Abstract
This study describes the clinical presentation and diagnosis in all Danish patients (49, 41 unrelated) with Wilson disease (WND). On the basis of the number of diagnosed patients from 1990–2008, the prevalence was estimated to be 1:49 500. Among routinely used diagnostic tests, none were consistently indicative of WND, with the exception of the 24-h urine-Cu test, which is always outside the normal range. Mutations were identified in 100% of the screened ATP7B alleles (70 unrelated), including five novel mutations: p.1021K; p.G1158V; p.L1304F; IVS20-2A>G; Ex5_6del. In all, 70% of mutations were found in exons 8, 14, 17, 18, and 20. The most frequent mutation, p.H1069Q, comprised 18%. We propose a new and simple model that correlates genotype and age of onset. By assuming that the milder of two mutations is ‘functionally dominant' and determines the age of onset, we classified 25/27 mutations as either severe (age of onset <20 years) or moderate (age of onset >20 years), and correctly predicted the age of onset in 37/39 patients. This method should be tested in other Wilson populations.
Keywords: Wilson disease, ATP7B, mutations, genotype/phenotype, age of onset, Denmark
Introduction
Wilson disease (WND; MIM# 27790) is an autosomal recessive disorder of copper transport caused by mutations in the ATP7B gene that encodes a P-type copper ATPase, ATP7B. It has been suggested that the prevalence of WND is between 1:30 000 and 1:100 000,1 but in the Scandinavian countries2 it may be as low as 1:250 000.
Copper (Cu) is the third most abundant trace element in the body after iron and zinc. Athough essential, Cu may be highly toxic.1, 3 The liver has a central role in Cu homeostasis, and a strict regulation is vitally important.4, 5 From the liver, Cu is either secreted to the blood bound to ceruloplasmin (Cp) or via bile excreted to the stool. The ATP7B protein has a dual role in controlling both processes; (1) it mediates the biosynthesis of holoceruloplasmin (HoloCp) by delivering Cu to apoceruloplasmin (ApoCp) in the trans-golgi network; and (2) it facilitates biliary excretion of excess Cu.4, 6, 7 In WND, a mutated dysfunctional ATP7B leads to a progressive accumulation of Cu in the liver and brain. Clinically, WND shows considerable phenotypic variability including fulminant hepatic failure, hemolysis, chronic liver disease, such as hepatitis and cirrhosis, and neuro-psychiatric disease with or without hepatic involvement.
The classical diagnosis of WND rests on the Sternlieb criteria1, 8, 9 and includes at least two of the following: (a) typical neurological symptoms, (b) the presence of a Kayser–Fleisher (KF) ring, (c) low Cp, and (d) elevated hepatic Cu. However, the diagnosis is still being challenged, and the significance attributed to the different tests is under debate.1, 9, 10
The ultimate confirmation of a WND diagnosis lies in the identification of the disease-causing mutations. More than 500 mutations have been described (http://www.wilsondisease.med. ualberta.ca/database.asp). A better understanding of the genotype–phenotype relationship in WND would be of great clinical importance, but so far, no clear relationship has been established.1, 11, 12
The management of WND in Denmark has been centralized since 2001 at Aarhus University Hospital. This paper describes the Danish WND population, the clinical presentations, the identified mutations, means of diagnosis, and provides an estimate of the incidence and prevalence of the disease in Denmark. The genotypes were identified in 35 out of a total of 41 families, facilitating the study of phenotype–genotype relationships. Finally, we propose a simple hypothesis for the prediction of age of onset from the identified mutations.
Materials and methods
The Danish database for Wilson patients
The Danish Wilson Centre, situated at the Aarhus University Hospital, is responsible for maintaining a database for all Danish patients, for treating them in collaboration with local hospitals, and facilitates diagnosis, including molecular testing.
Although clinical and genetic data were collected as part of a database required by the National Board of Health, the remaining investigations were carried out after obtaining informed consents and approval from the Scientific Ethical Committee.
Sequence analysis
Genomic DNA was extracted from peripheral blood lymphocytes or cultured fibroblasts using the NaCl method.13 PCR amplification of each of the 21 coding exons, including 20 base-pair flanking sequences was carried out with sequence-specific primers. The sequences of the oligoes are available on request. Amplification was carried out under standard conditions using Amplitaq Gold polymerase (Applied Biosystems, Naerum, Denmark). Purified PCR products were subsequently sequenced with the Big Dye Terminator v.3.1 Cycle Sequencing Kit and analysed using an Applied Biosystems 3130XL Genetic Analyzer with SeqScape Software (Applied Biosystems). NM_000053, NCBI was used as the reference sequence.
DNA samples in which two mutations in the ATP7B gene could not be identified by sequencing were subsequently analyzed for partial exon deletions or duplications using the ATP7B Multiplex Ligation-dependent Probe Amplification (MLPA) Kit (Salsa P098, MRC-Holland, Amsterdam, The Netherlands).
In cases with apparent homozygosity for a mutation, the mutations in trans were verified by either testing the parents, or by testing for exon deletions with MLPA in order to exclude any masking of one of the two alleles. Similar methodology was used to ensure that patients with two different mutations had one on each allele.
Results
The Danish Wilson database
The database included 49 patients from 41 unrelated families. One family included four affected siblings and five families had two. Their characteristics and identified mutations are listed in Table 1, which is organized according to the clinical presentations.
