Abstract
Ras-deregulated cells require reactive oxygen species for proliferation. They survive the resultant proteotoxic stress by maintaining sufficient levels of reduced glutathione and optimally functioning stress response machinery. In this issue of Cancer Cell, De Raedt et al. identify a novel strategy that utilizes this dependency to cause cell death.
The small GTPase protein Ras is tethered to the plasma membrane and relays signals from cell surface receptors to cytosolic effectors in order to promote cell growth and survival. When deregulated by mutation or other means, Ras proteins are crucial mediators of malignant transformation (Downward, 2003). Even though significant efforts have been made for more than two decades to develop Ras-targeted therapies, these initiatives have proven less successful than expected (Matallanas and Crespo, 2010). Moreover, Ras-driven tumors are often highly aggressive and are resistant to conventional chemotherapy, emphasizing the unmet need to identify novel strategies to target this oncogene. In this issue of Cancer Cell, De Raedt et al describe an effective strategy that combines two drugs targeting different molecules, the molecular chaperone heat shock protein 90 (Hsp90) and the mammalian target of rapamycin (mTOR), to induce catastrophic endoplasmic reticulum (ER) stress by exploiting the dependence of Ras-driven tumors on reactive oxygen species (ROS) (De Raedt et al., 2011).
Under normal conditions, Ras is activated when it is bound to GTP and inactivated when its GTPase activity is stimulated by proteins termed RasGAPs (Ras-GTPase activating proteins), one of which is the tumor suppressor NF1 (neurofibromatosis type 1). Somatic inactivating mutations of NF1 that result in aberrant Ras activation, have been found in sporadic glioblastoma, non-small cell lung cancer (NSCLC), and malignant peripheral nerve sheath tumors (MPNSTs) (Jett and Friedman, 2010). As a consequence of Ras deregulation, mTOR is activated in NF1-deficient tumors, and mTOR inhibitors such as rapamycin suppress tumor growth in vitro but have demonstrated only cytostatic activity in vivo. While searching for agents to enhance the efficacy of rapamycin, De Raedt and colleagues found that ER disrupting reagents, such as tunicamycin and thapsigargin, synergized with rapamycin to induce the death of Ras-driven cancer cells (but not of untransformed cells). However, neither tunicamycin, which interferes with protein glycosylation in the ER, nor thapsigargin, which promotes calcium release from the ER, are clinically viable. De Raedt et al. found that several Hsp90 inhibitors currently undergoing extensive clinical evaluation as anti-cancer drugs, including IPI-504 (retaspimycin), also were synergistic with rapamycin. A previous study identified the ER transmembrane kinases and stress response effectors inositol requiring enzyme-1 (IRE-1) and protein kinase RNA-like endoplasmic reticulum kinase (PERK) as Hsp90-dependent proteins (Marcu et al., 2002). De Raedt et al. confirm this earlier finding and further confirm that Hsp90 inhibitors such as IPI-504 abrogate a cell’s ability to mount an effective ER response when faced with severe proteotoxic stress.
A certain level of ER proteotoxic stress is a common characteristic of cancer cells, and is caused by a variety of factors including hypoxia, oxidative stress, and high mutational load. Thus, cancer cells depend on optimal function of the cellular stress response machinery, because catastrophic ER stress causes cell death. Unlike the reducing environment of the cytosol, the ER provides a unique oxidizing environment that promotes formation of protein disulfide bonds. Accumulating evidence indicates that ROS generation is a byproduct of protein oxidation in the ER. Since oxidative stress also induces ER stress, persistent ROS elevation in conjunction with compromised ER stress response machinery initiates a vicious cycle leading to ER collapse and cell death.
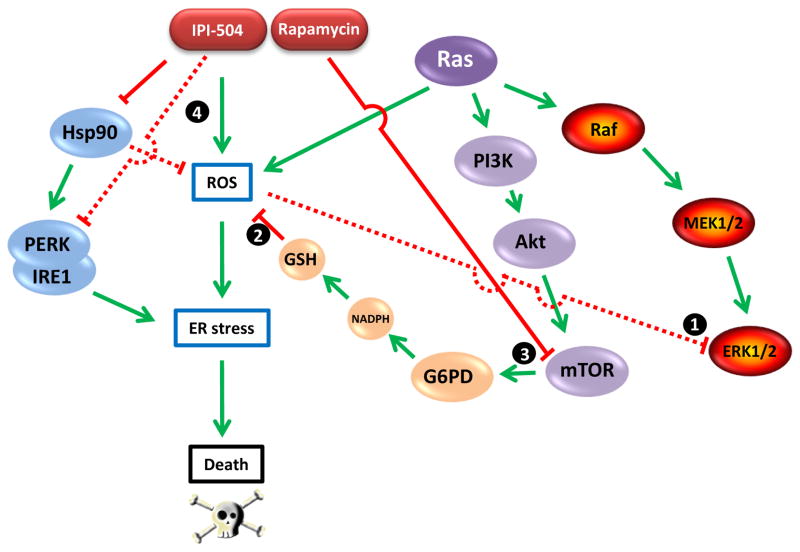
The dependence of Ras-driven cancers on elevated ROS levels makes them particularly sensitive to this strategy, as shown by De Raedt et al. Importantly, ROS is required for Ras-dependent cell proliferation. Ras deregulation promotes tonic activation of the Raf-MEK-ERK signal transduction cascade. While low levels of ERK activation promote proliferation, high levels of activation result in growth arrest (Meloche and Pouyssegur, 2007). Thus, Ras-activated cancer cells must buffer the level of ERK activation to be compatible with proliferation, and they employ mitochondrial ROS for this purpose (see ‘1’ in Figure 1; Weinberg et al., 2010). On the other hand, the ROS level must be tightly regulated since too much oxidative stress is harmful for the reasons described above. Thus, Ras-driven cancers are particularly dependent on maintaining an appropriate but not excessive ROS level. To achieve this balance, cancer cells must maintain sufficient reducing capability which they accomplish in part by metabolizing glucose via the pentose phosphate pathway to generate NADPH that in turn promotes accumulation of reduced glutathione (GSH). Indeed, glucose metabolism via the pentose phosphate pathway is essential for Ras-stimulated growth under normoxic conditions (Weinberg et al., 2010). The key enzyme in this pathway is glucose 6-phosphate dehydrogenase (G6PD). As shown by De Raedt and colleagues, G6PD is regulated by mTOR, a component of a second Ras-driven signaling pathway (Figure 1). Thus, Ras-dependent mTOR activation is essential to maintain sufficient levels of reduced glutathione (see ‘2’ in Figure 1). It follows that inhibition of mTOR by rapamycin interferes with an important ROS scavenging mechanism (see ‘3’ in Figure 1).
