoblots show proliferating cell nuclear antigen and β-actin levels in hMSChi-1α and hMSClo-1α cultures after 3 days in the absence or presence of 1,25(OH)2D3 or 25(OH)D3. (D) Western immunoblots show p21, cyclin D1, and β-actin in hMSChi-1α and hMSClo-1α cultures after 3 days in the absence or presence of 1,25(OH)2D3 or 25(OH)D3.
Relative effects of 25(OH)D3 and 1,25(OH)2D3 on Bax/Bcl-2 ratios in hMSCs
Mechanisms involved in the relative effects of 25(OH)D3 and 1,25(OH)D3 were studied by analysis of expression of apoptosis-associated proteins. First, effects of metabolites were compared in hMSCshi-1α and hMSCslo-1α. In hMSCshi-1α, both 25(OH)D3 and 1,25(OH)2D3 induced a downregulation of Bax and an upregulation of the Bcl-2 protein (Fig. 3A); these effects resulted in lower Bax/Bcl-2 ratios with 100 nM 1,25(OH)2D3 (20% compared with vehicle control) and with 1000 nM 25(OH)D3 (43%). In hMSCslo-1α, the Bax/Bcl-2 ratio was lower with 100 nM 1,25(OH)2D3 (19%), but there was essentially no effect by 25(OH)D3 (95%). The effects on mRNA levels of Bax and Bcl-2 (Fig. 3B) corresponded with the changes observed for protein levels (Fig. 3A).
Fig. 3.
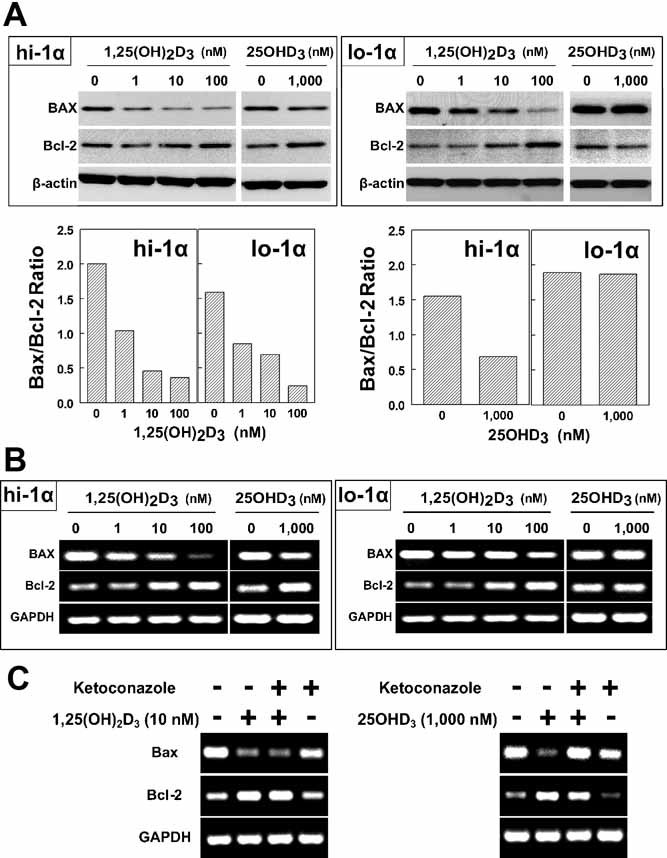
Relative effects of 25(OH)D3 and 1,25(OH)2D3 on Bax/Bcl-2 ratios in hMSCs. (A) Western immunoblots show Bax, Bcl-2, and β-actin in hMSChi-1α and hMSClo-1α cultures after 3 days in the absence or presence of 1,25(OH)2D3 or 25(OH)D3. The bar graphs represent the Bax/Bcl-2 ratios after each densitometric value was normalized to β-actin. (B) Gel electrophoretograms show RT-PCR products for Bax, Bcl-2, and GAPDH in hMSChi-1α and hMSClo-1α cultures after 3 days in the absence or presence of 1, 10, or 100 nM 1,25(OH)2D3 or 1000 nM 25(OH)D3. (C) Gel electrophoretogram shows RT-PCR products for Bax, Bcl-2, and GAPDH in hMSCshi-1α after 3 days in the absence or presence of 10 nM 1,25(OH)2D3 or 1000 nM 25(OH)D3 ± 10 µM ketoconazole.
