Abstract
Bone mineral density (BMD) is an important factor linked to bone health. Little is known of the prevalence of low BMD and its associated risk factors in an urban underserved population. Between 2001 and 2004, we recruited 338 subjects who completed drug use and medical history questionnaires, underwent hormonal measurements, and underwent whole-body dual-energy X-ray absorptiometry (DXA) for evaluation of BMD and body composition. Of these, 132 subjects had site-specific DXA (lumbar spine and hip) performed. Osteoporosis was defined as a T-score of –2.5 or less for men 50 years of age and older and postmenopausal women and a Z-score of –2.0 or less in men younger than 50 years of age and premenopausal women at either the lumbar spine, total hip, or femoral neck, according to National Osteoporosis Foundation (NOF) guidelines. The cohort consisted of mostly African-American, middle-aged people with a high prevalence of illicit drug use, 50% HIV+, and 39% hepatitis C+. Osteoporosis was identified in 22% of subjects (24 men, 5 women), with the majority of cases (90%) attributable to osteoporosis at the lumbar spine. Osteoporosis was more common in men than in women. Lower whole-body BMD among women was associated with multiple risk factors, but only with lower lean mass among men. Osteoporosis was highly prevalent in men, mainly at the spine. The risk factors for bone loss in this population need to be further clarified. Screening men for osteoporosis starting at age 50 might be warranted in this population given the multiple risk factors and the unexpectedly high prevalence of low BMD. © 2011 American Society for Bone and Mineral Research.
Keywords: OSTEOPOROSIS, BONE MINERAL DENSITY, HIV, BMI, INNER CITY
Introduction
Osteoporosis is a disease of the bone characterized by decreased bone mineral density (BMD) and bone quality with a resulting increased risk in skeletal fragility and fracture.(1) Fractures related to osteoporosis represent a large burden for society and high health care costs for prolonged hospitalization and complications of increased morbidity and mortality, as well as indirect costs of declining functioning, nursing home placement, and reduced quality of life.(2) Public health awareness of osteoporosis has lead to increased interest in identifying the fracture risk factors because fractures are preventable by screening appropriate populations and initiating effective therapy. Most of the data regarding osteoporosis has been derived from postmenopausal female white populations. Only recently is osteoporosis in men gaining attention(3,4) and, to a lesser extent, osteoporosis in African Americans.
Men and women living in the inner city have multiple risk factors for osteoporosis, but the prevalence of low BMD is not well defined in this population. Human immunodeficiency virus (HIV) infection, hepatitis C infection, alcohol abuse, smoking, illicit drug use, hypogonadism, homelessness, and poor nutritional status are common in inner-city populations(5–9) and have been associated with increased risk of osteoporosis and fractures.(10–13)
In this study, we aimed to determine the prevalence of osteoporosis and reduced BMD in a cohort of inner-city Baltimore's population and investigate the risk factors associated with BMD.
Subjects and Methods
Study population
The Study of HIV, Injection Drug Use, Nutrition and Endocrinology (SHINE) is a cross-sectional study of volunteers in Baltimore designed to evaluate the effects of HIV infection and drug use on multiple endocrine and metabolic parameters. Between 2001 and 2004, subjects were recruited from local medical and HIV clinics, community methadone maintenance programs, an existing cohort of injection drug users, homeless shelters, and by word of mouth. Subjects between 18 and 65 years of age were eligible for recruitment, which was stratified by gender and HIV status. Individuals with any of the following characteristics were excluded: (1) known gonadal dysfunction by medical history, (2) other significant medical problems resulting in a serum creatinine concentration more than three times the upper limit of normal (ULN), transaminases more than three times ULN, or hematocrit less than 25%, and (3) untreated endocrine problems, such as hypothyroidism, as indicated by medical history. All volunteers had provided written informed consent before participation. The study was approved by the Johns Hopkins University Institutional Review Board.
Drug and alcohol use classification
Drug-use status was divided into three groups of (1) no use, (2) occasional (<3 times/week), and (3) heavy drug users (≥ 3 times/week) over the past 6 months. Drug use included heroin (injection, snorting, or smoking), cocaine (snorting or injection), speedball (injection), crack/freebase, marijuana, and street methadone. Those who were enrolled in a methadone treatment program for at least 3 months were grouped separately. Subjects also were stratified by alcohol use into three groups of (1) no use, (2) moderate (<3 drinks/day), and (3) heavy use (≥ 3 drinks/day) during the past 6 months. Drinking equivalents for one drink included 8 fluid ounces of beer or wine, 4 fluid ounces of liquor or malt, or 1 fluid ounce of vodka. Subjects who reported smoking in the past 6 months were considered smokers.
HIV and hepatitis C infection
HIV status was determined by (1) self-reported serostatus, (2) self-report of antiretroviral therapy, or (3) ELISA testing followed by Western blot confirmation for subjects without documentation of HIV status. Hepatitis C status was self-reported.
Biochemical measurements
Hormonal measurements included free testosterone (fT), follicle-stimulating hormone (FSH), luteinizing hormone (LH), dehydroepiandrosterone (DHEA), and estradiol from morning serum samples collected between 8:00 and 10:00 a.m. in the Clinical Trials Unit at the Johns Hopkins University School of Medicine. Free testosterone was measured by equilibrium dialysis (Esoterix, Inc., Austin, TX, USA) with a normal range of 52 to 280 ng/dL for men. Intraassay coefficients of variation (CV) ranged from 6.6% to 9.4%, and interassay CV ranged from 9.1% to 11.9%. Males were considered hypogonadal if they had an fT level of less than 52 ng/dL or were on testosterone-replacement therapy. Estradiol was measured by radioimmunoassay (Esoterix, Inc.) in which intra- and interassay CVs were 5.2% and 8.0%, respectively. This variable was divided into tertiles from lowest (1) to highest (3) when evaluating its relation to BMD. LH and FSH were measured by immunochemiluminometric assay (ICMA), for which the intra- and interassay CVs were 3.4% and 3.8%, respectively, for LH, and 3.2% and 6.7%, respectively, for FSH. Females were considered postmenopausal if they had a FSH concentration of less than 50 mIU/mL, age of 51 years of greater, or they self-reported when the FSH level was greater than 30 and 50 mIU/mL or less.
