Abstract
O2-(2,4-Dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (JS-K) and O2-{2,4-dinitro-5-[4-(N-methylamino)be nzoyloxy]phenyl} 1-(N,N-dimethylamino)diazen-1-ium-1,2-diolate (PABA/NO) are O2-arylated diazeniumdiolates that have shown promising in vivo activity in a variety of rodent cancer models, including prostate cancer, leukemia, liver cancer, multiple myeloma, and ovarian cancer. This compound class was designed to be activated for anti-cancer effects by glutathione-S-transferase (GST)-induced release of cytotoxic nitric oxide (NO), but mechanistic studies have implicated a variety of pathways, some GST/NO-related, some not. Current work is focused on improving formulations and other drug development activities, as well as exploring possible new applications of these agents and their analogs. The selectivity of these drugs for attacking tumors while exhibiting little toxicity toward normal tissues suggests considerable promise for the treatment of various tumor types.
Keywords: nitric oxide, JS-K, arylating agents, glutathione, PABA/NO
I. INTRODUCTION
This paper reviews published work about a compound class known as “arylated diazeniumdiolates,” which are showing increasing promise as anti-cancer drugs.
I.A. The Starting Point
In comparing notes across the chemistry/biology interface some years ago with my University of Utah hematologist/oncologist colleague Paul Shami, he mentioned some pioneering results he and other colleagues had published earlier indicating that leukemia cells are substantially more sensitive to nitric oxide (NO) toxicity than most other mammalian cell types.1 It turns out that NO is so crucial for so many normal life processes that evolution has provided us with mechanisms for protecting ourselves from its potential toxicity, much as we have evolved means to shield ourselves from another inherently toxic species that is essential for our well-being: oxygen. Apparently, leukemia cells have lost some of that protection against NO.
That being the case, we reasoned, it might be possible to administer an NO-releasing drug systemically in a leukemia patient, allowing it to distribute throughout the body but expecting it to selectively annihilate the sensitive leukemia cells without inflicting harm on other tissues and cell types.
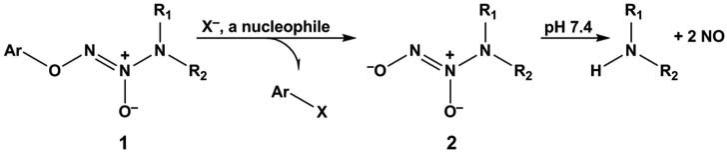
It happened that my chemist colleagues Joe Saavedra and Aloka Srinivasan had earlier shown in a basic research effort that arylated diazeniumdiolates of general structure 1 could serve as caged NO sources whose doors could be opened by reaction with attacking nucleophiles such as isopropylamine and hydroxide ion to free structure 2, an ion known to hydrolyze spontaneously to form NO (Fig. 1).2 Given the success they reported in their first paper describing the fundamental chemistry of this compound type, it occurred to us that even stronger nucleophiles, such as glutathione (GSH) and other thiols that are abundant in the body, might easily provoke NO release in vivo, and thus compounds of structure 1 might be candidates for anti-leukemic drug discovery.
FIGURE 1.
O2-Arylated diazeniumdiolate 1 as a prodrug of NO. A nucleophile X- displaces ionic diazeniumdiolate 2, which then spontaneously releases up to two equivalents of NO at physiological pH.