As a second approach, the cytochrome P450 inhibitor ketoconazole was used to determine the importance of hydroxylation on 25(OH)D3 effects on proliferation. Ketoconazole (10 µM) diminished the effects of 25(OH)D3 (1000 nM) and not the effects of 1,25(OH)2D3 (10 nM) on Bax and Bcl-2 in hMSCshi-1α (Fig. 3C). In the presence of 25(OH)D3, the Bax/Bcl-2 ratio was 27% of that with vehicle control, and the decrease by 25(OH)D3 was blocked by ketoconazole (90%). In the presence of 1,25(OH)2D3, the Bax/Bcl-2 ratio was 36% of that with vehicle control, similar to that with 1,25(OH)2D3 and ketoconazole (37%).
Relative effects of 25(OH)D3 and 1,25(OH)2D3 on osteoblast differentiation in hMSCs
Regulation of osteoblast differentiation was quantified first by AlkP enzymatic activity assays in osteogenic medium with 1% FBS-HI (Fig. 4A). In hMSCshi-1α, there was similar stimulation of AlkP activity by 25(OH)D3 (2.16-fold, p = .0003) and by 1,25(OH)2D3 (1.77-fold, p < .0001). In contrast, with hMSCslo-1α, 25(OH)D3 had no effect (0.96-fold, p = .577), and 1,25(OH)2D3 stimulated AlkP activity (1.86-fold, p < .0001).
Fig. 4.
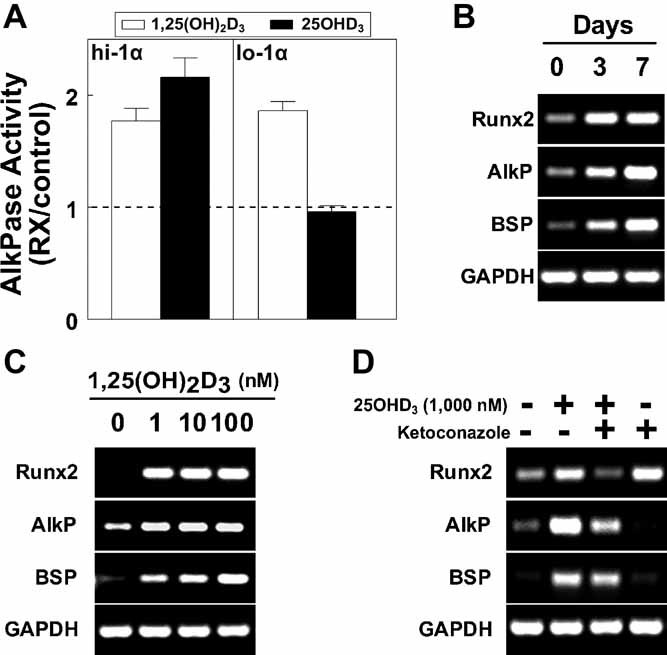
Comparison of effects of 25(OH)D3 and 1,25(OH)2D3 on osteoblast differentiation in hMSCs. (A) Alkaline phosphatase enzymatic activity (6 replicate wells) was measured in hMSCshi-1α and hMSCslo-1α in the absence or presence of 10 nM 1,25(OH)2D3 (open bars) or 25(OH)D3 (closed bars) in osteogenic medium with 1% FBS-HI for 7 days. Results are reported relative to control (Rx/control) with horizonal dashed line as 1.0; mean ± SEM. (B) Gel electrophoretogram shows RT-PCR products of osteoblast signature genes (Runx2, AlkP, and BSP) and GAPDH in hMSCshi-1α after 0, 3, and 7 days in standard osteogenic medium with 10% FBS-HI. (C) Gel electrophoretogram shows RT-PCR products of osteoblast signature genes (Runx2, AlkP, and BSP) and GAPDH in hMSCshi-1α after 3 days in the absence or presence of 1, 10, or 100 nM 1,25(OH)2D3 in standard growth medium with 10% FBS-HI. (D) Gel electrophoretogram shows RT-PCR products of osteoblast signature genes (Runx2, AlkP, and BSP) and GAPDH in hMSCshi-1α after 3 days in the absence or presence of 1000 nM 25(OH)D3 ± 10 µM ketoconazole in standard growth medium with 10% FBS-HI.