Using frozen serum samples, parathyroid hormone (PTH) and vitamin D levels were obtained later on 126 subjects of the subset with site-specific dual-energy X-ray absorptiometry (DXA) scans. Intact PTH (iPTH) was measured by two-site ELISA (Alpco Diagnostics, Salem, NH, USA) with intraassay CVs of 2.8% to 3.0% and interassay CVs of 5.1% to 5.5%. Reading the assay at 450 nm, iPTH concentrations were valid up to about 200 pg/mL as the upper limit. Secondary hyperparathyroidism was defined as iPTH greater than 65 pg/mL. 25-Hydroxyvitamin D [25(OH)D] level was measured by radioimmunoassay (DiaSorin, Stillwater, MN, USA) with an intraassay CV of 8.6% to 11.7% and interassay CV of 8.2% to 11.0%. The lower limit was measured at 1.5 ng/mL or less. Vitamin D deficiency was defined as 25(OH)D concentration of 15 ng/mL or less.
Measurement of serum inflammatory markers, including tumor necrosis factor α (TNF-α), high-sensitivity interleukin-6 (hs-IL6), and high-sensitivity C-reactive protein (hs-CRP), were completed at the Advanced Chemistry Laboratory at the Johns Hopkins University using an ALPO assay (Windham, NH, USA). TNF-α was measured by ELISA, where intra- and interassay CVs were 8.2% and 6.1%, respectively. hs-IL6 was measured by ELISA with intra- and interassay CVs of 5.1% and 5.0%, respectively. hs-CRP was measured by ELISA with intra- and interassay CVs of 6.3% and 2.2%, respectively. These markers were divided into tertiles from lowest (1) to the highest (3) level to evaluate the relation between BMD and inflammatory markers.
Body composition and BMD measurements
Body mass index [BMI = weight (kg)/height2 (m2)] was calculated for all subjects
Whole-body DXA scans were performed on a Hologic 4500A machine with QDA4500A software Version 9.03 (Hologic Inc, Bedford, MA, USA) to obtain measures of total lean body mass (LBM, kg), fat body mass (kg), as well as whole-body BMD (WBMD, g/cm2). A subset of the participants had BMD assessed regionally at the lumbar spine and hip (total and femoral neck). For this subset of patients, T-scores and Z-scores were calculated from the site-specific BMD measures using normative data from the manufacturer matched for gender and race.(14,15) Subjects who specified race other than African American, white, or Hispanic (n = 5) were considered white for consistency of definition (non-African American) because data are available only for whites, African Americans, and Hispanics. Osteoporosis was defined as a T-score of –2.5 or less for men 50 years of age or older and postmenopausal women and a Z-score of –2.0 or less in men younger than 50 years of age and premenopausal women at either the lumbar spine, total hip, or femoral neck, according to National Osteoporosis Foundation (NOF) guidelines.(16) Reduced BMD was defined as a T-score of less than –1.0. Osteopenia was defined as –2.5 < T-score < –1.
Statistical analysis
Data were stratified by gender, and the demographic characteristics and BMD measures of the study participants were described. Normality of the distributions for each variable was checked by plotting histograms. The first part of the analysis included the larger cohort (total study population) with WBMD (continuous variable) as the dependent variable in order to obtain more power and perform correlative analysis stratified by gender. WBMD closely reflects assessment by site-specific measures and has an excellent correlation with spine BMD in predicting osteoporosis.(17) The analysis examined the relationship between WBMD and various independent variables (risk factors) and was stratified by gender. One-way ANOVA was used to determine the association between WBMD and categorical independent variables, and linear regression analysis was used to assess the relation between WBMD and continuous independent variables. The association between WBMD and multiple variables was done by stepwise backward regression analysis using a p value of .25 to enter and .1 to remain in the model. Variables tested included age, gender, race (African American or other), smoking status (yes/no), alcohol/drug use (none, moderate, or heavy), methadone program (yes/no), hypogonadal/menopausal, hepatitis C status, HIV status, BMI or LBM + fat mass, and lab parameters (ie, DHEA, estradiol, TNF-α, IL6, and CRP) in tertiles. The final model locked in age and race and was stratified by gender because these variables are known risk factors for low BMD.
The second part of the analysis investigated the subset of participants with site-specific DXA, where osteoporosis (as defined earlier) was considered the dependent variable (dichotomous). A chi-square test was used to determine the group differences of osteoporosis among categorical independent variables. Regression analysis was used to determine the relation between osteoporosis and continuous independent variables. Multivariate backward stepwise regression was performed similar to the WBMD model described earlier. Smaller sample size limited stratification by gender, but the final model locked in gender, along with age and race. Risk factors tested were the same as used for WBMD analysis. In addition, we evaluated the relationship between osteoporosis and vitamin D deficiency status [25(OH)D ≤ 15 ng/mL) and the presence of secondary hyperparathyroidism (iPTH > 65 pg/mL).
p Values less than .05 were considered significant. Statistical analysis was performed on JMP Statistical Software 7.0.2 (2007, SAS Institute, Inc., Cary, NC, USA).
Results
Demographic characteristics of study participants
The cohort consisted of a total of 338 subjects who had measures of WBMD, including 187 males and 151 females. Demographic characteristics stratified by gender are presented in Table 1, with similar distributions among women and men. The population consisted of a relatively young group (mean age 42.8 years) of mostly African-American (93.8%) inner-city men and women of low socioeconomic status (SES); 27% of the population was homeless in the prior year, 15% were employed at the time of the study, 51% reported income of $10,000 or less in the prior year, and 28% reported no legal income. Seventy percent reported moderate or heavy drug use, and 11% reported the consumption of three or more alcohol drinks per day. Most were smokers (84%). Ninety-nine subjects (30%) were enrolled in a methadone program, of which 66 also were active drug users. Half (50%) the population was HIV+, and 38.7% were hepatitis C+. The prevalence of hypogonadism was 25% in men and 21% in women (postmenopausal). One subject was on testosterone-replacement therapy (fT = 2.2 ng/dL, therefore hypogonadal by biochemical definition), one subject reported corticosteroid use; and three subjects were taking anticonvulsant medications. Men had a statistically significant lower BMI than women (24.8 and 27.4 kg/m2, respectively, p < .05) as well as lower fat mass but higher LBM and WBMD (Table 1).