Figure 1.
The relationship between Ras-driven signaling pathways, ROS, Hsp90, and ER stress. Ras-driven proliferation requires ROS buffering of Ras-activated ERK1/2 activity (1). ROS level is regulated in part by reduced glutathione (GSH), requiring sufficient NADPH generated by G6PD-mediated glucose oxidation via the pentose phosphate pathway (2). G6PD expression is regulated by mTOR, a component of a second Ras-driven signaling pathway that is inhibited by rapamycin (3). Hsp90 buffers cellular ROS and is essential for stabilization and activity of the ER transmembrane kinases PERK and IRE1, two components of the ER stress response machinery. The Hsp90 inhibitor IPI-504 causes ROS levels to increase while also abrogating the ER stress response (4). The combination of IPI-504 and rapamycin disrupts the balance between ROS and GSH, causing catastrophic ER stress and cell death.
Hsp90 has been reported to buffer cellular ROS in other systems, although its mechanism of action remains poorly understood (Yang et al., 2011). Nevertheless, these data support the observations made by De Raedt and colleagues that IPI-504 and other Hsp90 inhibitors increase ROS in Ras-driven cancer cells (see ‘4’ in Figure 1). In addition to this activity and its abrogation of a robust ER stress response, Hsp90 inhibition also suppresses mTOR signaling (Ohji et al., 2006), explaining the current finding that combined administration of rapamycin and IPI-504 has a more dramatic impact on G6PD expression and GSH levels in MPNSTs in vivo than does either agent alone. Further, these data are in agreement with, and perhaps explain the underlying basis of a recent report that Ras mutation in NSCLC confers enhanced dependence on Hsp90 (Sos et al., 2009). De Raedt and colleagues found that the cytotoxicity (both in vitro and in vivo) of the Hsp90/mTOR inhibitor combination was ameliorated by administration of the ROS scavenger ascorbic acid. This is certainly consistent with the hypothesis that abrogation of the ER stress response concomitantly with suppression of ROS regulation is responsible for the dramatic anti-tumor activity reported. A clinical trial to evaluate the activity of IPI-504 and the mTOR inhibitor everolimus in Ras-mutated NSCLC is currently enrolling patients (http://www.infi.com/product-candidates-pipeline-ipi-504.asp).
Simultaneous targeting of Hsp90 and mTOR also demonstrates synergistic activity in several other cancer models, including hepatocellular carcinoma, breast cancer, and multiple myeloma. It will be intriguing to determine if a similar underlying mechanism involving ROS deregulation and ER collapse pertains in these cases. Given that increased oxidative stress is characteristic of most cancers, strategies to take advantage of this common liability might help improve the less than expected clinical activity of Hsp90 inhibitors when used as single agents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- De Raedt, et al. Cancer Cell. 2011;(this issue) [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010;12:1–11. doi: 10.1097/GIM.0b013e3181bf15e3. [DOI] [PubMed] [Google Scholar]
- Marcu MG, Doyle M, Bertolotti A, Ron D, Hendershot L, Neckers L. Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha. Mol Cell Biol. 2002;22:8506–8513. doi: 10.1128/MCB.22.24.8506-8513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matallanas D, Crespo P. New druggable targets in the Ras pathway? Curr Opin Mol Ther. 2010;12:674–683. [PubMed] [Google Scholar]
- Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- Ohji G, Hidayat S, Nakashima A, Tokunaga C, Oshiro N, Yoshino K, Yokono K, Kikkawa U, Yonezawa K. Suppression of the mTOR-raptor signaling pathway by the inhibitor of heat shock protein 90 geldanamycin. J Biochem. 2006;139:129–135. doi: 10.1093/jb/mvj008. [DOI] [PubMed] [Google Scholar]
- Sos ML, Michel K, Zander T, Weiss J, Frommolt P, Peifer M, Li D, Ullrich R, Koker M, Fischer F, et al. Predicting drug susceptibility of non-small cell lung cancers based on genetic lesions. J Clin Invest. 2009;119:1727–1740. doi: 10.1172/JCI37127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Yang C, Xiao L, Liao X, Lan A, Wang X, Guo R, Chen P, Hu C, Feng J. Novel insights into the role of HSP90 in cytoprotection of H2S against chemical hypoxia-induced injury in H9c2 cardiac myocytes. Int J Mol Med. 2011;28:397–403. doi: 10.3892/ijmm.2011.682. [DOI] [PubMed] [Google Scholar]