Osteoblast differentiation was also monitored by osteoblast signature genes (ie, Runx2, AlkP, and BSP) after transfer to standard osteogenic medium (10% FBS-HI) or after addition of 1,25(OH)2D3 to standard growth medium (10% FBS-HI). As expected, there was time-dependent upregulation of Runx2, AlkP, and BSP in hMSCshi-1α in standard osteogenic medium (Fig. 4B). Addition of 1,25(OH)2D3 to standard growth medium also upregulated osteoblast genes in hMSCshi-1α (Fig. 4C). Addition of 25(OH)D3 to standard growth medium also upregulated osteoblast genes in hMSCshi-1α, but its effect was diminished by ketoconazole (Fig. 4D).
Effect of CYP27B1 siRNA on the stimulation of osteoblast differentiation by 25(OH)D3
As another approach to assess the mechanism by which 25(OH)D3 can stimulate osteoblast differentiation, hMSCshi-1α were engineered to have reduced constitutive expression of CYP27B1. There were no noticeable differences in cell density or appearance of control cells (electroporation with PBS), cells treated with nonsilencing control siRNA, and cells with 10 or 100 pmol CYP27B1 siRNA (Fig. 5A). Transient transfection of CYP27B1 siRNA into hMSCshi-1α resulted in reductions of CYP27B1 mRNA (2% of control; Fig. 5B) and CYP27B1 protein (11% of control; Fig. 5C). No effect was shown with a nonsilencing, scrambled siRNA sequence (lane NC in Fig. 5B, C). The amount of 1,25(OH)2D3 synthesized by the cells transfected with CYP27B1 siRNA was 22% of that for cells transfected with nonsilencing siRNA (1075 versus 4786 fmol/mg protein per hour, p < .0001; Fig. 5D). Treatment with 25(OH)D3 upregulated Runx2, AlkP, and BSP in both control preparations of hMSCshi-1α. With cells transfected with CYP27B1 siRNA, however, 25(OH)D3 had no effect on osteoblast genes (Fig. 5E). As a functional marker of osteoblast differentiation, we measured AlkP enzymatic activity after 7 days in osteogenic medium (1% FBS-HI). Whereas 25(OH)D3 stimulated AlkP activity of control cells (1.87-fold, p < .0001), there was no effect in cells transfected with CYP27B1 siRNA (1.04-fold, p = .093; Fig. 5F).
Fig. 5.
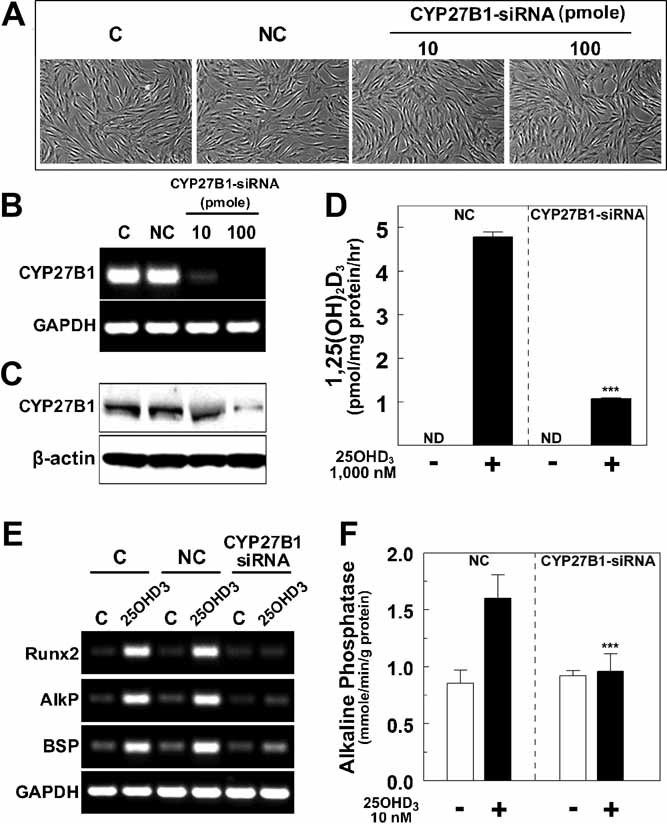
Effect of CYP27B1 siRNA on the stimulation of osteoblast differentiation by 25(OH)D3. Four groups were treated by electroporation with PBS (C = control), with nonsilencing control siRNA (NC), or with 10 or 100 pmol of CYP27B1 siRNA. (A) Photomicrographs show cultures of control and transfected hMSCshi-1α (×200 magnification). (B) Gel electrophoretogram shows CYP27B1 and GAPDH in controls and in transfected cells. (C) Western immunoblot shows CYP27B1 and β-actin protein levels in controls and in transfected cells. (D) Cells transfected with nonsilencing siRNA and 100 pmol of CYP27B1 siRNA were treated with or without 1000 nM 25(OH)D3 in serum-free α-MEM supplemented with 1% ITS+1, 10 µM 1,2-dianilinoethane (N,N'-diphenylethylene diamine) for 24 hours. Cellular 1,25(OH)2D3 production was determined by EIA as described under “In vitro biosynthesis of 1,25(OH)2D3 by hMSCs.” Results are shown as the mean ± SEM (3 replicate wells). There was no detectable (ND) 1,25(OH)2D3 in cultures without 1000 nM 25(OH)D3 exogenous substrate. ***p< .001. (E) Gel electrophoretogram shows Runx2, AlkP, BSP, and GAPDH in controls and in transfected hMSCshi-1α after 3 days ± 1000 nM 25(OH)D3. (F) Alkaline phosphatase enzymatic activity was measured in control and transfected hMSCshi-1α (100 pmol of CYP27B1 siRNA) after 7 days ± 10 nM 25(OH)D3 in osteogenic medium. Values represent the mean ± SEM (6 replicate wells). ***p< .001.
Discussion