Table 1.
Population Demographics and Biochemical Measures
| WBMD cohort (n = 338) | Site-specific DXA cohort (n = 132) | |||
|---|---|---|---|---|
| Variable | M (187) | F (151) | ||
| Age (mean, SD) | 43.3 (7.2) | 42.1 (7.6) | 42.5 (8) | |
| Male | — | — | 83 (63%) | |
| African American | 175 (94%) | 141 (93%) | 124 (94%) | |
| Homeless (in the past year) | 48 (26%) | 41 (28%) | 35 (27%) | |
| Employed (at time of study) | 32 (17%) | 19 (13%) | 12 (9%) | |
| High school or GED completion | 114 (62%) | 77 (51%) | 72 (55%) | |
| Smokinga | 145 (82%) | 128 (87%) | 102 (80%) | |
| Alcoholb | ||||
| No use | 74 (41%) | 73 (50%) | 55 (43%) | |
| <3 drinks/day | 85 (48%) | 58 (39%) | 57 (45%) | |
| ≥3 drinks/day | 20 (11%) | 16 (11%) | 16 (13%) | |
| Methadonec | 43 (24%) | 56 (37%) | 33 (25%) | |
| Drug used | ||||
| No use | 49 (28%) | 47 (33%) | 23 (18%) | |
| <3 times/week | 74 (42%) | 44 (31%) | 47 (37%) | |
| ≥3 times/week | 54 (30%) | 53 (37%) | 58 (45%) | |
| Hepatitis Ce | 71 (39%) | 58 (40%) | 49 (38%) | |
| HIVf | 103 (56%) | 65 (43%) | 67 (51%) | |
| Hypogonadalg/menopausalh | 44 (25%) | 32 (21%) | 36 (28%) | |
| BMI (kg/m2) (mean, SD) | 24.8 (4.4) | 27.4 (6.6) | 25.1 (5) | |
| Lean body mass (kg) (mean, SD) | 59.3 (7.9) | 47.2 (8.1) | 54.4 (9.8) | |
| Fat body mass (kg) (mean, SD) | 13.4 (7.4) | 22.3 (11) | 15.8 (8.9) | |
| 25 (OH) D (ng/ml) (mean, SD) | — | — | 16.1 (0.65) | |
| 25 (OH)D< 15 | — | — | 61 (48%) | |
| iPTH (pg/ml) (mean, SD) | — | — | 51.3 (2.2) | |
| iPTH> 65 | — | — | 28 (22%) | |
| TNF-α (pg/ml) (mean, SE) N = 243 | 2.9 (0.26) | 2.6 (0.3) | 2.8 (0.3) | |
| hsCRP (ng/ml) (mean, SE) N = 243 | 3.9 (0.6) | 5.1 (0.9) | 6.3 (1.1) | |
| hsIL-6 (pg/ml) (mean, SE) N= 238 | 3.3 (0.23) | 3.8 (0.3) | 3 (0.2) | |
| M | F | |||
| Free T (ng/dL) (mean, SE) N = 318 | 89.1 (4.9) | 5.5 (1.4) | 83.4 (6.8) | 2.6 (0.3) |
| Estradiol (pg/ml) (mean, SE) N = 327 | 32.7 (1.5) | 52.5 (4.5) | 37.1 (2.2) | 52.4 (6.4) |
| LH (mIU/ml) (mean, SE) N = 328 | 5.6 (0.26) | 12 (1.2) | 5.7 (0.4) | 12.9 (2.4) |
| FSH (mIU/ml) (mean, SE) N = 328 | 5.8 (0.5) | 15.9 (1.7) | 5.9 (0.8) | 19.3 (3.5) |
| DHEA (ng/dL) (mean, SE) N = 328 | 161 (8.5) | 107 (7.8) | 178 (15) | 119 (15) |
Note: Items in boldface denote statistically significant difference (p < .05) between males and females.
Determined by answering yes to having smoked cigarettes in the past 6 months (irrespective of quantity).
Equivalent of 1 drink is 8 fluid ounces of beer or wine, 4 fluid ounces of liquor or malt, and 1 fluid ounce of vodka.
Includes subjects who are enrolled in a methadone treatment program for at least 3 months.
Includes marijuana, crack/freebase, cocaine (snorting, injection), speedball (injection), heroin (smoking, snorting, injection), and street methadone in the past 6 months.
Self-report.
Self-report, HIV-positive by testing, or history of past or present use of antiretroviral therapy.
Defined as having a free testosterone level < 52 pg/mL or currently on testosterone-replacement therapy.
Defined as having FSH level > 50 mIU/mL, age ≥ 51 years, or FSH level > 30 mIU/mL and ≤ 50 mIU/mL and answered yes to having gone through menopause.
Factors associated with WBMD
When stratified by gender, the relation of WBMD to various independent risk factors showed major differences among men and women (Table 2). Whereas only LBM was positively related with BMD in men, multiple risk factors showed significant associations in women. In females, older age, non-African American race, postmenopausal status, lower LBM, and methadone use showed significant association with lower BMD in univariate analysis. Similar results were obtained when BMI replaced fat mass and LBM, with BMI as a significant determinant. Methadone use was an independent determinant of WBMD in women, where the association was not affected by simultaneous adjustment for other factors. Moderate (<3 drinks/day) consumption of alcohol was significantly associated with higher BMD in women. Women having the highest levels of DHEA and estradiol had significantly higher BMD measures, consistent with the known benefits of estrogen on bone. Independent determinants of lower BMD in women included increasing age, non-African-American race, methadone, and lower LBM (Table 3). There was a trend toward lower BMD by HIV status in women (p = .11). Among HIV-infected women (n = 65), we explored whether the self-report of antiretroviral therapy was associated with WBMD. After adjustment for age, non-African-American race, methadone use, and LBM, HIV therapy was not associated with lower WBMD (–0.002, p = .89).
Table 2.