II. RESULTS AND DISCUSSION
Accordingly, Paul screened a library of Joe's arylated diazeniumdiolates for their in vitro cytotoxic activity toward two human leukemia cell lines, HL-60 and U937. He identified O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (JS-K) as the most active compound of the series. Paul then implanted HL-60 cells subcutaneously in the flanks of immune-compromised (non-obese diabetic severe combined immune deficient, or NOD-SCID) mice incapable of rejecting the xenografted cells, and charted the growth of the resulting tumor mass over time in animals receiving tail vein bolus injections of either JS-K or the vehicle in which the JS-K was dissolved. The JS-K dose he chose was the maximum that could be administered without inducing NO-mediated hypotension in these mice, 4 μmol/kg. This was given three times per week, and tumor volume was estimated by measuring the dimensions of the tumor implants every other day for the duration of the experiment. The results showed that JS-K cut the tumor growth rate in half, and histochemical examination of tumor explants revealed that the drug had induced substantial tumor necrosis as opposed to vehicle-treated control animals.3 This initial set of experiments that Paul conducted was the first to demonstrate the anti-cancer activity of arylated diazeniumdiolates, and showed their potential for introduction to the clinic. Microarray analysis conducted later in collaboration with Jie Liu and Mike Waalkes of the National Cancer Institute showed the involvement of numerous pathways, including apoptosis and differentiation-related genes, acute phase protein genes, and genes related to angiogenesis.4
II.A. Solid Tumor Surprises
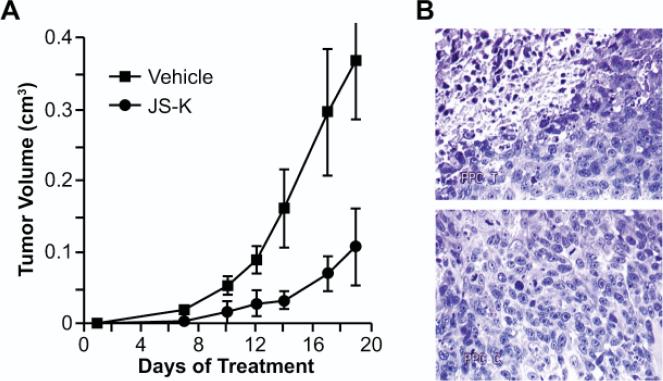
Very interestingly, quite similar results were observed in a prostate tumor cell line not known to be particularly sensitive to NO-induced toxicity. As with the leukemia line, Paul implanted PPC-1 human prostate cancer cells in the flanks of NOD-SCID mice and made similar observations as with the leukemia cells. Here, too, explants from JS-K-treated mice showed extensive tumor necrosis compared with vehicle-treated animals (Fig. 2).3
FIGURE 2.
JS-K significantly slowed the rate of prostate xenograft growth (A) and induced necrosis in the remaining tumor mass (B). Note that the tumor tissue shown in the JS-K-treated section at the top shows more necrotic areas than the untreated control tissue section shown at the bottom. (Adapted from Shami et al., 2003.3)
Meanwhile, Brian Carr of the University of Pittsburgh began studying the action of JS-K on human Hep3B hepatoma cells. Mechanistic studies conducted by Brian et al. implicated the mitogen-activated protein-kinase (MAPK) pathways in the apoptotic demise of these cells.5 Subsequently, using an orthotopic syngeneic rat hepatoma model with JM-1 cells implanted in the liver of Fischer rats, Brian showed that JS-K inhibited the growth of liver cancer cells in vivo. Tumor growth inhibition was dose dependent.6
II.B. Multiple Myeloma and JS-K
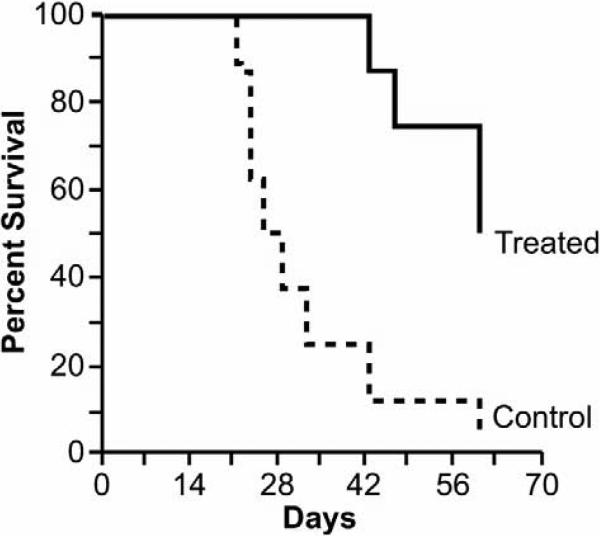
Having noted Paul's successes in the leukemia model, Ken Anderson and Tanyel Kiziltepe of Harvard began a study of JS-K's potential as a therapy for multiple myeloma. Using a treatment protocol similar to the one Paul employed, Tanyel found an even more dramatic effect on the growth of OPM-1 multiple myeloma xenografts in mice, observing a growth delay of about 2.8-fold relative to control, as well as significant survival prolongation (Fig. 3). In vitro experiments showed activation of apoptosis, JS-K-induced DNA double-strand breaks, and elimination of the growth advantage imparted to myeloma cells by bone marrow stromal cells.7
FIGURE 3.
Intravenously administered JS-K signifi cantly improved survival of mice bearing xenografts of OPM-1 multiple myeloma cells. (Adapted from Kiziltepe et al., 2007.7 Copyright American Society of Hematology.)