Table 1. Clinical presentation and identified mutations.
| WD NO | Sex | Vital status | Age at onset (year) | Age at diagnosis (year) | Diagnostic Delay (months) | Year of diagnosis | Country of origin | Variant 1 | Variant 2 |
|---|---|---|---|---|---|---|---|---|---|
| FHF | |||||||||
| 15 | M | Alive | 30 | 30 | 0.2 | 1993 | Denmark | p.H1069Q | p.G1158V |
| 20-S | M | Dead (FHF) | 13 | 13 | NA | <1966 | Denmark | p.I1021K | p.T1220M |
| 25 | F | Alive | 21 | 21 | 0.2 | 1991 | Denmark | p.W779X | p.H1069Q |
| 25-S | F | Dead (FHF) | 16 | 16 | 0.2 | 1990 | Denmark | p.W779X | p.H1069Q |
| 34 | M | Alive-TX | 12 | 12 | 0.2 | 1994 | Denmark | p.W779X | p.M769V |
| 43 | M | Alive-TX | 12 | 12 | 0.2 | 2005 | Denmark | p.W779X | p.T977M |
| 41 | F | Alive-TX | 23 | 23 | 0.5 | 2007 | Iceland | c.2007_2013delATATGCT | c2007_2013delATATGCT, |
| 44 | M | Dead (FHF) | 9 | 9 | NA | 1974 | Denmark | p.G1266R | c.1708-?_1946+?del |
| B | M | Alive-TX | 11 | 11 | 0.2 | 1994 | Denmark | NA | NA |
| F-S1 | F | Dead (FHF) | 13 | 13 | 0.2 | 1978 | Denmark | NA | NA |
| Hepatitis+Hemolysis | |||||||||
| 9a | M | Alive | 11 | 11 | 4 | 1981 | Turkey | c.4022-?_4398+?del | c.4022-?_4398+?del |
| 20 | M | Alive | 8 | 11 | 36 | 1966 | Denmark | p.I1021K | T1220M |
| 24 | F | Alive | 33 | 34 | 8 | 2001 | Iceland | c2007_2013delATATGCT | c2007_2013delATATGCT |
| 31 | M | Alive | 11 | 11 | 2 | 2003 | Denmark | p.W779X | G943D |
| 37 | F | Alive | 14 | 16 | 24 | 2004 | Denmark | p.W779X | G1266R |
| 42 | F | Alive | 15 | 15 | 6 | 1992 | Denmark | p.H1069Q | G1266R |
| 45 | M | Alive | 16 | 16 | 6 | 2000 | Denmark | p.P1273 L | c.2304insC |
| CLD | |||||||||
| F | F | Alive | 17 | 17 | NA | 1969 | Denmark | NA | NA |
| 10 | M | Alive | 13 | 14 | 13 | 1994 | Pakistan | p.L1304F | p.L1304F |
| 11 | F | Alive | 39 | 39 | 2 | 1989 | Denmark | p.R1041W | p.T1220M |
| 19 | M | Alive-TX | 39 | 39 | NA | 1990 | Israel | p.D642H | p.D642H |
| 22 | F | Alive | 13 | 13 | 6 | 1969 | Denmark | p.G943D | p.S1363F |
| 32 | M | Alive | 7 | 7 | 3 | 2004 | Denmark | p.H1069Q | p.P1273 L |
| 38 | M | Alive | 13 | 13 | 6 | 1995 | Denmark | p.H1069Q | p.S1363F |
| 39 | F | Alive | 9 | 10 | 14 | 2007 | Denmark | p.I1021K | p.H1069Q |
| 40 | M | Alive | 7 | 7 | 2 | 2007 | Denmark | p.H1069Q | p.H1069Q |
| 17 | M | Dead (CLD) | 30 | 32 | 21 | 1995 | Denmark | p.M769V | p.G943D |
| C | M | Alive-TX | 33 | 34 | 14 | 1989 | Denmark | NA | NA |
| Neurological symptoms | |||||||||
| 6 | M | Alive | 12 | 12 | 0 | 1969 | Denmark | p.W779X | p.W779X |
| 13 | F | Alive | 23 | 33 | 120 | 1978 | Denmark | p.T1220M | p.Q1351X |
| 14 | M | Alive | 26 | 26 | 4 | 1979 | Denmark | p.P1273 L | c.4022-2A>G |
| 16 | M | Alive | 14 | 14 | 1 | 1995 | Turkey | p.N1270S | p.N1270S |
| 21 | F | Alive | 17 | 18 | 13 | 1987 | Denmark | p.H1069Q | p.S1363F |
| 23 | F | Alive | 38 | 38 | 5 | 1997 | Denmark | p.W779X | p.R1041W |
| 26 | M | Alive | 34 | 35 | 7 | 1988 | Denmark | p.H1069Q | p.A1003T |
| 27 | F | Alive | 10 | 17 | 84 | 1993 | Denmark | p.H1069Q | p.S1363F |
| 29 | F | Alive-TX | 14 | 19 | 60 | 1994 | Turkey | p.H1069Q | p.G1341D |
| 33 | F | Alive | 23 | 24 | 14 | 2004 | Turkey | p.M1169T | p.M1169T |
| 35 | M | Alive | 17 | 17 | 6 | 2004 | Korea | p.R778 L | p.R778 L |
| 36 | M | Alive | 28 | 30 | 27 | 2004 | Serbia | p.G591D | p.R616Q |
| 2 | M | Alive | 19 | 19 | NA | 1978 | Denmark | p.T1220M | p.G1266R |
| 6-S | M | Alive | NA | 15 | NA | 1968 | Denmark | p.W779X | p.W779X |
| A | F | Dead-TX (HCC) | 27 | 30 | 37 | 1984 | Denmark | NA | NA |
| D | F | Alive | 23 | 29 | 72 | 1996 | NA | NA | |
| E | F | Dead (Other) | 20 | 20 | NA | 1959 | NA | NA | |
| Identified as asymptomatic siblings who started treatment before symptomatic disease | |||||||||
| 2-S | F | Dead (accident) | 17 | 17 | NA | 1978 | Denmark | p.T1220M | p.G1266R |
| F-S2 | M | Dead (accident) | 20 | 20 | NA | NA | Denmark | NA | NA |
| F-S3 | F | Alive | 10 | 10 | 36 | 1975 | Denmark | NA | NA |
| 27-S | M | Alive | 15 | 15 | 0 | 2000 | Denmark | p.H1069Q | p.S1363F |
Abbreviations: CLD, chronic liver disease; FHF, fulminant hepatic failure; HCC, hepatocellular carcinoma; NA, Not available; TX, liver transplantation
Patients belonging to the same family have the same number or letter. ‘S' denotes sibling to the first patient diagnosed in the particular family.