Potential Determinants of Whole-Body BMD: Analysis by Gender
| Male | Female | |||
|---|---|---|---|---|
| Variable | Univariate β estimate | Multivariatea β estimate | Univariate β estimate | Multivariateb β estimate |
| Lean body mass (kg) | 0.005*** | 0.006*** | 0.007*** | 0.006*** |
| Age (per increase of 5 years) | 0.003 | 0.002 | −0.03** | −0.02*** |
| Race (non-African American/African American) | −0.04 | 0.04 | −0.18*** | −0.14*** |
| Methadone | −0.03 | NS | −0.05* | −0.05*** |
| HIVc | 0.02 | NS | −0.04 | −0.03 |
| Smokingd | 0.02 | NS | −0.04 | NS |
| Alcohole | NS | NS | ||
| 0 (no use) | Ref | Ref | ||
| 1 (<3 drinks/day) | 0.03 | 0.05* | ||
| 2 (≥3 drinks/day) | −0.007 | 0.01 | ||
| Drug usef | NS | NS | ||
| 0 (no use) | Ref | Ref | ||
| 1 (<3 times/week) | 0.005 | 0.01 | ||
| 2 (≥3 times/week) | −0.02 | 0.05 | ||
| Hypogonadalg/menopausalh | −0.01 | NS | −0.1*** | NS |
| Hepatitis Ci | 0.005 | NS | −0.02 | NS |
| Fat body mass (kg) | <0.01 | NS | < 0.01* | NS |
| BMI (kg/m2) | 0.007** | NS | 0.005** | (Not included) |
| DHEA (tertiles)j | NS | NS | ||
| 1 | Ref | Ref | ||
| 2 | −0.04 | 0.05 | ||
| 3 | 0.0004 | 0.07** | ||
| Estradiol (tertiles)j | NS | NS | ||
| 1 | Ref | Ref | ||
| 2 | −0.003 | 0.04 | ||
| 3 | 0.02 | 0.08** | ||
| IL-6 (tertiles)j | NS | NS | ||
| 1 | Ref | Ref | ||
| 2 | −0.006 | −0.01 | ||
| 3 | −0.004 | −0.02 | ||
| CRP (tertiles)j | NS | NS | ||
| 1 | Ref | Ref | ||
| 2 | −0.03 | 0.03 | ||
| 3 | −0.007 | 0.03 | ||
| TNF-α (tertiles)j | NS | NS | ||
| 1 | Ref | Ref | ||
| 2 | −0.03 | −0.03 | ||
| 3 | 0.006 | −0.03 | ||
NS = nonsignificant.
Note: Items in boldface denote significant p values:
p < .05.
p < .01.
p < .0001.
Final model R2 value = 0.12, R2 adjusted = 0.1, p < .0001, n = 186.
Final model R2 value = 0.38, R2 adjusted = 0.36, p < .0001, n = 150.
Self-report, HIV+ by testing, or history of past or present use of antiretroviral therapy.
Determined by answering yes to having smoked cigarettes in the past 6 months (irrespective of quantity).
Equivalent of 1 drink is 8 fluid ounces of beer or wine, 4 fluid ounces of liquor or malt, and 1 fluid ounce of vodka.
Includes marijuana, crack/freebase, cocaine (snorting, injection), speedball (injection), heroin (smoking, snorting, injection), and street methadone in the past 6 months.
Defined as having free testosterone level < 52 pg/mL or currently on testosterone-replacement therapy.
Defined as having an FSH level > 50 mIU/mL, age ≥ 51 years, or an FSH level >30 and ≤50 mIU/mL and answered yes to having gone through menopause.
Self-report.
Obtained by plotting continuous lab values, 0 representing the lowest tertile, 1 the middle tertile, and 2 the highest tertile.
Table 3.
Bone Mineral Density Measures
| Site Measure | Male | Female | Total |
|---|---|---|---|
| Whole-body data (n = 338) | (n = 187) | (n = 151) | |
| Total BMD mean (g/cm2) (SD) | 1.27 (0.13) | 1.19 (0.13) | 1.23 (0.14) |
| Site-specific data (n = 132) | (n = 83) | (n = 49) | |
| Total hip | |||
| BMD mean (g/cm2) (SD) | 1.02 (0.15) | 0.98 (0.15) | 1 (0.15) |
| Proportion with reduced BMDa | 42 (50.6%) | 12 (24.5%) | 54 (41%) |
| Proportion with osteopeniab | 40 (48.2%) | 11 (22.4%) | 51 (38.6%) |
| Proportion with osteoporosisc | 2 (2.4%) | 1 (2%) | 3 (2.3%) |
| Hip-femoral neck | |||
| BMD mean (g/cm2) (SD) | 0.94 (0.15) | 0.90 (0.15) | 0.93 (0.15) |
| Proportion with reduced BMDa | 39 (46.9%) | 12 (24.5%) | 51 (38.6%) |
| Proportion with osteopeniab | 37 (44.6%) | 11 (22.4%) | 48 (36.4%) |
| Proportion with osteoporosisc | 2 (2.4%) | 1 (2%) | 3 (2.3%) |
| Lumbar spine | |||
| BMD mean (g/cm2) (SD) | 1.04 (0.15) | 1.04 (0.15) | 1.04 (0.15) |
| Proportion with reduced BMDa | 54 (65%) | 25 (51%) | 79 (60%) |
| Proportion with osteopeniab | 39 (47%) | 21 (42.9%) | 60 (45.5%) |
| Proportion with osteoporosisc | 23 (27.7%) | 5 (10.2%) | 28 (21.2%) |
| Reduced BMD any sited | 63 (75.9%) | 28 (57%) | 91 (68.9%) |
| Osteopenia any sited | 46 (55.4%) | 23 (46.9%) | 69 (52.3%) |
| Osteoporosis any sited | 24 (28.9%) | 5 (10.2%) | 29 (22%) |
Note: Items in boldface denote statistically significant difference (p < .05) between males and females.
Reduced BMD defined as having a T-score < –1.
Osteopenia defined as having –2.5 < T-score < –1.
Osteoporosis defined as having a Z-score ≤ –2 for pre-menopausal women and men < 50 years of age and T-score ≤ –2.5 for postmenopausal women and men ≥ 50 years of age.
Total hip, hip femoral neck, or lumbar spine.