II.C. Tumor-targeting Selectivity
A very important property of JS-K identified in the multiple myeloma study was the greater toxicity it showed toward tumor cells relative to normal human peripheral blood mononuclear cells (PBMCs). Tanyel reported IC50 (the concentration required to inhibit cell proliferation by 50%) values for primary patient isolates as well as therapy-resistant and -responsive cell lines in the 0.3 to 2.5 μM range. She also observed that, at a concentration of 2.5 μM, JS-K was not toxic to PBMCs. At a concentration of 5 μM, JS-K was toxic to over 20% of PBMCs. That concentration of JS-K was toxic to the majority of myeloma cells tested.7
Subsequently, Paul set up bone marrow transplant experiments in mice in collaboration with his colleague Thai Cao at the University of Utah. Paul and Thai showed that pretreatment of normal mouse hematopoietic stem cells with JS-K (at concentrations in the range of its IC50 for leukemia cells) did not affect survival or engraftment when these cells were used to rescue lethally irradiated mice.8
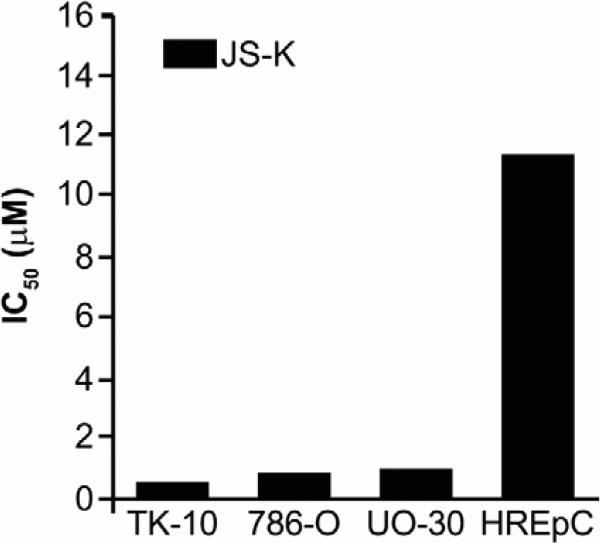
Meanwhile, Anna Maciag and Hari Chakrapani of NCI were following up results from the “NCI-60” tumor cell line screen,6 suggesting activity on the part of JS-K against kidney cancer. They found IC50 values in the low micromolar range for three kidney cancer lines, but a value 20-fold less potent for the normal renal epithelial cell line HREpC (Fig. 4).9
FIGURE 4.
Selective inhibitory activity of JS-K against a panel of renal cancer cell lines (TK-10, 786-O, and UO-30) compared with the normal renal epithelial cell line HREpC. (Adapted from Chakrapani et al., 2008.9)
It is perhaps relevant to note that the only toxic effect (other than those on the tumor) reported in any of the in vivo studies mentioned above was the hypotension expected for an NO-releasing drug at doses higher than the therapeutic doses. The mechanistic basis for this selectivity toward malignant cells as opposed to their normal counterparts is not yet known, but whatever its origin it is a most welcome attribute for an anti-cancer drug.
II.D. Synergies
Another desirable attribute in a drug candidate is the ability to augment the action of existing drugs. JS-K has been found to synergize with several. NCI's Liu-Waalkes team showed it to inhibit the multidrug resistance-related protein (MRP-1) efflux pump, allowing liver cancer cells resistant to the anti-leukemic agents cisplatin and sodium arsenite to accumulate the drugs in the cells and increase their toxicity.10 Tanyel and colleagues reported JS-K's synergy with bortezomib in multiple myeloma cultures.7 Paul found a synergistic anti-leukemic effect between cytarabine and JS-K, though his studies showed antagonism with other drugs.11 Because prevailing clinical practice calls for new anti-cancer drugs to be introduced into human trials in combination with other agents, these early demonstrations of synergy could prove to be of great value in the future.