As seen in Table 1, the age of onset was higher in 17 patients with neurological WDN (median 20 years; range 12–38), than in 11 with chronic liver disease (13 years; 7–39), 7 with hepatitis and hemolysis (14 years; 8–33) and 10 with fulminant hepatic failure (12.5; 9–30). The time from first symptom to diagnosis (diagnostic delay) was longer in neurological (median 14 months, range 0–120), than in chronic liver disease or hepatitic presentation (6 months; 2–24) (Table 1).
By June 2009, 9 of the 49 patients had died. Causes of death included fulminant hepatic failure in four patients, accidents in two, complications related to cirrhosis in one, complications related to other chronic diseases in one, and one patient who died of recurrent hepatocellular carcinoma after a liver transplant. Altogether, eight patients received a liver transplant (due to fulminant hepatic failure in four patients, complications related to cirrhosis in two, hepatocellular carcinoma in one, and one with progressive disease during zinc therapy and intolerance to both penicillamine and trientine). Apart from the patient with hepatocellular carcinoma, all transplanted patients are still alive.
The diagnostic awareness increased with time, as 21 were diagnosed during 29 years from 1960–1989 and 28 during 19 years from 1990–2009. From 28 new diagnoses during 19 years, a population of 5.5 mio, and a life expectancy of 70 years in treated patients, we estimated a theoretical prevalence of 1:49 500.
Diagnosis of Wilson disease
The database included information about the diagnostic tests used to diagnose WND. The individual diagnostic criteria are provided in Supplementary Table 1. None of the tests were consistently indicative of Wilson disease. In short, only the 24-h urine-Cu was consistently above the normal range (<1.6 μmol/24 h) in all 32 out of 32 patients where this was available. Of these, 23 patients had clearly elevated values (>5 μmol/24 h) and nine patients had values in the intermediate range (1.6–5 μmol/24 h). Hepatic Cu was above the upper limit of normal in 23/23 but only above the conventional diagnostic limit for WND (five times upper limit of normal) in 12/23 patients.
None of the other tests were consistently abnormal in WND patients. Thus, a KF ring was only reported in 26 out of 41 patients in whom this diagnostic examination was available, a decreased Cp in 37/41, and low plasma Cu (P-Cu) in 34/39. A penicillamine-stimulated 24-h urine-Cu above 25 mmol has been suggested as diagnostic for WND; however, this was only seen in 16 out of 24 patients in whom it was measured.
The identified mutations
DNA was available from 40 WND patients from 35 unrelated families. The mutations were identified in all investigated alleles, corresponding to a 100% detection rate (Table 1). As expected, the most frequent mutations were p.H1069Q with an allele frequency of 18% (12 out of 70 unrelated alleles), and p.W779X with an allele frequency of 16% (8/70). Three mutations (p.S1363F, p.T1220M, and p.G1266R) were each identified in 6–7% of the alleles. Approximately 70% of the mutations were located in exons 8, 14, 17, 18, and 20. In patient WD9, a homozygous deletion of exon 21–22 (c.4022-?_4398+?del) was observed as described previously,14 and a deletion of exon 5–6 (c.1708-?_1946+?del) in the heterozygous form was found in patient WD44. Thus, partial deletions of the ATP7B gene were observed in 4% of the alleles (3/70).
The mutations p.D642H, p.R778L, p.W779X, p.H1069Q, p.M1169T, p.N1270S, p.L1304F, c.2007-2013delATATCT, and c.4022-?+_4398+?del were all identified in the homozygous form. Most of these patients had an ethnic background with a tradition of consanguineous marriages in small communities. Only the patients with the p.W779X and p.H1069Q alterations in homozygous form were ethnic Danes. The parents of these patients were not related, but these mutations are the most common in the Danish population.
Five of the identified mutations, p.1021K, p.G1158V, p.L1304F, c.4022-2A>G, and c.1708-?_1946+?del have not been reported before. These are marked in bold letters in Table 2. Analysis of the missense mutations by the Sorting Intolerant From Tolerant (SIFT) homology tool (Ng and Henikoff) (http://blocks.fhcrc.org/sift/SIFT.html) clearly suggested their functional significance (Table 2). This was further supported by testing for conservation between ATP7B proteins from different mammals (human, rat, mouse, and dog), and for conservation between the human ATP7A and ATP7B, ATPases (data not shown). The splice site mutation IVS20-2A>G was analyzed with the tools provided at link http://www.fruitfly.org/cgi-bi n/seq_tools/splice.pl. Although the wild-type sequence was predicted as a useful 3′splice site with a score of 0.97, the mutated sequence was not recognized as a splice site, again confirming the functional significance of the mutation.