Demographic and BMD characteristics of the subpopulation with site-specific DXA
Of the 338 subjects, 132 subjects received site-specific DXA scans of the spine and hip. Demographic characteristics of this group were similar to those of the larger cohort (Table 1), with similar distribution among men and women (not shown). Men had lower BMI values than women (24.4 versus 26.2 kg/m2, respectively), lower fat mass, but higher LBM (59.3 versus 46 kg, respectively) as well as higher WMBD (1.26 versus 1.20 g/cm2, respectively), all with statistically significant differences between men and women (p < .05). Regression analysis revealed strong correlation between WMBD and spine BMD (r = 0.57, p < .0001), between WMBD and total-hip BMD (r = 0.53, p < .0001), and between WBMD and hip femoral neck BMD (r = 0.46, p < .0001). Reduced BMD was present in 69% of the population, with twice as many men having osteoporosis of the hip region (total or femoral neck) compared with women. Osteoporosis, as defined by the NOF, was identified in 22% of subjects (24 men and 5 women), with the majority of cases (90%) attributable to osteoporosis of the lumbar spine (Table 3, Fig. 1A, B).
Fig. 1.
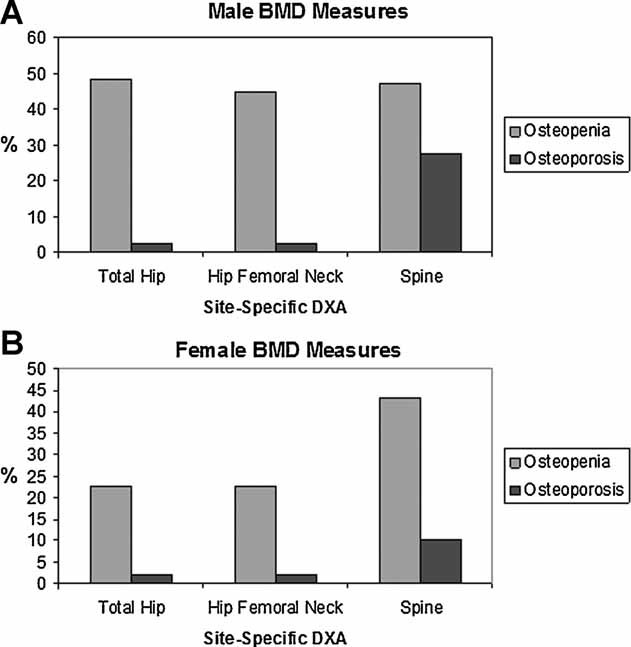
(A) Male BMD measures. (B) Female BMD measures
Risk factors associated with osteoporosis
The relation between osteoporosis and different independent variables is shown in Table 4. Men were nearly four times more likely to have osteoporosis than women [odds ratio (OR) = 3.6, 95% confidence interval (CI) 1.3–10]. Increasing LBM was the only other variable associated with lower risk of osteoporosis (OR = 0.84, 95% CI 0.76–0.94) in univariable analysis (Table 4). Similar results were obtained when BMI replaced lean and fat mass.
Table 4.
Potential Determinants of BMD by Site-Specific DEXA: Odds Ratio (95% CI) for having Osteoporosisa
| Variable | Univariate analysis | Multivariateb analysis |
|---|---|---|
| Gender (male/female) | 3.58 (1.27–10.12)* | 22.9 (5.01–105.5)*** |
| Lean body massc (kg) | 0.97 (0.93–1.01) | 0.88 (0.825–0.945)** |
| Age (per increase of 5 years) | 0.84 (0.65–1.08) | 0.86 (0.64−1.14) |
| Race (non-African American/African American) | 3.96 (0.93–16.94) | 3.32 (0.71−15.4) |
| Smokingd | 0.7 (0.26–1.89) | NS |
| Alcohole | NS | |
| 0 (no use) | Ref | |
| 1 (<3 drinks/day) | 1.07 (0.43–2.67) | |
| 2 (≥ 3 drinks/day) | 1.82 (0.52–6.32) | |
| Drug usef | NS | |
| 0 (no use) | Ref | |
| 1 (<3 times/week) | 0.74 (0.21–2.58) | |
| 2 (>3 times/week) | 1.26 (0.4–3.97) | |
| Hypogonadalg/menopausalh | 1.01 (0.4–2.53) | NS |
| Hepatitis Ci | 1.22 (0.53–2.84) | NS |
| HIVj | 1.77 (0.76–4.12) | NS |
| Fat body mass (kg) | 0.99 (0.99–0.99) | NS |
| BMI (kg/m2) | 0.84 (0.76–0.94)** | NS |
| Methadone | 1.52 (0.61–3.8) | NS |
| DHEA (tertiles)k | NS | |
| 1 | Ref | |
| 2 | 2.43 (0.85–6.95) | |
| 3 | 1.15 (0.39–3.42) | |
| Estradiol (tertiles)k | NS | |
| 1 | Ref | |
| 2 | 1.35 (0.47–3.82) | |
| 3 | 0.55 (0.17–1.77) | |
| IL-6 (tertiles)k | NS | |
| 1 | Ref | |
| 2 | 0.77 (0.25–2.35) | |
| 3 | 0.78 (0.23–2.63) | |
| CRP (tertiles)k | NS | |
| 1 | Ref | |
| 2 | 0.49 (0.15–1.56) | |
| 3 | 0.32 (0.1–1.06) | |
| TNF-α (tertiles)k | NS | |
| 1 | Ref | |
| 2 | 1.08 (0.35–3.27) | |
| 3 | 0.46 (0.13–1.6) | |
| Vitamin D deficientl | NS | |
| 0 | Ref | |
| 1 | 0.69 (0.29–1.59) | |
| Secondary hyperparathyroidismm | NS | |
| 0 | Ref | |
| 1 | 1.15 (0.41–2.97) |
NS = nonsignificant.
Note: Items in boldface denote significant p values
p < .05.
p < .01.
p < .0001.
Osteoporosis defined as having a T-score ≤ –2.5 for postmenopausal women and men aged ≥ 50 years of age or a Z-score ≤ –2 for premenopausal women and men < 50 years of age. T-score/Z-score measured at the lumbar spine, total hip, or hip femoral neck (lowest value taken).
