Abstract
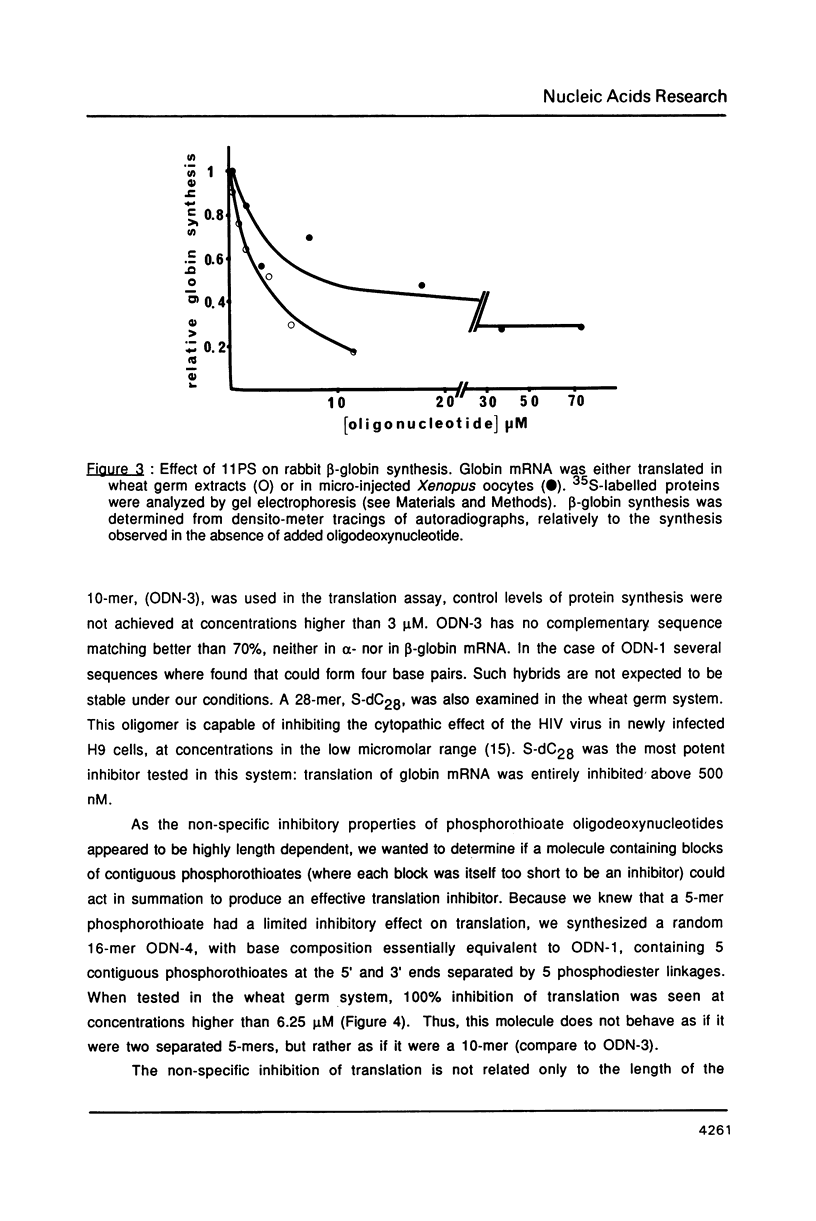
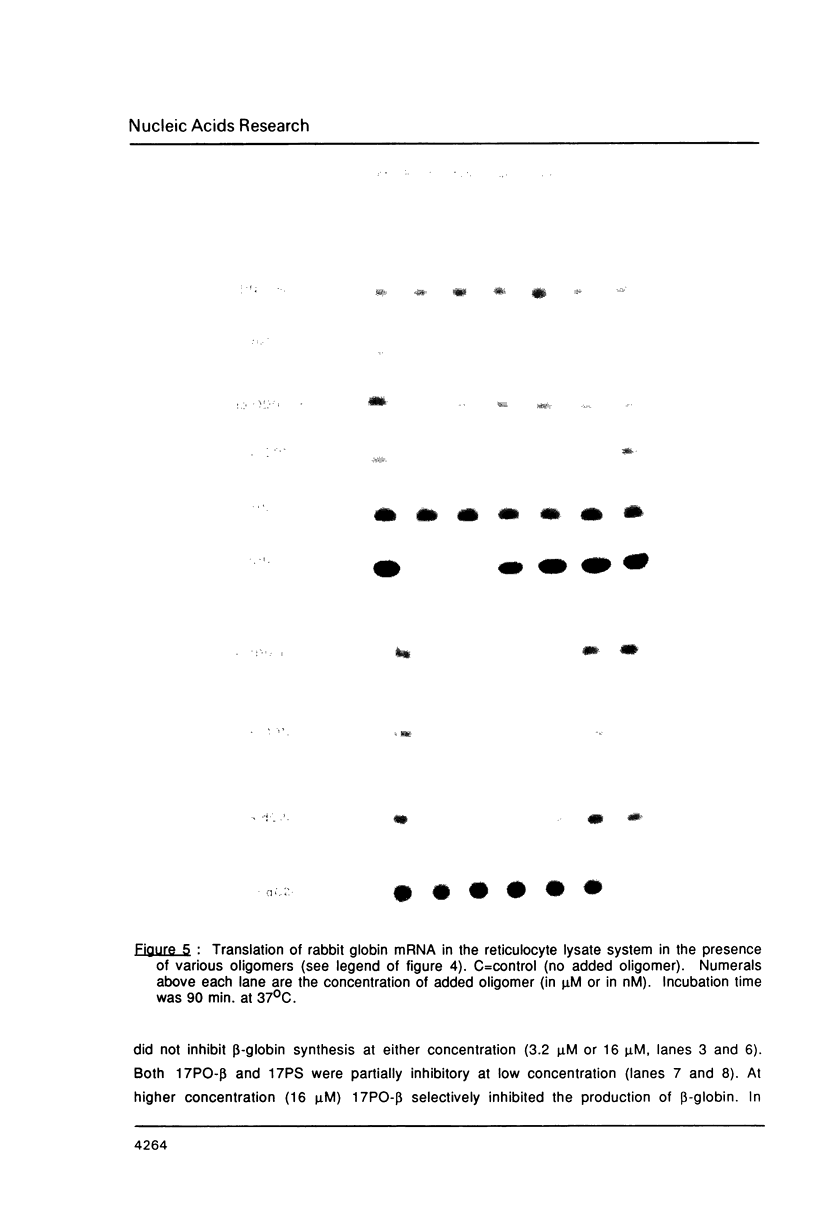
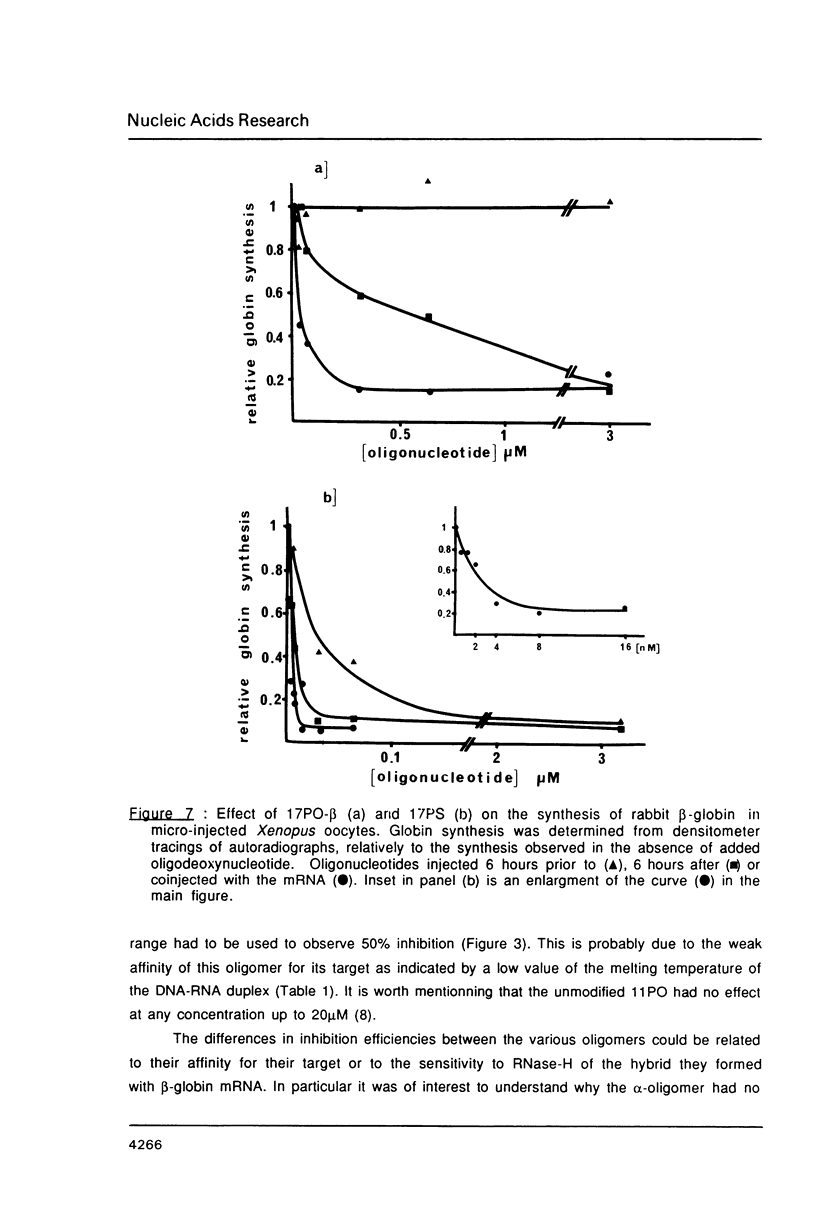
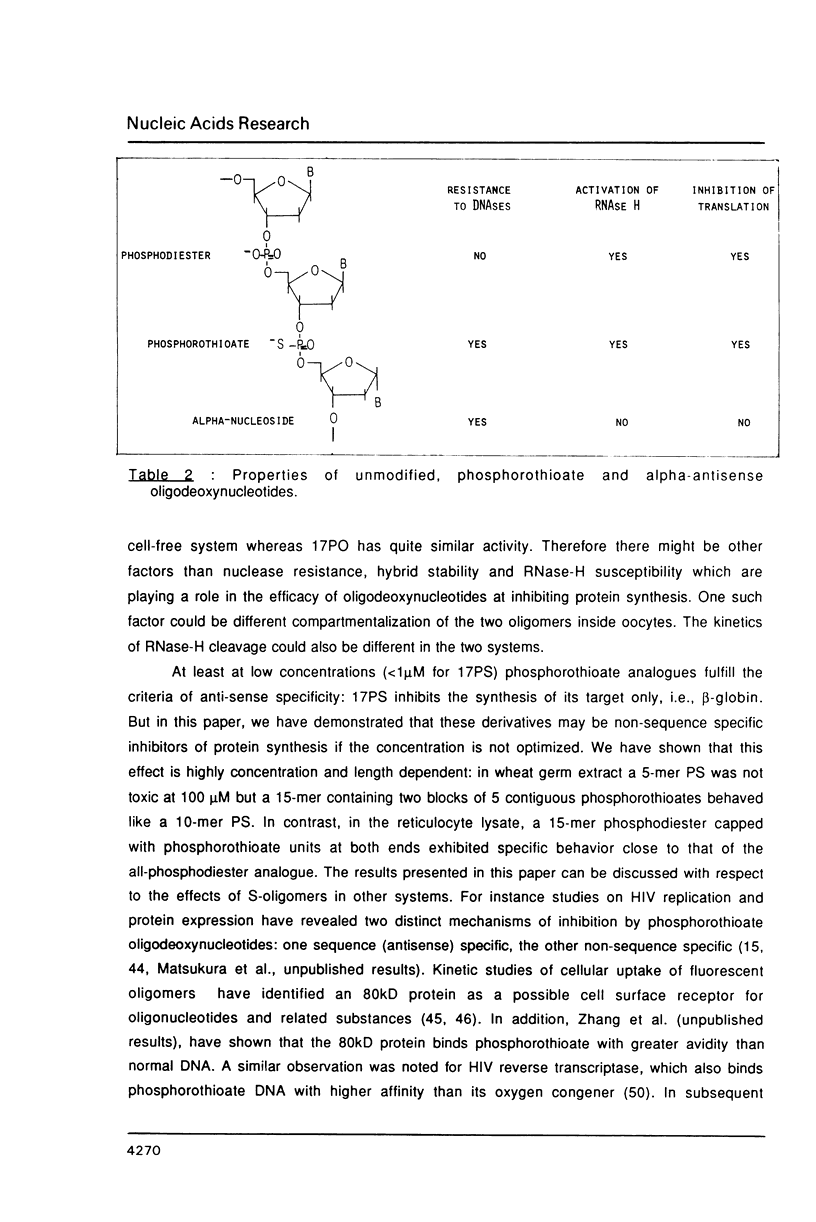
We have studied the translation of rabbit globin mRNA in cell free systems (reticulocyte lysate and wheat germ extract) and in microinjected Xenopus oocytes in the presence of anti-sense oligodeoxynucleotides. Results obtained with the unmodified all-oxygen compounds were compared with those obtained when phosphorothioate or alpha-DNA was used. In the wheat germ system a 17-mer sequence targeted to the coding region of beta-globin mRNA was specifically inhibitory when either the unmodified phosphodiester oligonucleotide or its phosphorothioate analogue were used. In contrast no effect was observed with the alpha-oligomer. These results were ascribed to the fact that phosphorothioate oligomers elicit an RNase-H activity comparable to the all-oxygen congeners, while alpha-DNA/mRNA hybrids were a poor substrate. Microinjected Xenopus oocytes followed a similar pattern. The phosphorothioate oligomer was more efficient to prevent translation than the unmodified 17-mer. Inhibition of beta-globin synthesis was observed in the nanomolar concentration range. This result can be ascribed to the nuclease resistance of phosphorothioates as compared to natural phosphodiester linkages, alpha-oligomers were devoid of any inhibitory effect up to 30 microM. Phosphorothioate oligodeoxyribonucleotides were shown to be non-specific inhibitors of protein translation, at concentrations in the micromolar range, in both cell-free systems and oocytes. Non-specific inhibition of translation was dependent on the length of the phosphorothioate oligomer. These non-specific effects were not observed with the unmodified or the alpha-oligonucleotides.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Goodchild J., Civeira M. P., Thornton A. H., Sarin P. S., Zamecnik P. C. Oligodeoxynucleoside phosphoramidates and phosphorothioates as inhibitors of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7079–7083. doi: 10.1073/pnas.85.19.