Table 2. Classification of mutations.
| Varianta | Mutationa | Severity groupc | SIFTd | Exon | Type, location | Functional effecte | Allele frequencies |
|---|---|---|---|---|---|---|---|
| p.G591D | c.1772G>A | Moderate | 0.42 | 5 | Missense, Cu 6 | Extensive mislocalization | 1% (1/70) |
| p.R616Q | c.1847G>A | Moderate | 0.07 | 5 | Missense | 1% (1/70 | |
| Ex5-6delb | c.1708-?_1946+?del | Severe | — | 5–6 | Deletion, Cu 6 | 1% (1/70) | |
| p.D642H | c.1924G>C | Moderate | 0.05 | 6 | Missense, Cu6/TM1 | 3% (2/70) | |
| p.I669I-fs | c.2007_2013delATATGCT | Moderate | — | 7 | Deletion, TM1 | 6% (4/70) | |
| p.M769H-fs | c.2304insC | Severe | — | 8 | Insertion, TM4 | 1% (1/70) | |
| p.M769V | c.2305A>G | Moderate | 0.52 | 8 | Missense, TM4 | Normal rescue ΔCcc2 yeast. Normal cellular localization | 3% (2/70) |
| p.R778 L | c.2333G>T | Severe | 0.01 | 8 | Missense, TM4 | Reduced rescue ΔCcc2 yeast. Extensive mislocalization | 3% (2/70) |
| p.W779X | c.2336G>A | Severe | 0.00 | 8 | Non-sense, TM4 | 12% (8/70) | |
| p.G943D | c.2828G>A | Severe | 0.02 | 12 | Missense, TM5 | 4% (3/70) | |
| p.T977M | c.2930C>T | Severe | 0.00 | 13 | Missense, TM6 | No rescue ΔCcc2 yeast | 1% (1/70) |
| p.A1003T | c.3007G>A | Moderate | 0.00 | 13 | Missense, TM6 | 1% (1/70) | |
| p.I1021 Kb | c.3062T>A | Severe | 0.00 | 14 | Missense, ATP | 3% (2/70) | |
| p.R1041W | c.3121C>T | Moderate | 0.03 | 14 | Missense, ATP loop, N-domain | 3% (2/70) | |
| p.H1069Q | c.3207C>A | Severe | 0.00 | 14 | Missense, ATP loop, N-domain | No/reduced rescue ΔCcc2 yeast. Extensive mislocalization | 18% (12/70) |
| p.G1158Vb | c.3473G>T | Moderate | 0.03 | 16 | Missense, ATP loop, N-domain | 1% (1/70) | |
| p.M1169T | c.3506T>C | Moderate | 0.01 | 16 | Missense, ATP loop, N-domain | 3% (2/70) | |
| p.T1220M | c.3659C>T | Severe | 0.00 | 17 | Missense, ATP binding P-domain | 6% (4/70) | |
| p.G1266R | c.3796G>A | Severe | 0.00 | 18 | Missense, ATP hinge, P-domain | 6% (4/70) | |
| p.N1270S | c.3620A>G | Severe | 0.00 | 18 | Missense, ATP hinge, P-domain | Reduced ATP hydrolysis activity | 3% (2/70) |
| p.P1273 L | c.3818C>T | Severe | 0.00 | 18 | Missense, ATP hinge, P-domain | 3% (3/70) | |
| p.L1304Fb | c.3912G>T | Severe | 0.00 | 19 | Missense, between ATP hinge and TM7 | 3% (2/70) | |
| p.G1341D | c.4022G>A | Severe | 0.02 | 20 | Missense, TM7 | 1% (1/70) | |
| p.Q1351X | c.4051C>T | Moderate | 0.00 | 20 | Non-sense, loop between TM7-TM8 | 1% (1/70) | |
| p.S1363F | c.4088C>T | Severe | 0.00 | 20 | Missense, TM 8 | 6% (4/70) | |
| IVS20-2A>Gb | c.4022-2A>G | Moderate | — | Intron 20 | Splice-site, skipping of exon 21 | 1% (1/70) | |
| Ex20-21del | c.4022-?_4398+?del | Severe | — | 20-21 | TM8, C-ter | 3% (2/70) |
Nucleotide numbering refers to the cDNA numbering, where +1 is the A of the ATG translation initiation codon in the reference sequence. Codon 1 is the initiation codon, ATG.
New mutations in bold letters, reported for the first time in this study.
Severe, age of onset<20 years; moderate, age of onset>20 years.
SIFT: predicts whether a substitution can be tolerated or not: >0.05 tolerated; <0.05 affect protein function.
Reviewed in de Bie et al.36
Genotype–phenotype correlation
So far there are no reports on any clear association between the genotype and the clinical presentation of WND. One problem is that most patients carry two different mutations. We hypothesized that in WND there is a correlation between the residual activity of the enzyme and the clinical severity of the disease as expressed by the age of onset. A similar relation is reported in other genetic metabolic diseases.15, 16 We assumed that the least severe mutation is ‘functionally dominant' that is, that the age of onset is determined by the least severe of the two ATP7B mutations. The known mutations were divided into ‘severe' or ‘moderate' according to whether they caused clinical symptoms before or after the age of 20. The procedure consisted of the following steps:
Step one
First we looked at the age of clinical presentation in patients homozygous for a single mutation (patients WD6, WD-6S, WD9, WD10, WD16, WD19, WD24, WD33, WD35, WD41, and WD40) and categorized the following mutations as severe: p.R778L, p.W779X, p.H1069Q, p.N1270S, p.L1304F, c.4022-?_4398+?del, and as moderate; p.D642H, p.M1169T, and c2007_2013del.