Final model, R2 = 0.193; max-rescaled R2 = 0.297, p = .0001, n = 132.
In kilograms, measured by whole-body DXA scan.
Determined by answering yes to having smoked cigarettes in the past 6 months (without account to quantity).
Equivalent of 1 drink is 8 fluid ounces of beer or wine, 4 fluid ounces of liquor or malt, and 1 fluid ounce of vodka.
Includes marijuana, crack/freebase, cocaine (snorting, injection), speedball (injection), heroin (smoking, snorting, injection), and street methadone in the past 6 months.
Defined as having a free testosterone level < 52 pg/mL or currently on testosterone-replacement therapy
Defined as having an FSH level > 50 mIU/mL, age ≥ 51 years, or having FSH > 30 or ≤ 50 mIU/mL and answered yes to having gone through menopause.
Self-report.
Self-report, HIV+ by testing, or history of past or present use of antiretroviral therapy.
Obtained by plotting continuous lab values: 0 = the lowest tertile, 1 = the middle tertile, and 2 = the highest tertile.
Deficiency defined as having 25(OH)D level ≤ 15 ng/mL.
Defined as having an iPTH level > 65 pg/mL.
Of the 126 subjects with site-specific DXA and biochemical measurements of 25(OH)D and iPTH, 124 (98%) had 25(OH)D level < 32 ng/mL, 72% had 25(OH)D level ≤ 20 ng/mL, and 48% had 25(OH)D level ≤ 15 ng/mL. Secondary hyperparathyroidism (iPTH > 65 pg/mL) was present in 22% of subjects. 25(OH)D was inversely related to iPTH (r = 0.08, p = .001). However, the prevalence of secondary hyperparathyroidism was similar in those with and without vitamin D deficiency (28% versus 17%, p = .14). Osteoporosis was not associated with vitamin D deficiency or secondary hyperparathyroidism (Table 4). Site-specific Z-scores were similar regardless of the presence of vitamin D deficiency and/or secondary hyperparathyroidism (Table 5).
Table 5.
Site-Specific Z-score (Mean, SD) by Vitamin D, PTH Status
| Normal PTH | High PTH (>65 pg/mL) | |||
|---|---|---|---|---|
| Site | Low vitamin Da (n = 44) | Normal vitamin D (n = 54) | Low vitamin Da (n = 17) | Normal vitamin D (n = 11) |
| Total hip | −0.35 (1.0) | −0.22 (0.90) | −0.19 (0.9) | −0.47 (1.0) |
| Femoral neck | −0.04 (1.1) | 0.04 (0.99) | 0.24 (1.1) | −0.23 (0.8) |
| Lumbar spine | −0.81 (1.5) | −0.99 (1.3) | −0.92 (1.3) | −1.36 (1.4) |
Values presented as mean (SD).
Defined as 25(OH)D ≤ 15 ng/mL.
Discussion
In this predominately middle-aged, low-income, African-American, inner-city population in Baltimore, MD, we found a very high prevalence of osteoporosis, particularly at the lumbar spine. BMD in the osteoporotic range was more common in men than in women. Among men, only lower LBM was a significant positive determinant of lower WBMD, whereas in women, multiple factors were associated with lower WBMD, including age, white race, methadone use, and lower LBM.
The prevalence of osteoporosis was higher than expected in this population, which was 94% African American. Race plays an important role in BMD, where African Americans attain higher peak mass than whites, Hispanics, and Asians during puberty.(18,19) In population-based studies, African Americans have 13% to 18% higher hip BMD measures(20–24) and 2% to 12% higher spine BMD measures(20–25) than whites. The National Health and Nutrition Examination Survey III (NHANES III) data, consisting of 7116 subjects, similarly reported 12.3% higher femoral neck BMD measures in African-American compared with white men.(26,27) Compared with African Americans in a random sample from Boston (mean age 48 years), where the majority had good health, our African-American men had similar WBMD and femoral neck BMD measures, but our men had 6.4% lower total-hip and 6.3% lower spine BMD measures.(20) Other populations of African Americans also have shown high rates of osteoporosis. In a population of African-American male veterans (mean age 64 years, BMI 29 kg/m2), the prevalence of osteopenia (39%) and osteoporosis (7%) was unexpectedly high in a relatively low-risk population.(28) The contributing factors associated with low BMD in this cohort were not described. Together with our findings, these observations suggest that clinicians should be aware that African-American race is not necessarily a protective factor for low BMD. While we cannot conclude that African-American race is associated with lower BMD because our cohort was overwhelmingly African American and no similar cohort of white subjects is available for comparison, our data suggest that more aggressive bone density screening may be appropriate in some African-American populations.
Another major finding in our study was the higher prevalence of osteoporosis in men than in women. Rates of BMD lower in men than in women have been described among groups with depression(29) and in subjects on methadone maintenance, where in one study the prevalence of osteoporosis was higher in men than in women (61% versus 20%).(9) Plausible explanations for such high rates among men included secondary causes of osteoporosis such as reduced physical activity and diet. In our study, we investigated some secondary causes such as hypogonadism, vitamin D deficiency, and alcohol and tobacco abuse; however, none was significantly related to lower BMD.
The prevalence of vitamin D deficiency was high in our DXA subpopulation [48% with 25(OH)D < 15 ng/mL], a finding that is common among African Americans.(30–32) However, neither vitamin D deficiency nor secondary hyperparathyroidism could account for the high prevalence of osteoporosis in our population. It has been suggested that the adverse effects of vitamin D deficiency on bone in African Americans are less pronounced than in whites, possibly owing to relative PTH resistance.(33) This hypothesis requires further investigation because it has clear implications for the definition of vitamin D deficiency in African-American populations.
Most of the osteoporosis in our cohort (>90%) was found in the spine. The spine consists mainly of trabecular bone, which is highly metabolically active and is characterized by increased bone remodeling. In young men, loss of trabecular bone has been related to low physical activity.(34) Lower-spine BMD can have important clinical consequences. In the Osteoporotic Fractures in Men (MrOS) study, a prospective community study of 5995 men aged 65 years and older, a significant association was observed between lumbar spine BMD and vertebral fractures and a weaker but significant association between lumbar spine BMD and risk of hip fracture.(35,36) In contrast to the population of MrOs, which was predominantly white (90%), our population was 94% African American. It is unknown whether the low spine BMD observed in our cohort is predictive of higher rates of clinical vertebral and nonvertebral fracture because historical data regarding incidence fractures after DXA assessment were not captured.