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agris C. H., Blake K. R., Miller P. S., Reddy M. P., Ts'o P. O. Inhibition of vesicular stomatitis virus protein synthesis and infection by sequence-specific oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1986 Oct 7;25(20):6268–6275. doi: 10.1021/bi00368a065. [DOI] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Miller P. S. Inhibition of rabbit globin mRNA translation by sequence-specific oligodeoxyribonucleotides. Biochemistry. 1985 Oct 22;24(22):6132–6138. doi: 10.1021/bi00343a015. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Thuong N. T., Toulmé J. J., Hélène C. Enzymatic amplification of translation inhibition of rabbit beta-globin mRNA mediated by anti-messenger oligodeoxynucleotides covalently linked to intercalating agents. Nucleic Acids Res. 1987 Jun 25;15(12):4717–4736. doi: 10.1093/nar/15.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Toulmé J. J., Hélène C. Anti-messenger oligodeoxynucleotides: specific inhibition of rabbit beta-globin synthesis in wheat germ extracts and Xenopus oocytes. Biochimie. 1986 Sep;68(9):1063–1069. doi: 10.1016/s0300-9084(86)80180-8. [DOI] [PubMed] [Google Scholar]
- Cornelissen A. W., Verspieren M. P., Toulmé J. J., Swinkels B. W., Borst P. The common 5' terminal sequence on trypanosome mRNAs: a target for anti-messenger oligodeoxynucleotides. Nucleic Acids Res. 1986 Jul 25;14(14):5605–5614. doi: 10.1093/nar/14.14.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P., Lotan I., Knapp M., Kandel E. R., Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnor C., Bertrand J. R., Thenet S., Lemaître M., Morvan F., Rayner B., Malvy C., Lebleu B., Imbach J. L., Paoletti C. alpha-DNA. VI: Comparative study of alpha- and beta-anomeric oligodeoxyribonucleotides in hybridization to mRNA and in cell free translation inhibition. Nucleic Acids Res. 1987 Dec 23;15(24):10419–10436. doi: 10.1093/nar/15.24.10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild J., Carroll E., 3rd, Greenberg J. R. Inhibition of rabbit beta-globin synthesis by complementary oligonucleotides: identification of mRNA sites sensitive to inhibition. Arch Biochem Biophys. 1988 Jun;263(2):401–409. doi: 10.1016/0003-9861(88)90652-2. [DOI] [PubMed] [Google Scholar]