This study used three approaches to examine the role of CYP27B1 on the effects of 25(OH)D3 in hMSCs. First, we compared cells with high and low constitutive expression of CYP27B1. Finding a wide range of expression in hMSCs from 22 subjects is consistent with our previous studies.(17) The level of CYP27B1 expression was found to be related to the vitamin D status(17) and, more recently, to age(25) of the subjects. There is growing evidence that hMSCs(17) and human bone cells(26) are both sources and targets of 1,25(OH)2D3, and thus vitamin D may have multiple autocrine/paracrine actions in bones.
It was important to control for 24-hydroxylation in these studies because differences in inactivation of added vitamin D metabolites could confound interpretation. The activities of CYP27B1 and CYP24A1 are important for the maintenance of appropriate levels of 1,25(OH)2D3 and 25(OH)D3. Therefore, two hMSCs were selected and studied in detail on the basis of having extremes in expression of CYP27B1 and equivalent expression of CYP24A1 and VDR. Further, the expression of CYP24A1 was found to be regulated with equivalence in both specimens of hMSCs; this observation reduces concerns of confounding effects of 24-hydroxylation in these studies. In addition, fresh metabolites were added daily.
There were substantial differences in the synthesis of 1,25(OH)2D3 by hMSCshi-1α and hMSCslo-1α. The difference also held for hMSCs with and without CYP27B1 gene silencing. Although these experiments cannot be used to estimate what would be the steady-state concentration of 1,25(OH)2D3 in the bone marrow in different subjects whose cells have high or low expression of CYP27B1, they provide evidence for a potential autocrine/paracrine role for 25(OH)D3 metabolism in osteoblast differentiation. Similar ideas have been proposed for 25(OH)D3 metabolism in regulating bone matrix formation by differentiated human osteoblasts.(23)
There was dose-dependent inhibition of proliferation by 25(OH)D3 with hMSCs that had a high level of expression of CYP27B1; 25(OH)D3 reduced their proliferation and downregulated PCNA. There is some information about antiproliferative actions of 25(OH)D3 in other human cell types. In human primary prostate epithelial cells that expressed CYP27B1, low concentrations of 25(OH)D3 suppressed cell growth.(27) In prostatic cancer cells lacking CYP27B1, 25(OH)D3 failed to demonstrate antiproliferative action.(28) There are several mechanisms mediating the antiproliferative effects of 1,25(OH)2D3. In U937 myelomonocytic cells, 1,25(OH)2D3 induces an arrest in the G1 phase of the cell cycle that depends on upregulation of the cyclin-dependent kinase inhibitor p21Waf1/Cip1.(29) More recently, p21Waf1/Cip1 was shown to be a primary antiproliferative mediator for the VDR in the presence of its ligand, 1,25(OH)2D3.(30) Cyclin D1 is increased in dividing cells during the G1 phase and is necessary for the transition from G1 to S phase.(31) Vitamin D decreases cyclin D1 abundance and/or activity by different mechanisms in different cell types. For example, in human epidermoid A431 cells, 1,25(OH)2D3 inhibited transforming growth factor α (TGF-α)/endothelial growth factor receptor (EGFR) transactivation of cyclin D1.(32) We found that 25(OH)D3 downregulated cyclin D1 and upregulated the negative regulator p21Waf1/Cip1 in hMSCshi-1α. In contrast, 25(OH)D3 had no such effects in hMSCslo-1α. The upregulation of p21Waf1/Cip1 and decreased expression of cyclin D1 in hMSCshi-1α provide mechanisms for the antiproliferative effect of 25(OH)D3.