Step two
The next step was to identify heterozygous patients with one mutation already categorized as severe (WD15, WD34, WD43, WD31, WD37, WD42, WD32, WD38, WD39, WD21, WD23, WD26, WD27, WD29, and WD27-S). If the age of onset was <20 years, the other mutation was then also regarded as severe; else, as moderate. This step identified the following mutations as severe: p.T977M, p.G943D, p.G1266R, p.P1273L, p.S1363F, p.I1021K, and p.G1341D, and the following as moderate: p.G1158V, p.R1041W, and p.A1003T. Even though some of these were found in several patients (ie, p.S1363F), their classification was always consistent.
Step three and subsequent steps
Compound heterozygous patients with one mutation categorized as severe according to step two (WD2, WD20, WD44, WD45, and WD2-S) categorized p.T1220M, c.1708-?_1946+?del, and c.2304insC as severe mutations. Subsequently the mutations p.Q1351X, c.4022-2A>G, and p.M769V in WD13, WD14, and WD17, respectively, could be classified as moderate. The age of onset of patient WD11 (39 years) with one severe (p.T1220M) and one moderate (p.R1041W) fits with the prediction.
Most mutations had a SIFT score of 0.00, but with a tendency towards higher values for mutations classified as moderate (Table 2). SIFT seems therefore to be a useful supportive tool for classification.
The data from WD36 could not be used for this analysis. This patient whose age of onset was 28 years was compound heterozygous for the mutations p.G591D and p.R161Q. As these mutations were not seen in any of the other patients, we could not deduce their severity. Their SIFT values indicate that possibly both mutations are moderate.
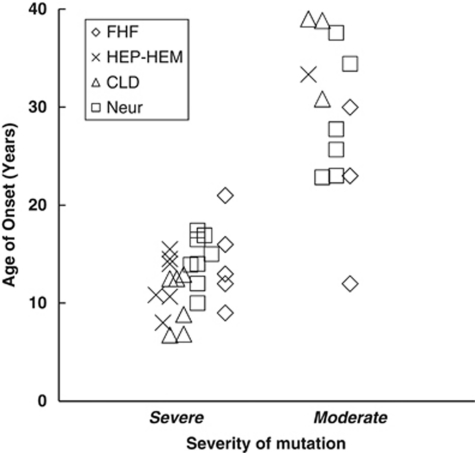
The genotype–phenotype relation constructed in this way is illustrated in Figure 1. As seen, only 2 out of 39 patients do not match the predictions: WD25 with two mutations classified as severe (p.W779X/p.H1069Q) with age of onset at 21, and WD34 with one mutation (p.M769V) classified as moderate (p.M769V/p.G943D) with onset at 12 years.
Figure 1.
Relationship between the severity of mutation and age of onset (years) of WND. FHF, fulminant hepatic failure; HEP-HEM, hepatitis + hemolysis; CLD, chronic liver disease; Neur, neurological symptoms. Mutations were classified as severe (age of onset <20 years) or moderate (>20 years) on the assumption that the least severe mutation was functionally dominant. The individual classification of mutations is displayed in Table 2.
In contrast to the correlation between genotype and age of onset, there was no clear association between type of clinical presentation (hepatic versus neurological) and genotype. This is illustrated by the four patients WD27-S, WD27, WD21 and WD38, all with the genotype H1069Q/S1363F and similar age of onset, but with four different clinical presentations: asymptomatic, neurological, neurologic-hepatic, and hepatic, respectively.
Discussion
This paper describes the clinical presentation, diagnostic methodology, and the genetic aberrations in the Danish WND population. We propose a simple and efficient method to establish a relationship between genotype and age of onset based on the hypothesis that the least severe mutation is functionally dominant. As the study included the total WND population from an entire country, it should be devoid of selection bias.
Incidence and prevalence of WND in Denmark
On the basis of the number of diagnosed WND patients between 1990–2008, we estimated the prevalence of WND to be 1:49 500, which are not different from the general expectation of 1:30 000 −1:100 000 in the literature.1, 4, 17 A Swedish study2 that used a different methodology, suggested a lower incidence in Sweden maybe as low as 1:250 000. It therefore seems that WND may be more common in Denmark than in Sweden.
On the basis of the theoretical prevalence of 1:49 500 one would expect 107 WND patients in Denmark. The fact that only 49 cases were identified may reflect that a number of undiagnosed and untreated patients died before 1990. With higher diagnostic awareness and adequate treatment, the number of WDN patients in Denmark will increase over the next 2–3 decades.
The so-called diagnostic delay, that is, the time from first symptoms to diagnosis, was median 10.5 months with a wide range. In other studies this period varied between 13 and 25 months on average.18, 19 An increasing diagnostic awareness is warranted to reduce this delay, because the disease if untreated may cause permanent damage to the liver and brain.
The clinical spectrum and diagnosis of WND
The spectrum of clinical presentations was not different from that reported in similar studies.19, 20 Although hepatic symptoms generally appeared earlier than neurological,19, 21 we also observed a group of patients with liver disease and late onset (32–39 years), that may have been overlooked in earlier studies.