II.E. Anti-angiogenic Effects
In separate experiments, Gurmeet Kaur from NCI showed that JS-K inhibits different aspects of angiogenesis in vitro. These include inhibition of human umbilical vein endothelial cell (HUVEC) proliferation, migration, and cord formation. Furthermore, JS-K completely suppressed angiogenesis (induced by vascular endothelial growth factor or fibroblast growth factor) in the chick aortic ring assay. These in vitro observations were confirmed with the finding that JSK inhibited tumor angiogenesis in vivo in Tanyel's multiple myeloma xenografts.12
II.F. Further Mechanistic Insights
In follow-up experiments, Paul showed that JS-K's anti-leukemic activity was dependent on NO release. He also showed that JS-K activates the intrinsic apoptosis pathway in leukemia cells directly through the loss of mitochondrial membrane potential and release of cytochrome c in the cytoplasm. Furthermore, Paul showed that JS-K activates the extrinsic apoptosis pathway indirectly.3 Ana Tari, then of M.D. Anderson Cancer Center in Texas, studied JS-K's influence on the invasive properties of breast cancer cell lines, and reported that it inhibited matrigel invasion by breast cancer cells through the induction of tissue inhibitor of matrix metalloproteinase-2 (TIMP-2).13 Ana also showed that JS-K induces autophagic cell death (cytoplasmic cell death or type II cell death) in a dose-dependent manner (concentrations higher than 1 μM) in all of the breast cancer cell lines tested.14
NCI's Yili Yang and Jirouta Kitagaki showed JS-K to inhibit ubiquitin ligase E1 in a variety of cell lines, reducing ubiquitylation and thus degradation of p53, and enhancing JS-K's ability to kill p53 wild-type cancer cells.15
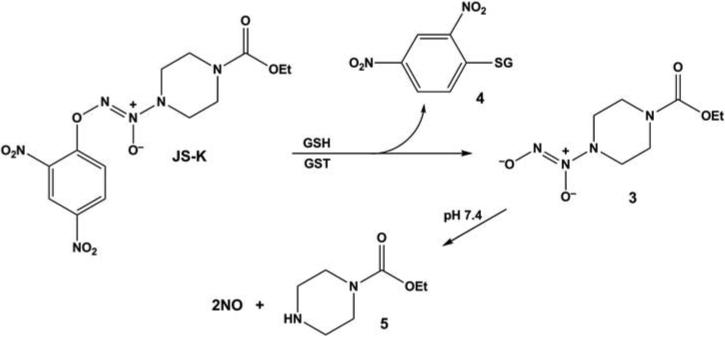
While many of the effects seen in the various cell lines investigated so far in the JS-K studies are as expected for an NO-releasing drug, there is at least one other chemical pathway at work. Consider the activation mechanism that Joe and Aloka designed into the structure of this molecule, shown in Figure 5. In order to generate NO, JS-K must react with cellular thiol groups or other nucleophilic species (shown here as GSH for the sake of illustration) to displace ionic diazeniumdiolate 3, which then is freed to release NO spontaneously in the aqueous cellular environment. However, the attacking thiol group gets arylated to produce ionic diazeniumdiolate 4 in the process, effectively irreversibly. If the attacking nucleophile is a protein (PSH instead of GSH) whose function depends upon keeping its thiol group(s) free to maintain proper structure and reactivity, then that protein can be essentially taken out of action. Evidence that this pathway serves as a major factor in mediating JS-K's biological effects was seen in Paul's work with control compounds in HL-60 cells. The “pure” arylating agent 1-chloro-2,4-dinitrobenzene inhibited leukemia cell growth (IC50 1.4 μM) and was somewhat better than spontaneously NO-generating ion 3 (IC50 4 μM). Surprisingly, carbamoylated piperazine 5, the carrier molecule that is left after the NO is released, was much more potent than expected (IC50 8.6 μM), suggesting the possibility that a trans-carbamoylation pathway contributes to the mechanism of action. JS-K's submicromolar IC50 of 0.5 μM suggests that it combines all of these effects into a multifaceted chemical mechanism of action.6
FIGURE 5.
Metabolic activation pathway converting JS-K to carbamoylated piperazine 5, an arylated thiol moiety 4 (in this case that of GSH under catalysis by GST), and diazeniumdiolate ion 3, which spontaneously hydrolyzes at physiological pH to produce up to two equivalents of NO.
Signaling pathways implicated in JS-K's activity are also clearly multifaceted, as summarized in Tables 1 and 2. Some would dismiss this richness of activity as the properties of a “dirty drug,” one that hits too many targets to be worthy of further development. But it is increasingly clear that with the extent of genetic complexity observed in malignant cells there is great redundancy in the pathophysiologic mechanisms of cancer. Thus, with the notable exception of chronic myelogenous leukemia in the chronic phase, so-called “targeted therapies” have not held the promise that was hoped they would achieve. It may be that JS-K's multitude of molecular effects will prove to be a major advantage in our bench-to-bedside effort. It is also worth repeating that JS-K has so far shown little or no toxicity to the normal counterparts of two malignant cell types (leukemia and renal cancer) against which it was tested.