1,25(OH)2D3 also affects the levels of proapoptotic (ie, Bax and Bak) and antiapoptotic (ie, Bcl-2 and Bcl-XL) proteins, resulting in apoptosis in several tumor models, including human carcinomas of the breast, colon, and prostate.(4,10,33) This study with hMSCs indicates that the antiproliferation effects of 1,25(OH)2D3 or 25(OH)D3 are not explained by increases in Bax or decreases in Bcl-2. In fact, the ratio of Bax/Bcl-2 decreases at both the mRNA and protein levels. Two lines of evidence indicate that those effects of 25(OH)D3 on Bax and Bcl-2 depend on CYP27B1. First, they were not detected in hMSCslo-1α. Second, they were blocked in hMSCshi-1α by the pan-cytochrome P450 inhibitor ketoconazole, not like the effects of 1,25(OH)2D3, which were not affected by ketoconazole. The antiapoptotic effects of 1,25(OH)2D3 or 25(OH)D3 in hMSCshi-1α are different from the proapoptotic effects in some cancer cells(4,10,33) and are similar to the effects in other cell types. In ovarian cancer cells, 1,25(OH)2D3 inhibits apoptosis that is mediated by death receptors.(34) In rat osteoblast-like osteosarcoma UMR 106 cells, 1,25(OH)2D3 elicited antiapoptotic effects by decreasing the Bax/Bcl-2 ratio.(11) There are other antiapoptotic signals, as was reported for nongenotropic mechanisms in osteoblasts and osteocytes.(35) In sum, the data indicate that the antiproliferative effects of 25(OH)D3 in hMSCshi-1α and of 1,25(OH)2D3 in both samples of hMSCs are explained by cell cycle arrest and not by increased apoptosis.
Calcitriol induces differentiation of many types of benign and malignant cells.(12,13,18,36–38) The differentiation of various cells, including human myelomonocytic cells,(9,29) induced by 1,25(OH)2D3 depends on the induction of p21Waf1/Cip1. Further, 1,25(OH)2D3 is a key regulator of the reciprocal relationship between proliferation and differentiation during the osteoblast development sequence.(39) Vitamin D or its analogues promote osteoblastic differentiation, as shown for osteosarcoma cell lines MG-63,(40) HOS,(23) SAOS,(41) and TE85.(41) Osteoblast differentiation of human MSCs is stimulated by 1,25(OH)2D3 or 25(OH)D3 in hMSCshi-1α, as shown here and elsewhere.(17,18,37,38,42) As expected, 25(OH)D3 failed to stimulate osteoblast differentiation in hMSCslo-1α. Upregulation of osteoblast genes by 25(OH)D3 in hMSCshi-1α was diminished by ketoconazole. Thus experiments with ketoconazole indicate that both the antiproliferative and prodifferentiation effects of 25(OH)D3 depend on CYP27B1.