The diagnosis of WND is rarely straightforward,9, 10 as also confirmed by our data. The discussion will focus on the diagnosis of non-fulminant cases, as the additional difficulties in fulminant WND are well recognized.1, 19
The KF ring was absent in 36% (15/41) of the examined patients, including 4/15 with neurological WND. Thus, as also reported by others,20, 22, 23 the absence of KF rings cannot be used to exclude WND. Low Cp and low P-Cu values were observed more consistently, but then again, 4/37 patients without fulminant hepatic failure had normal Cp values and 5/39 patients had normal P-Cu values. The 24-h urine-Cu excretion was above the normal range in all 32 patients from whom it was attainable, suggesting its use as a screening test. This was, however, questioned in a German study,19 in which 24-h urine-Cu was elevated in only 87% of WND patients. In addition, moderately elevated values are also seen in patients with cholestasis or chronic hepatitis.18, 24, 25 The penicillamine challenged 24-h urine-Cu was only available in 24 patients without fulminant hepatic failure; eight had values below the limit of 25 μmol/24 h that has been proposed as diagnostic for symptomatic children.26 Our data suggest that a lower threshold might be more appropriate.
Genetic analysis
We were able to identify the mutation in all 70 analyzed alleles. The high proportion of identified alleles was obtained by sequencing all exons and, in cases where no point mutation could be identified a search for partial gene deletions or duplications was carried out. Interestingly, partial gene deletions were much less common (4%) in WND than in Menkes disease, where partial gene deletions represent about 17% of the mutations in ATP7A.27
H1069Q is the most common mutation in patients from Central, Eastern, and northern Europe. The allele frequency was 18% among Danish Wilson patients. The frequency is higher in several other countries, including Sweden (38%), Hungary (47%), Poland (72%),28 Bulgaria (59%), Greece (35%)29 former Yugoslavia (49%),30 Australia (34%),12 northern America (38%),31 and Spain (25%).31 In the United Kingdom, the frequency is 17%32 like in Denmark.
Five of the identified mutations (p.I1021K, p.G1158V, p.L1304F, c.4022-2A>G, and c.1708-?_1946+?del) have not been previously reported. As described in the results section, these mutations were subjected to further analysis to verify that, they indeed caused disease (Table 2).
Genotype–phenotype correlation
As in other studies,21, 29, 30, 33, 34 we could not establish a correlation between genotype and clinical presentation. The difficulties may reflect that the phenotype could be modified by an interaction of the mutated ATP7B proteins with other proteins, such as ATOX1 and COMMD1,35, 36 or by environmental factors like exposure to metals, including levels of copper in the diet or drinking water, oxidative stress, and the consumption of alcohol.
However, this study represents a new attempt to develop a simple correlation between ATP7B genotype and age of onset. As is the case with phenylketonuria15 and carnitine palmitoyltransferase II,16 we assumed that disease severity defined by the age of onset was determined by the less severe of the two ATP7B mutations, which is then regarded as ‘functionally dominant'. Assuming that this indeed is the case, we were able to classify 25/27 mutations as either severe (onset <20 years of age) or moderate (onset>20 years). The predictions fitted with the age of onset in 37/39 patients (Figure 1).
A relationship between the type of mutation and the age of onset has been suggested in studies of patients with identical genotype.21, 29, 37 Gu et al37 found that the age of onset was between 10 and 20 years in 32/37 patients who were homozygous for the p.R778L mutation, in accordance with our classification of p.R778L as Severe. In studies of patient with the p.H1069Q mutation, Vrabelova et al21 reported the mean age of onset to be 16.2±5.7 years in 94 patients from 75 unrelated families, and Panagiotakaki et al29 reported 18.7 years±6.1 in 13 patients. This is not in conflict with our method, which classified p.H1069Q as severe with age of onset before 20 years of age.
It has also previously been suggested that the age of onset could potentially be related to the residual functional activity of the ATP7B mutants. It was claimed that truncating mutations in ATP7B are associated with an early onset of Wilson disease. Merle et al11 found that patients with two severe truncating mutations had an onset at 13 years with an interquartile range of 9–13 years, whereas patients with two missense mutations had an onset at 22 years with an interquartile range of 14–27 years. Similar results were published by Gromadzka et al,38 who reported that in the presence of two severe truncating mutations the age of onset was 14±7 years, while in the presence of two missense mutations the age of onset was 29±9 years. They reported an intermediate age of onset in the presence of one missense mutation and one severe truncating mutation (25±9 years).38 Interestingly, we found that the p.Q1351X mutation in our material could be classified as moderate with late onset, although it leads to a termination of the protein. Truncating mutations associated with relatively late onset has also been described with p.C271X (age of onset 17–19 years)39 and p.S932X (age of onset 24 years).40 One explanation for the apparently mild effects of some truncating mutations could be translational read through by insertion of an amino acid despite a stop codon, as observed for the p.R201X mutation in the homologous ATP7A gene.41 Taken together, these studies allow no robust predictions about the relationship between truncating mutations and age of onset.
Gupta et al39 made an observation that seems to fit with a third possibility, namely the age of onset is determined by the most severe of the two mutations, but these data were rather limited and based on only six patients.
A better understanding of the genotype–phenotype relationship would be of great clinical importance. Compared with previous attempts to establish a relation between the mutation and age of onset, our simple model performed remarkably well. Therefore, our proposed model should be evaluated in other and larger WND populations. With a larger number of patients, it might even be possible to categorize the mutations into more than two groups.