TABLE 1.
Genes Up-regulated by JS-K in HL-60 Human Leukemia Cells4
| Apoptosis-related genes |
| caspase 3 |
| caspase8 |
| caspase9 |
| BAX |
| TNF-α |
| Monocytic differentiation-related genes |
| CD14 |
| CD11b |
| vimentin |
| Acute-phase genes |
| c-jun |
| EGR-1 |
| Migration-related genes |
| TIMP-1 |
| TIMP-2 |
| TIMP-3 |
| Anti-angiogenesis genes |
| thrombospondin-1 |
| CD36 |
TABLE 2.
Examples of Other Signaling Pathways Affected, Including Some That Are Cell Type-Dependent
II.G. Lead Optimization
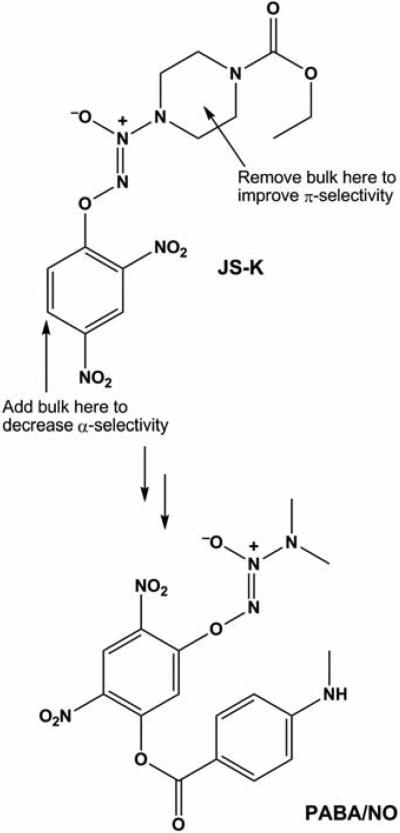
Having discovered JS-K in something of a random screening process, thought has been given to systematically modifying its structure to develop even more targeted anti-cancer action. Structural biologist Xinhua Ji of NCI knew that glutathione-S-transferase (GST) catalyzes NO release by JS-K, and that the Π isoform of this enzyme is overexpressed in many cancers. He was also intimately familiar with the active site characteristics of the three principal isoforms of GST (α, μ, and π) that are expressed to different extents in leukemia cells isolated from patients.16 Kinetic studies have shown JS-K to be metabolized 100-fold more efficiently by α relative to Π.3 Reasoning that a reversal of this selectivity ratio would greatly increase the extent of cytolytic metabolism in the Π-overexpressing cancer cells, Xinhua modeled the accommodation of JS-K in the active sites of each. Based on this structure-based drug design exercise, he suggested that altering the JS-K molecule as shown in (Fig. 6) would accomplish that goal.17 Shrinking the size of the amino group to which the diazeniumdiolate group is attached should ease the accommodation in the Π active site, while increasing the two-dimensional steric bulk of the aryl group should make metabolism by α more difficult. In response, Joe prepared PABA/NO, whose structure is shown in Figure 6. Kinetic studies showed this compound to be metabolized by Π and α at comparable rates, an improvement of about 100-fold in the Π/α ratio.17
FIGURE 6.
Results of molecular modeling experiments leading to the design of PABA/ NO as a potential GSTπ-selective improvement over JS-K. (Reproduced from Saavedra et al., 2006.17)
II.H. In Vivo Activity Rivaling That of Cisplatin
PABA/NO has been studied in some detail by Ken Tew and Danyelle Townsend, currently of the University of South Carolina. They first established that its mechanism of toxic action is GSH-, GSTΠ-, and MRP-1-dependent. They then performed an in vivo experiment in mice, establishing human ovarian cancer xenografts and studying PABA/NO's effect on tumor growth rate. Here, too, the arylated diazeniumdiolate greatly retarded growth of the tumor mass relative to the vehicle control. Very significantly, PABA/NO's potency was comparable to that of the positive control cisplatin, which is used clinically for treating ovarian cancer.18
II.I. Current Problems and Future Plans
Our next goals in the effort to move JS-K and its analogs through the drug-development process and into the clinic are to increase the compounds’ stability in the bloodstream and to increase their efficacy. To address these goals, we are examining a variety of formulations designed to shield the molecule from GSH and other nucleophiles encountered in the circulatory system, thereby slowing drug activation. Importantly, such a formulation should be scalable to clinical quantities and should be by itself safe. Paul has now developed such a formulation using nanoscale micelles. We are also continuing lead optimization studies with the hope that systematically fine-tuning the structure will lead to further improvements in the drugs’ pharmacokinetics and, eventually, to the needed cures.