Ketoconazole is a strong but differential inhibitor of both CYP24A1 and CYP27B1(43) and may be cytotoxic for some cells.(23) Those confounders may complicate interpretation of results obtained with this agent. We therefore used the highly specific technique of RNA interference to inhibit CYP27B1 expression in hMSCshi-1α. The level of synthesized 1,25(OH)2D3 in the cells transfected with CYP27B1 siRNA was reduced to 22% of that for cells transfected with nonsilencing siRNA. Osteoblast differentiation of hMSCshi-1α by 25(OH)D3 was prevented upon transfection with CYP27B1 siRNA, as indicated by osteoblast signature gene expression and by AlkP enzymatic activity. These findings are consistent with those from a study with HOS human osteosarcoma cells in which silencing of CYP27B1 resulted in a suppression of 25(OH)D3's effects on those cells.(23,44)
In conclusion, 25(OH)D3 has multiple effects in normal hMSCs; it inhibits proliferation and promotes osteoblast differentiation by mechanisms similar to those for 1,25(OH)2D3. Our data indicate that antiproliferative and prodifferentiation effects of 25(OH)D3 in hMSCs require 1α-hydroxylase. There are suggestions that other effects of 25(OH)D3 in other cell types, such as induction of 24-hydroxylase in prostatic cells, may not require 1α-hydroxylase.(45) Three lines of evidence indicate that CYP27B1 is required for the effects of 25(OH)D3 on hMSCs. Those effects were not seen (1) in hMSCs with low constitutive expression of CYP27B1, (2) in hMSCs treated with ketoconazole, or (3) in hMSCs in which CYP27B1 expression was silenced. These findings suggest that local osteoblast differentiation in vivo may be promoted by 25(OH)D3 if the progenitor/precursor cells in marrow express CYP27B1/1α-hydroxylase. We found that many of hMSCs' in vitro behaviors and baseline characteristics depend on clinical features of the subjects from whom the cells were isolated. The level of CYP27B1 expression in those cells depends on vitamin D status and can be regulated by a number of factors, including vitamin D.(17) The combined presence of CYP27B1 and the VDR in hMSCs indicates possible autocrine/paracrine roles for 25(OH)D3 to regulate osteoblast differentiation and skeletal homeostasis.
Acknowledgments
We greatly appreciate Drs Zhenggang Bi and Shuichi Mizuno for advice and Ms Sara Anderson for assistance. This work is based on a thesis by Shuo Geng for MD and PhD degrees from Harbin Medical University, China. Shuo Geng was supported by the China Scholarship Council (CSC). This project was supported by NIH Grants AG025015 and AG028114.
Disclosures
All the authors state that they have no conflicts of interest.
References
- 1.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 2.Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- 3.Verlinden L, Verstuyf A, Convents R, Marcelis S, Van Camp M, Bouillon R. Action of 1,25(OH)2D3 on the cell cycle genes cyclin D1, p21, and p27 in MCF-7 cells. Mol Cell Endocrinol. 1998;142:57–65. doi: 10.1016/s0303-7207(98)00117-8. [DOI] [PubMed] [Google Scholar]
- 4.Vandewalle B, Wattez N, Lefebvre J. Effects of vitamin D3 derivatives on growth, differentiation and apoptosis in tumoral colonic HT 29 cells: possible implication of intracellular calcium. Cancer Lett. 1995;97:99–106. doi: 10.1016/0304-3835(95)03958-y. [DOI] [PubMed] [Google Scholar]
- 5.Ylikomi T, Laaksi I, Lou YR, et al. Antiproliferative action of vitamin D. Vitam Horm. 2002;64:357–406. doi: 10.1016/s0083-6729(02)64010-5. [DOI] [PubMed] [Google Scholar]
- 6.Beer TM, Myrthue A. Calcitriol in cancer treatment: from the lab to the clinic. Mol Cancer Ther. 2004;3:373–381. [PubMed] [Google Scholar]
- 7.Shannan B, Seifert M, Boothman DA, Tilgen W, Reichrath J. Clusterin over-expression modulates proapoptotic and antiproliferative effects of 1,25(OH)2D3 in prostate cancer cells in vitro. J Steroid Biochem Mol Biol. 2007;103:721–725. doi: 10.1016/j.jsbmb.2006.12.068. [DOI] [PubMed] [Google Scholar]
- 8.Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994;54:805–810. [PubMed] [Google Scholar]