In conclusion, we describe that the total Danish WND population is composed of 49 known patients diagnosed since 1959. We suggest that the diagnostic awareness has improved with time, but with a median diagnostic delay of 10.5 months, there is still room for improvement. The theoretical prevalence was estimated to be 1:49 500. The data confirmed that no single diagnostic test can stand alone when diagnosing WND, but a normal 24-h urine-Cu may exclude WND and be useful as a screening test. By searching for mutations in 35 patients in all 20 coding exons and assessing deletions, it was possible to identify 100% of the mutations. In this paper we hypothesize that the age of onset is related to the milder of the two mutations. This enabled a rather sharp discrimination between mutations associated with clinical presentation below or above 20 years of age. This new hypothesis needs validation in other patient populations.
Acknowledgments
This study was supported by The Lundbeck Foundation, Novo Nordisk Foundation, Apotekerfonden af 1991, Ludvig og Sara Elsass Fond, Fonden af 1870, Jeppe Juhl og Ovita Juhls Mindelegat and Unilabs. We thank Susan Peters for assistance with the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089–2111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- Olsson C, Waldenstrom E, Westermark K, Landegre U, Syvanen AC. Determination of the frequencies of ten allelic variants of the Wilson disease gene (ATP7B), in pooled DNA samples. Eur J Hum Genet. 2000;8:933–938. doi: 10.1038/sj.ejhg.5200566. [DOI] [PubMed] [Google Scholar]
- Gitlin N. Wilson's disease: the scourge of copper. J Hepatol. 1998;28:734–739. doi: 10.1016/s0168-8278(98)80302-4. [DOI] [PubMed] [Google Scholar]
- Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson's disease. Lancet. 2007;369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- Turnlund JR. Human whole-body copper metabolism. Am J Clin Nutr. 1998;67 (5 Suppl:960S–964S. doi: 10.1093/ajcn/67.5.960S. [DOI] [PubMed] [Google Scholar]
- Vanderwerf SM, Cooper MJ, Stetsenko IV, Lutsenko S. Copper specifically regulates intracellular phosphorylation of the Wilson's disease protein, a human copper-transporting ATPase. J Biol Chem. 2001;276:36289–36294. doi: 10.1074/jbc.M102055200. [DOI] [PubMed] [Google Scholar]
- Møller LB, Bukrinsky JT, Molgaard A, et al. Identification and analysis of 21 novel disease-causing amino acid substitutions in the conserved part of ATP7A. Hum Mutat. 2005a;26:84–93. doi: 10.1002/humu.20190. [DOI] [PubMed] [Google Scholar]
- Sternlieb I. Perspectives on Wilson's disease. Hepatology. 1990;12:1234–1239. doi: 10.1002/hep.1840120526. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Caca K, Loudianos G, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003;23:139–142. doi: 10.1034/j.1600-0676.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Nicastro E, Ranucci G, Vajro P, Vegnente A, Iorio R. Re-evaluation of the diagnostic criteria for Wilson disease in children with mild liver disease. Hepatology. 2010;52:1948–1956. doi: 10.1002/hep.23910. [DOI] [PubMed] [Google Scholar]
- Merle U, Weiss KH, Eisenbach C, Tuma S, Ferenci P, Stremmel W. Truncating mutations in the Wilson disease gene ATP7B are associated with very low serum ceruloplasmin oxidase activity and an early onset of Wilson disease. BMC Gastroenterol. 2010;10:8. doi: 10.1186/1471-230X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci P. Regional distribution of mutations of the ATP7B gene in patients with Wilson disease: impact on genetic testing. Hum Genet. 2006;120:151–159. doi: 10.1007/s00439-006-0202-5. [DOI] [PubMed] [Google Scholar]
- Grimberg J, Nawoschik S, Belluscio L, McKee R, Turck A, Eisenberg A. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res. 1989;17:8390. doi: 10.1093/nar/17.20.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller LB, Ott P, Lund C, Horn N. Homozygosity for a gross partial gene deletion of the C-terminal end of ATP7B in a Wilson patient with hepatic and no neurological manifestations. Am J Med Genet A. 2005b;138:340–343. doi: 10.1002/ajmg.a.30977. [DOI] [PubMed] [Google Scholar]
- Guldberg P, Rey F, Zschocke J, et al. A European multicenter study of phenylalanine hydroxylase deficiency: classification of 105 mutations and a general system for genotype-based prediction of metabolic phenotype. Am J Hum Genet. 1998;63:71–79. doi: 10.1086/301920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isackson PJ, Bennett MJ, Lichter-Konecki U, et al. CPT2 gene mutations resulting in lethal neonatal or severe infantile carnitine palmitoyltransferase II deficiency. Mol Genet Metab. 2008;94:422–427. doi: 10.1016/j.ymgme.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Terada K, Schilsky ML, Miura N, Sugiyama T. ATP7B (WND) protein. Int J Biochem Cell Biol. 1998;30:1063–1067. doi: 10.1016/s1357-2725(98)00073-9. [DOI] [PubMed] [Google Scholar]
- Walshe JM, Yealland M. Wilson's disease: the problem of delayed diagnosis. J Neurol Neurosurg Psychiatry. 1992;55:692–696. doi: 10.1136/jnnp.55.8.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle U, Schaefer M, Ferenci P, Stremmel W. Clinical presentation, diagnosis and long-term outcome of Wilson's disease: a cohort study. Gut. 2007;56:115–120. doi: 10.1136/gut.2005.087262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steindl P, Ferenci P, Dienes HP, et al. Wilson's disease in patients presenting with liver disease: a diagnostic challenge. Gastroenterology. 1997;113:212–218. doi: 10.1016/s0016-5085(97)70097-0. [DOI] [PubMed] [Google Scholar]