The risk factors for lower BMD in our cohort differed between men and women. In both men and women, LBM was an important correlate of BMD. However, among women, other factors were associated with lower BMD, including age, non-African-American race, methadone use, and HIV status. Other studies have shown differences in risk factors among women and men. The Framingham Osteoporosis Study, which examined risk factors for longitudinal bone loss, found only weight to be related to lower BMD in men, whereas weight, alcohol intake, and estrogen therapy constituted risk factors in women.(37) Consistent risk factors for low BMD in a systematic review of healthy men aged 50 years or older included advanced age, smoking, and low weight.(38) Since our population was enriched by injection drug users having multiple risk factors that are different from the populations studied in the literature, other risk factors might be present that merit further investigation as low BMD determinants among men in this population.
Consistent with the literature, BMI was a significant determinant of BMD in both men and women.(39,40) When evaluating the components of BMI that contribute to the relation with BMD, our analysis showed that LBM rather than fat mass predicted BMD in both women and men compared with some studies that found LBM in men but fat mass in women to be BMI mediators.(41–43) Despite the high prevalence of many osteoporosis risk factors, surprisingly, we found no association between heavy alcohol, drug, and tobacco use; hepatitis C infection; hypogonadism; and inflammatory markers and lower BMD.
Implications and Conclusion
Osteoporosis of the spine was strikingly high in this cohort of inner-city, high-risk African-American males. Although the causes of this finding in our population are unclear, the high prevalence of osteoporosis in this young population could indicate even higher rates in similar older subjects. There is evidence of suboptimal recognition and diagnosis of osteoporosis among African Americans and in men,(44–46) and such disparities in detection can be more pronounced in underserved populations such as ours. Current osteoporosis guidelines suggest DXA screening in postmenopausal women and men aged 50 to 70 years based on clinical risk factors. Given the high burden of osteoporosis in this population, screening for osteoporosis could be warranted in this high-risk subpopulation at a younger age (50 years). It is uncertain that such high rates of osteoporosis could translate to increased fracture rate in the future, but further studies are needed to better understand and assess the implications.
Acknowledgments
This work was supported by Grants 1R01DA/DKR814–6141, 1R01DA14098, K23 AT002862 (to TTB), and P30 DA013868 (National Institute of Drug Abuse) from the NIH.
Disclosures
JC has served on an advisory board for Gilead Sciences. All the other authors state that they have no conflicts of interest.
References
- 1.Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement 2000. Bethesda, MD, January 1, 2000. [PubMed]
- 2. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General, 2004, 2009. [PubMed]
- 3.Ebeling PR. Clinical practice: osteoporosis in men. N Engl J Med. 2008;358:1474–1482. doi: 10.1056/NEJMcp0707217. [DOI] [PubMed] [Google Scholar]
- 4.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cofrancesco J, Jr, Scherzer R, Tien PC, et al. Illicit drug use and HIV treatment outcomes in a US cohort. AIDS. 2008;22:357–365. doi: 10.1097/QAD.0b013e3282f3cc21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santolaria-Fernandez FJ, Gomez-Sirvent JL, Gonzalez-Reimers CE, et al. Nutritional assessment of drug addicts. Drug Alcohol Depend. 1995;38:11–18. doi: 10.1016/0376-8716(94)01088-3. [DOI] [PubMed] [Google Scholar]
- 7.Wisniewski AB, Brown TT, John M, et al. Hypothalamic-pituitary-gonadal function in men and women using heroin and cocaine, stratified by HIV status. Gend Med. 2007;4:35–44. doi: 10.1016/s1550-8579(07)80007-6. [DOI] [PubMed] [Google Scholar]
- 8.Hollenbach KA, Barrett-Connor E, Edelstein SL, Holbrook T. Cigarette smoking and bone mineral density in older men and women. Am J Public Health. 1993;83:1265–1270. doi: 10.2105/ajph.83.9.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TW, Alford DP, Malabanan A, Holick MF, Samet JH. Low bone density in patients receiving methadone maintenance treatment. Drug Alcohol Depend. 2006;85:258–262. doi: 10.1016/j.drugalcdep.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coin A, Sergi G, Beninca P, et al. Bone mineral density and body composition in underweight and normal elderly subjects. Osteoporos Int. 2000;11:1043–1050. doi: 10.1007/s001980070026. [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 12.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260:76–87. doi: 10.1111/j.1365-2796.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- 14. Hologic I. Hologic Data Dictionary and Calculations. 2000 Nov 30.
- 15. Hologic I. Ethnic Normals Reference Databases. 2007.