- 9.Rots NY, Iavarone A, Bromleigh V, Freedman LP. Induced differentiation of U937 cells by 1,25-dihydroxyvitamin D3 involves cell cycle arrest in G1 that is preceded by a transient proliferative burst and an increase in cyclin expression. Blood. 1999;93:2721–2729. [PubMed] [Google Scholar]
- 10.Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:367–376. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 11.Morales O, Samuelsson MK, Lindgren U, Haldosen LA. Effects of 1alpha,25-dihydroxyvitamin D3 and growth hormone on apoptosis and proliferation in UMR 106 osteoblast-like cells. Endocrinology. 2004;145:87–94. doi: 10.1210/en.2003-0718. [DOI] [PubMed] [Google Scholar]
- 12.Abe E, Miyaura C, Sakagami H, et al. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangelsdorf DJ, Koeffler HP, Donaldson CA, Pike JW, Haussler MR. 1,25-Dihydroxyvitamin D3-induced differentiation in a human promyelocytic leukemia cell line (HL-60): receptor-mediated maturation to macrophage-like cells. J Cell Biol. 1984;98:391–398. doi: 10.1083/jcb.98.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manolagas SC, Provvedini DM, Murray EJ, Tsoukas CD, Deftos LJ. The antiproliferative effect of calcitriol on human peripheral blood mononuclear cells. J Clin Endocrinol Metab. 1986;63:394–400. doi: 10.1210/jcem-63-2-394. [DOI] [PubMed] [Google Scholar]
- 15.Adams JS, Beeker TG, Hongo T, Clemens TL. Constitutive expression of a vitamin D 1-hydroxylase in a myelomonocytic cell line: a model for studying 1,25-dihydroxyvitamin D production in vitro. J Bone Miner Res. 1990;5:1265–1269. doi: 10.1002/jbmr.5650051212. [DOI] [PubMed] [Google Scholar]
- 16.Howard GA, Turner RT, Sherrard DJ, Baylink DJ. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J Biol Chem. 1981;256:7738–7740. [PubMed] [Google Scholar]
- 17.Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010;151:14–22. doi: 10.1210/en.2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu P, Oyajobi BO, Russell RG, Scutt A. Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-beta and 1,25(OH)(2) vitamin D(3) In vitro. Calcif Tissue Int. 1999;65:173–180. doi: 10.1007/s002239900678. [DOI] [PubMed] [Google Scholar]
- 19.Manolagas SC, Burton DW, Deftos LJ. 1,25-Dihydroxyvitamin D3 stimulates the alkaline phosphatase activity of osteoblast-like cells. J Biol Chem. 1981;256:7115–7117. [PubMed] [Google Scholar]
- 20.Zhou S, Lechpammer S, Greenberger JS, Glowacki J. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-beta/Smad3 signaling. J Biol Chem. 2005;280:22688–22696. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou S, Greenberger JS, Epperly MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tirado OM, Mateo-Lozano S, Notario V. Rapamycin induces apoptosis of JN-DSRCT-1 cells by increasing the Bax : Bcl-xL ratio through concurrent mechanisms dependent and independent of its mTOR inhibitory activity. Oncogene. 2005;24:3348–3357. doi: 10.1038/sj.onc.1208471. [DOI] [PubMed] [Google Scholar]
- 23.Atkins GJ, Anderson PH, Findlay DM, et al. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone. 2007;40:1517–1528. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Aslan H, Zilberman Y, Arbeli V, et al. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006;12:877–889. doi: 10.1089/ten.2006.12.877. [DOI] [PubMed] [Google Scholar]
- 25.Geng S, Zhou S, Glowacki J. Age-related Declines in Expression of CYP27B1 and in Osteoblast Differentiation in Human MSCs. J Bone Miner Res. 2010;25:S107. [Google Scholar]
- 26.van Driel M, Koedam M, Buurman CJ, et al. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. Faseb J. 2006;20:2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 27.Barreto AM, Schwartz GG, Woodruff R, Cramer SD. 25-Hydroxyvitamin D3, the prohormone of 1,25-dihydroxyvitamin D3, inhibits the proliferation of primary prostatic epithelial cells. Cancer Epidemiol Biomarkers Prev. 2000;9:265–270. [PubMed] [Google Scholar]
- 28.Hsu JY, Feldman D, McNeal JE, Peehl DM. Reduced 1alpha-hydroxylase activity in human prostate cancer cells correlates with decreased susceptibility to 25-hydroxyvitamin D3-induced growth inhibition. Cancer Res. 2001;61:2852–2856. [PubMed] [Google Scholar]