- Vrabelova S, Letocha O, Borsky M, Kozak L. Mutation analysis of the ATP7B gene and genotype/phenotype correlation in 227 patients with Wilson disease. Mol Genet Metab. 2005;86:277–285. doi: 10.1016/j.ymgme.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Merle U, Eisenbach C, Weiss KH, Tuma S, Stremmel W. Serum ceruloplasmin oxidase activity is a sensitive and highly specific diagnostic marker for Wilson's disease. J Hepatol. 2009;51:925–930. doi: 10.1016/j.jhep.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Gheorghe L, Popescu I, Iacob S, et al. Wilson's Disease: a challenge of diagnosis. The 5-year experience of a tertiary centre. Rom J Gastroenterol. 2004;13:179–185. [PubMed] [Google Scholar]
- LaRusso NF, Summerskill WH, McCall JT. Abnormalities of chemical tests for copper metabolism in chronic active liver disease: differentiation from Wilson's disease. Gastroenterology. 1976;70:653–655. [PubMed] [Google Scholar]
- Frommer DJ. Urinary copper excretion and hepatic copper concentrations in liver disease. Digestion. 1981;21:169–178. doi: 10.1159/000198559. [DOI] [PubMed] [Google Scholar]
- Muller T, Koppikar S, Taylor RM, et al. Re-evaluation of the penicillamine challenge test in the diagnosis of Wilson's disease in children. J Hepatol. 2007;47:270–276. doi: 10.1016/j.jhep.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Møller LB, Mogensen M, Horn N. Molecular diagnosis of Menkes disease: genotype-phenotype correlation. Biochimie. 2009;91:1273–1277. doi: 10.1016/j.biochi.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Gromadzka G, Schmidt HH, Genschel J, et al. p.H1069Q mutation in ATP7B and biochemical parameters of copper metabolism and clinical manifestation of Wilson's disease. Mov Disord. 2006;21:245–248. doi: 10.1002/mds.20671. [DOI] [PubMed] [Google Scholar]
- Panagiotakaki E, Tzetis M, Manolaki N, et al. Genotype-phenotype correlations for a wide spectrum of mutations in the Wilson disease gene (ATP7B) Am J Med Genet A. 2004;131:168–173. doi: 10.1002/ajmg.a.30345. [DOI] [PubMed] [Google Scholar]
- Loudianos G, Kostic V, Solinas P, et al. Characterization of the molecular defect in the ATP7B gene in Wilson disease patients from Yugoslavia. Genet Test. 2003;7:107–112. doi: 10.1089/109065703322146786. [DOI] [PubMed] [Google Scholar]
- Brage A, Tome S, Garcia A, Carracedo A, Salas A. Clinical and molecular characterization of Wilson disease in Spanish patients. Hepatol Res. 2007;37:18–26. doi: 10.1111/j.1872-034X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- Curtis D, Durkie M, Balac M, et al. A study of Wilson disease mutations in Britain. Hum Mutat. 1999;14:304–311. doi: 10.1002/(SICI)1098-1004(199910)14:4<304::AID-HUMU5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Shah AB, Chernov I, Zhang HT, et al. Identification and analysis of mutations in the Wilson disease gene (ATP7B): population frequencies, genotype-phenotype correlation, and functional analyses. Am J Hum Genet. 1997;61:317–328. doi: 10.1086/514864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann W, Caca K, Eggers B, et al. Genotype correlation with fine motor symptoms in patients with Wilson's disease. Eur Neurol. 2002;48:97–101. doi: 10.1159/000062992. [DOI] [PubMed] [Google Scholar]
- Hamza I, Schaefer M, Klomp LW, Gitlin JD. Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis. Proc Natl Acad Sci USA. 1999;96:13363–13368. doi: 10.1073/pnas.96.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bie P, van de Sluis B, Burstein E, et al. Distinct Wilson's disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B. Gastroenterology. 2007;133:1316–1326. doi: 10.1053/j.gastro.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu YH, Kodama H, Du SL, Gu QJ, Sun HJ, Ushijima H. Mutation spectrum and polymorphisms in ATP7B identified on direct sequencing of all exons in Chinese Han and Hui ethnic patients with Wilson's disease. Clin Genet. 2003;64:479–484. doi: 10.1046/j.1399-0004.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- Gromadzka G, Schmidt HH, Genschel J, et al. Frameshift and nonsense mutations in the gene for ATPase7B are associated with severe impairment of copper metabolism and with early clinical manifestations of Wilson's disease. Clin Genet. 2005;68:524–532. doi: 10.1111/j.1399-0004.2005.00528.x. [DOI] [PubMed] [Google Scholar]
- Gupta A, Aikath D, Neogi R, et al. Molecular pathogenesis of Wilson disease: haplotype analysis, detection of prevalent mutations and genotype-phenotype correlation in Indian patients. Hum Genet. 2005;118:49–57. doi: 10.1007/s00439-005-0007-y. [DOI] [PubMed] [Google Scholar]
- Deguti MM, Genschel J, Cancado EL, et al. Wilson disease: novel mutations in the ATP7B gene and clinical correlation in Brazilian patients. Hum Mutat. 2004;23:398. doi: 10.1002/humu.9227. [DOI] [PubMed] [Google Scholar]
- Kaler SG, Tang J, Donsante A, Kaneski CR. Translational read-through of a nonsense mutation in ATP7A impacts treatment outcome in Menkes disease. Ann Neurol. 2009;65:108–113. doi: 10.1002/ana.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.