- 16.National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008. [Google Scholar]
- 17.Melton LJ, III, Looker AC, Shepherd JA, et al. Osteoporosis assessment by whole body region vs. site-specific DXA. Osteoporos Int. 2005;16:1558–1564. doi: 10.1007/s00198-005-1871-y. [DOI] [PubMed] [Google Scholar]
- 18.Bryant RJ, Wastney ME, Martin BR, et al. Racial differences in bone turnover and calcium metabolism in adolescent females. J Clin Endocrinol Metab. 2003;88:1043–1047. doi: 10.1210/jc.2002-021367. [DOI] [PubMed] [Google Scholar]
- 19.Walker MD, Novotny R, Bilezikian JP, Weaver CM. Race and diet interactions in the acquisition, maintenance, and loss of bone. J Nutr. 2008;138:1256S–1260S. doi: 10.1093/jn/138.6.1256S. [DOI] [PubMed] [Google Scholar]
- 20.Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, McKinlay JB. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007;18:943–953. doi: 10.1007/s00198-006-0321-9. [DOI] [PubMed] [Google Scholar]
- 21.Daniels ED, Pettifor JM, Schnitzler CM, Russell SW, Patel DN. Ethnic differences in bone density in female South African nurses. J Bone Miner Res. 1995;10:359–367. doi: 10.1002/jbmr.5650100305. [DOI] [PubMed] [Google Scholar]
- 22.DeSimone DP, Stevens J, Edwards J, Shary J, Gordon L, Bell NH. Influence of body habitus and race on bone mineral density of the midradius, hip, and spine in aging women. J Bone Miner Res. 1989;4:827–830. doi: 10.1002/jbmr.5650040606. [DOI] [PubMed] [Google Scholar]
- 23.Kleerekoper M, Nelson DA, Peterson EL, et al. Pawluszka AS, Jacobsen G, Wilson P. Reference data for bone mass, calciotropic hormones, and biochemical markers of bone remodeling in older (55–75) postmenopausal white and black women. J Bone Miner Res. 1994;9:1267–1276. doi: 10.1002/jbmr.5650090817. [DOI] [PubMed] [Google Scholar]
- 24.Tobias JH, Cook DG, Chambers TJ, Dalzell N. A comparison of bone mineral density between Caucasian, Asian and Afro-Caribbean women. Clin Sci (Lond). 1994;87:587–591. doi: 10.1042/cs0870587. [DOI] [PubMed] [Google Scholar]
- 25.Meier DE, Luckey MM, Wallenstein S, Lapinski RH, Catherwood B. Racial differences in pre- and postmenopausal bone homeostasis: association with bone density. J Bone Miner Res. 1992;7:1181–1189. doi: 10.1002/jbmr.5650071010. [DOI] [PubMed] [Google Scholar]
- 26.Looker AC, Wahner HW, Dunn WL, et al. Proximal femur bone mineral levels of US adults. Osteoporos Int. 1995;5:389–409. doi: 10.1007/BF01622262. [DOI] [PubMed] [Google Scholar]
- 27.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 28.Sinnott B, Kukreja S, Barengolts E. Utility of screening tools for the prediction of low bone mass in African American men. Osteoporos Int. 2006;17:684–692. doi: 10.1007/s00198-005-0034-5. [DOI] [PubMed] [Google Scholar]
- 29.Mussolino ME, Jonas BS, Looker AC. Depression and bone mineral density in young adults: results from NHANES III. Psychosom Med. 2004;66:533–537. doi: 10.1097/01.psy.0000132873.50734.7d. [DOI] [PubMed] [Google Scholar]
- 30.Akhter N, Sinnott B, Mahmood K, Rao S, Kukreja S, Barengolts E. Effects of vitamin D insufficiency on bone mineral density in African American men. Osteoporos Int. 2009;20:745–750. doi: 10.1007/s00198-008-0746-4. [DOI] [PubMed] [Google Scholar]
- 31.Ginde AA, Liu MC, Camargo CA., Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988- 2004. Arch. Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng M, Giri V, Bruner DW, Giovannucci E. Prevalence and correlates of vitamin D status in African American men. BMC Public Health. 2009;9:191. doi: 10.1186/1471-2458-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cosman F, Morgan DC, Nieves JW, et al. Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res. 1997;12:958–966. doi: 10.1359/jbmr.1997.12.6.958. [DOI] [PubMed] [Google Scholar]
- 34.Tervo T, Nordstrom P, Neovius M, Nordstrom A. Reduced physical activity corresponds with greater bone loss at the trabecular than the cortical bone sites in men. Bone. 2009;45:1073–1078. doi: 10.1016/j.bone.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Cummings SR, Cawthon PM, Ensrud KE, Cauley JA, Fink HA, Orwoll ES. BMD and risk of hip and nonvertebral fractures in older men: a prospective study and comparison with older women. J Bone Miner Res. 2006;21:1550–1556. doi: 10.1359/jbmr.060708. [DOI] [PubMed] [Google Scholar]
- 36.Freitas SS, Barrett-Connor E, Ensrud KE, et al. Rate and circumstances of clinical vertebral fractures in older men. Osteoporos Int. 2008;19:615–623. doi: 10.1007/s00198-007-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hannan MT, Felson DT, wson-Hughes B, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–720. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 38.Papaioannou A, Kennedy CC, Cranney A, et al. Risk factors for low BMD in healthy men age 50 years or older: a systematic review. Osteoporos Int. 2009;20:507–518. doi: 10.1007/s00198-008-0720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dennison E, Eastell R, Fall CH, Kellingray S, Wood PJ, Cooper C. Determinants of bone loss in elderly men and women: a prospective population-based study. Osteoporos Int. 1999;10:384–391. doi: 10.1007/s001980050244. [DOI] [PubMed] [Google Scholar]
- 40.Edelstein SL, Barrett-Connor E. Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol. 1993;138:160–169. doi: 10.1093/oxfordjournals.aje.a116842. [DOI] [PubMed] [Google Scholar]
- 41.Douchi T, Kuwahata R, Matsuo T, Uto H, Oki T, Nagata Y. Relative contribution of lean and fat mass component to bone mineral density in males. J Bone Miner Metab. 2003;21:17–21. doi: 10.1007/s007740300003. [DOI] [PubMed] [Google Scholar]
- 42.Pluijm SM, Visser M, Smit JH, Popp-Snijders C, Roos JC, Lips P. Determinants of bone mineral density in older men and women: body composition as mediator. J Bone Miner Res. 2001;16:2142–2151. doi: 10.1359/jbmr.2001.16.11.2142. [DOI] [PubMed] [Google Scholar]
- 43.Reid IR, Ames R, Evans MC, et al. Determinants of total body and regional bone mineral density in normal postmenopausal women-a key role for fat mass. J Clin Endocrinol Metab. 1992;75:45–51. doi: 10.1210/jcem.75.1.1619030. [DOI] [PubMed] [Google Scholar]
- 44.Cheng H, Gary LC, Curtis JR, et al. Estimated prevalence and patterns of presumed osteoporosis among older Americans based on Medicare data. Osteoporos Int. 2009;20:1507–1515. doi: 10.1007/s00198-009-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller RG, Ashar BH, Cohen J, et al. Disparities in osteoporosis screening between at-risk African-American and white women. J Gen Intern Med. 2005;20:847–851. doi: 10.1111/j.1525-1497.2005.0157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohldin A, Floyd J. Unrecognized risks among Veterans with hip fractures: opportunities for improvements. J South Orthop Assoc. 2003;12:18–22. [PubMed] [Google Scholar]