- 29.Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 30.Saramaki A, Banwell CM, Campbell MJ, Carlberg C. Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Res. 2006;34:543–554. doi: 10.1093/nar/gkj460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pines J. Four-dimensional control of the cell cycle. Nat Cell Biol. 1999;1:E73–79. doi: 10.1038/11041. [DOI] [PubMed] [Google Scholar]
- 32.Cordero JB, Cozzolino M, Lu Y, et al. 1,25-Dihydroxyvitamin D down-regulates cell membrane growth- and nuclear growth-promoting signals by the epidermal growth factor receptor. J Biol Chem. 2002;277:38965–38971. doi: 10.1074/jbc.M203736200. [DOI] [PubMed] [Google Scholar]
- 33.Guzey M, Kitada S, Reed JC. Apoptosis induction by 1alpha,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther. 2002;1:667–677. [PubMed] [Google Scholar]
- 34.Zhang X, Li P, Bao J, et al. Suppression of death receptor-mediated apoptosis by 1,25-dihydroxyvitamin D3 revealed by microarray analysis. J Biol Chem. 2005;280:35458–35468. doi: 10.1074/jbc.M506648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vertino AM, Bula CM, Chen JR, et al. Nongenotropic, anti-apoptotic signaling of 1alpha,25(OH)2-vitamin D3 and analogs through the ligand binding domain of the vitamin D receptor in osteoblasts and osteocytes. Mediation by Src, phosphatidylinositol 3-, and JNK kinases. J Biol Chem. 2005;280:14130–14137. doi: 10.1074/jbc.M410720200. [DOI] [PubMed] [Google Scholar]
- 36.Provvedini DM, Deftos LJ, Manolagas SC. 1,25-Dihydroxyvitamin D3 promotes in vitro morphologic and enzymatic changes in normal human monocytes consistent with their differentiation into macrophages. Bone. 1986;7:23–28. doi: 10.1016/8756-3282(86)90148-1. [DOI] [PubMed] [Google Scholar]
- 37.Beresford JN, Joyner CJ, Devlin C, Triffitt JT. The effects of dexamethasone and 1,25-dihydroxyvitamin D3 on osteogenic differentiation of human marrow stromal cells in vitro. Arch Oral Biol. 1994;39:941–947. doi: 10.1016/0003-9969(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 38.Fromigue O, Marie PJ, Lomri A. Differential effects of transforming growth factor beta2, dexamethasone and 1,25-dihydroxyvitamin D on human bone marrow stromal cells. Cytokine. 1997;9:613–623. doi: 10.1006/cyto.1997.0209. [DOI] [PubMed] [Google Scholar]
- 39.Owen TA, Aronow MS, Barone LM, Bettencourt B, Stein GS, Lian JB. Pleiotropic effects of vitamin D on osteoblast gene expression are related to the proliferative and differentiated state of the bone cell phenotype: dependency upon basal levels of gene expression, duration of exposure, and bone matrix competency in normal rat osteoblast cultures. Endocrinology. 1991;128:1496–1504. doi: 10.1210/endo-128-3-1496. [DOI] [PubMed] [Google Scholar]
- 40.Finch JL, Dusso AS, Pavlopoulos T, Slatopolsky EA. Relative potencies of 1,25-(OH)(2)D(3) and 19-Nor-1,25-(OH)(2)D(2) on inducing differentiation and markers of bone formation in MG-63 cells. J Am Soc Nephrol. 2001;12:1468–1474. doi: 10.1681/ASN.V1271468. [DOI] [PubMed] [Google Scholar]
- 41.Mulkins MA, Manolagas SC, Deftos LJ, Sussman HH. 1,25-Dihydroxyvitamin D3 increases bone alkaline phosphatase isoenzyme levels in human osteogenic sarcoma cells. J Biol Chem. 1983;258:6219–6225. [PubMed] [Google Scholar]
- 42.Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11:312–324. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 43.Schuster I, Egger H, Bikle D, et al. Selective inhibition of vitamin D hydroxylases in human keratinocytes. Steroids. 2001;66:409–422. doi: 10.1016/s0039-128x(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 44.Anderson PH, Atkins GJ, Findlay DM, et al. RNAi-mediated silencing of CYP27B1 abolishes 1,25(OH)2D3 synthesis and reduces osteocalcin and CYP24 mRNA expression in human osteosarcoma (HOS) cells. J Steroid Biochem Mol Biol. 2007;103:601–605. doi: 10.1016/j.jsbmb.2006.12.084. [DOI] [PubMed] [Google Scholar]
- 45.Lou YR, Laaksi I, Syvala H, et al. 25-hydroxyvitamin D3 is an active hormone in human primary prostatic stromal cells. Faseb J. 2004;18:332–334. doi: 10.1096/fj.03-0140fje. [DOI] [PubMed] [Google Scholar]