- Gupta K. C. Antisense oligodeoxynucleotides provide insight into mechanism of translation initiation of two Sendai virus mRNAs. J Biol Chem. 1987 Jun 5;262(16):7492–7496. [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Frank R., Dobberstein B. Translation arrest by oligodeoxynucleotides complementary to mRNA coding sequences yields polypeptides of predetermined length. Nucleic Acids Res. 1986 Feb 11;14(3):1427–1448. doi: 10.1093/nar/14.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessus C., Cazenave C., Ozon R., Hélène C. Specific inhibition of endogenous beta-tubulin synthesis in Xenopus oocytes by anti-messenger oligodeoxynucleotides. Nucleic Acids Res. 1988 Mar 25;16(5):2225–2233. doi: 10.1093/nar/16.5.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S. Quantitative hybridization-arrest of mRNA in Xenopus oocytes using single-stranded complementary DNA or oligonucleotide probes. Nucleic Acids Res. 1985 Jul 11;13(13):4991–5004. doi: 10.1093/nar/13.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelot G., Guesnet J. L., Roig V., Thuong N. T. 2D-NMR studies of the unnatural duplex alpha-d(TCTAAAC)-beta-d(AGATTTG). Nucleic Acids Res. 1987 Sep 25;15(18):7531–7547. doi: 10.1093/nar/15.18.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T. G., Ray B. K., Dodds J. T., Grifo J. A., Abramson R. D., Merrick W. C., Betsch D. F., Weith H. L., Thach R. E. Influence of 5' proximal secondary structure on the translational efficiency of eukaryotic mRNAs and on their interaction with initiation factors. J Biol Chem. 1986 Oct 25;261(30):13979–13989. [PubMed] [Google Scholar]
- Liebhaber S. A., Cash F. E., Shakin S. H. Translationally associated helix-destabilizing activity in rabbit reticulocyte lysate. J Biol Chem. 1984 Dec 25;259(24):15597–15602. [PubMed] [Google Scholar]
- Loke S. L., Stein C., Zhang X., Avigan M., Cohen J., Neckers L. M. Delivery of c-myc antisense phosphorothioate oligodeoxynucleotides to hematopoietic cells in culture by liposome fusion: specific reduction in c-myc protein expression correlates with inhibition of cell growth and DNA synthesis. Curr Top Microbiol Immunol. 1988;141:282–289. doi: 10.1007/978-3-642-74006-0_38. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Comparative hybrid arrest by tandem antisense oligodeoxyribonucleotides or oligodeoxyribonucleoside methylphosphonates in a cell-free system. Nucleic Acids Res. 1988 Apr 25;16(8):3341–3358. doi: 10.1093/nar/16.8.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar C., Stein C. A., Cohen J. S., Broder S., Wilson S. H. Stepwise mechanism of HIV reverse transcriptase: primer function of phosphorothioate oligodeoxynucleotide. Biochemistry. 1989 Feb 7;28(3):1340–1346. doi: 10.1021/bi00429a060. [DOI] [PubMed] [Google Scholar]
- Marcus-Sekura C. J., Woerner A. M., Shinozuka K., Zon G., Quinnan G. V., Jr Comparative inhibition of chloramphenicol acetyltransferase gene expression by antisense oligonucleotide analogues having alkyl phosphotriester, methylphosphonate and phosphorothioate linkages. Nucleic Acids Res. 1987 Jul 24;15(14):5749–5763. doi: 10.1093/nar/15.14.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura M., Shinozuka K., Zon G., Mitsuya H., Reitz M., Cohen J. S., Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Hunt T. The use of single-stranded DNA and RNase H to promote quantitative 'hybrid arrest of translation' of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986 Aug 26;14(16):6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Chang D. K., Lown J. W. alpha-DNA-III. Characterization by high field 1H-NMR, anti-parallel self-recognition and conformation of the unnatural hexadeoxyribonucleotides alpha-[d(CpApTpGpCpG)] and alpha-[d(CpGpCpApTpG)]. Alpha-oligodeoxynucleotides as potential cellular probes for gene control. Nucleic Acids Res. 1987 May 26;15(10):4241–4255. doi: 10.1093/nar/15.10.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Chang D. K., Lown J. W. alpha-DNA. I. Synthesis, characterization by high field 1H-NMR, and base-pairing properties of the unnatural hexadeoxyribonucleotide alpha-[d(CpCpTpTpCpC)] with its complement beta-[d(GpGpApApGpG)]. Nucleic Acids Res. 1986 Jun 25;14(12):5019–5035. doi: 10.1093/nar/14.12.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Lee M., Hartley J. A., Chang D. K., Lown J. W. alpha-DNA-V. Parallel annealing, handedness and conformation of the duplex of the unnatural alpha-hexadeoxyribonucleotide alpha-[d(CpApTpGpCpG)] with its beta-complement beta-[d(GpTpApCpGpC)] deduced from high field 1H-NMR. Nucleic Acids Res. 1987 Sep 11;15(17):7027–7044. doi: 10.1093/nar/15.17.7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Thenet S., Bertrand J. R., Paoletti J., Malvy C., Paoletti C. alpha-DNA II. Synthesis of unnatural alpha-anomeric oligodeoxyribonucleotides containing the four usual bases and study of their substrate activities for nucleases. Nucleic Acids Res. 1987 Apr 24;15(8):3421–3437. doi: 10.1093/nar/15.8.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A., Blake K. R., Miller P. S. Characterization of sequence-specific oligodeoxyribonucleoside methylphosphonates and their interaction with rabbit globin mRNA. Biochemistry. 1985 Jul 16;24(15):4041–4046. doi: 10.1021/bi00336a036. [DOI] [PubMed] [Google Scholar]
- Pavlakis G. N., Lockard R. E., Vamvakopoulos N., Rieser L., RajBhandary U. L., Vournakis J. N. Secondary structure of mouse and rabbit alpha- and beta-globin mRNAs: differential accessibility of alpha and beta initiator AUG codons towards nucleases. Cell. 1980 Jan;19(1):91–102. doi: 10.1016/0092-8674(80)90391-8. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Chassignol M., Takasugi M., Le Doan T., Thuong N. T., Hélène C. Double helices with parallel strands are formed by nuclease-resistant oligo-[alpha]-deoxynucleotides and oligo-[alpha]-deoxynucleotides covalently linked to an intercalating agent with complementary oligo-[beta]-deoxynucleotides. J Mol Biol. 1987 Aug 20;196(4):939–942. doi: 10.1016/0022-2836(87)90416-5. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Perrouault L., Le Doan T., Chassignol M., Thuong N., Hélène C. Sequence-specific binding and photocrosslinking of alpha and beta oligodeoxynucleotides to the major groove of DNA via triple-helix formation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1349–1353. doi: 10.1073/pnas.85.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakin S. H., Liebhaber S. A. Destabilization of messenger RNA/complementary DNA duplexes by the elongating 80 S ribosome. J Biol Chem. 1986 Dec 5;261(34):16018–16025. [PubMed] [Google Scholar]
- Shuttleworth J., Colman A. Antisense oligonucleotide-directed cleavage of mRNA in Xenopus oocytes and eggs. EMBO J. 1988 Feb;7(2):427–434. doi: 10.1002/j.1460-2075.1988.tb02830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth J., Matthews G., Dale L., Baker C., Colman A. Antisense oligodeoxyribonucleotide-directed cleavage of maternal mRNA in Xenopus oocytes and embryos. Gene. 1988 Dec 10;72(1-2):267–275. doi: 10.1016/0378-1119(88)90152-7. [DOI] [PubMed] [Google Scholar]
- Smith R. C., Dworkin M. B., Dworkin-Rastl E. Destruction of a translationally controlled mRNA in Xenopus oocytes delays progesterone-induced maturation. Genes Dev. 1988 Oct;2(10):1296–1306. doi: 10.1101/gad.2.10.1296. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Stein C. A., Mori K., Loke S. L., Subasinghe C., Shinozuka K., Cohen J. S., Neckers L. M. Phosphorothioate and normal oligodeoxyribonucleotides with 5'-linked acridine: characterization and preliminary kinetics of cellular uptake. Gene. 1988 Dec 10;72(1-2):333–341. doi: 10.1016/0378-1119(88)90160-6. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M. L., Zamecnik P. C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):285–288. doi: 10.1073/pnas.75.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. S., Asseline U., Rouzaud D., Montenay-Garestier T., Nguyen T. T., Hélène C. Oligo-[alpha]-deoxynucleotides covalently linked to an intercalating agent. Double helices with parallel strands are formed with complementary oligo-[beta]-deoxynucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6149–6158. doi: 10.1093/nar/15.15.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. S., François J. C., Lavery R., Saison-Behmoaras T., Montenay-Garestier T., Thuong N. T., Hélène C. Sequence-targeted cleavage of nucleic acids by oligo-alpha-thymidylate-phenanthroline conjugates: parallel and antiparallel double helices are formed with DNA and RNA, respectively. Biochemistry. 1988 Aug 9;27(16):6039–6045. doi: 10.1021/bi00416a032. [DOI] [PubMed] [Google Scholar]
- Thuong N. T., Asseline U., Roig V., Takasugi M., Hélène C. Oligo(alpha-deoxynucleotide)s covalently linked to intercalating agents: differential binding to ribo- and deoxyribopolynucleotides and stability towards nuclease digestion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5129–5133. doi: 10.1073/pnas.84.15.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé J. J., Hélène C. Antimessenger oligodeoxyribonucleotides: an alternative to antisense RNA for artificial regulation of gene expression--a review. Gene. 1988 Dec 10;72(1-2):51–58. doi: 10.1016/0378-1119(88)90127-8. [DOI] [PubMed] [Google Scholar]
- Toulmé J. J., Krisch H. M., Loreau N., Thuong N. T., Hélène C. Specific inhibition of mRNA translation by complementary oligonucleotides covalently linked to intercalating agents. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1227–1231. doi: 10.1073/pnas.83.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder J. A., Eder P. S., Engman D. M., Brentano S. T., Walder R. Y., Knutzon D. S., Dorfman D. M., Donelson J. E. The 35-nucleotide spliced leader sequence is common to all trypanosome messenger RNA's. Science. 1986 Aug 1;233(4763):569–571. doi: 10.1126/science.3523758. [DOI] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5011–5015. doi: 10.1073/pnas.85.14.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]