II.J. A Path Toward Clinical Development
Our goals cannot be achieved by one research group working in isolation, and I would like to close by acknowledging the essential contribution of all of my gifted collaborators (the “JS-K consortium”) who have served as coauthors in the published works cited in the references section of my paper. Other invaluable collaborators are in the process of publishing newer work that will greatly advance the effort. In view of the enormous resources required to bring a new anti-cancer agent to the clinic, a commercialization effort is necessary. For this purpose, Paul and his colleague Thomas Kennedy from the University of Utah have founded JSK Therapeutics, Inc. JSKT has executed the necessary licensing agreements with the University of Utah and is leading the preclinical development of JS-K in preparation for an Investigational New Drug filing with the US Food and Drug Administration. JSKT has secured initial seed funding and is seeking further funding in order to start a phase I clinical trials program in about two years. Consequently, through the work of the multiple members of the “JS-K consortium” and a serious commercialization effort, it will hopefully be possible to bring to the clinic a new class of potent anti-cancer agents.
ACKNOWLEDGEMENT
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
REFERENCES
- 1.Magrinat G, Mason SN, Shami PJ, Weinberg JB. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood. 1992;80:1880–4. [PubMed] [Google Scholar]
- 2.Saavedra JE, Srinivasan A, Bonifant CL, Chu J, Shanklin AP, Flip-pen-Anderson JL, Rice WG, Turpin JA, Davies KM, Keefer LK. The secondary amine/nitric oxide complex ion R2N[N(O)NO]– as nucleophile and leaving group in SNAr reactions. J Org Chem. 2001;66:3090–8. doi: 10.1021/jo0016529. [DOI] [PubMed] [Google Scholar]
- 3.Shami PJ, Saavedra JE, Wang LY, Bonifant CL, Diwan BA, Singh SV, Gu Y, Fox SD, Buzard GS, Citro ML, Waterhouse DJ, Davies KM, Ji X, Keefer LK. JS-K, a glutathione/glutathione S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineo-plastic activity. Mol Cancer Ther. 2003;2:409–17. [PubMed] [Google Scholar]
- 4.Liu J, Malavya S, Wang X, Saavedra JE, Keefer LK, Tokar E, Qu W, Waalkes MP, Shami PJ. Gene expression profiling for nitric oxide pro-drug JS-K to kill HL-60 myeloid leukemia cells. Genomics. 2009;94:32–8. doi: 10.1016/j.ygeno.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren Z, Kar S, Wang Z, Wang M, Saavedra JE, Carr BI. JS-K, a novel non-ionic diazeniumdiolate derivative, inhibits Hep 3B hepatoma cell growth and induces c-Jun phosphorylation via multiple MAP kinase pathways. J Cell Physiol. 2003;197:426–34. doi: 10.1002/jcp.10380. [DOI] [PubMed] [Google Scholar]
- 6.Shami PJ, Saavedra JE, Bonifant CL, Chu J, Udupi V, Malaviya S, Carr BI, Kar S, Wang M, Jia L, Ji X, Keefer LK. Antitumor activity of JS-K [O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate] and related O2-aryl diazeniumdiolates in vitro and in vivo. J Med Chem. 2006;49:4356–66. doi: 10.1021/jm060022h. [DOI] [PubMed] [Google Scholar]
- 7.Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L, Li CQ, Trudel LJ, Yasui H, Vallet S, Kutok JL, Chauhan D, Mitsiades CS, Saavedra JE, Wogan GN, Keefer LK, Shami PJ, Anderson KC. JS-K, a GST-activated nitric oxide generator, induces DNA doublestrand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood. 2007;110:709–18. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shami PJ, Leukel HC, Kosak KM, Saavedra JE, Keefer LK, Cao TM. JS-K, an arylating nitric oxide (NO) generator, shows no toxicity towards normal hematopoietic cells (abstract); Proceedings of the American Association of Cancer Research; Los Angeles, CA; Philadelphia (PA): AACR. April 14-18, 2007; 2007. Abstract 1534. [Google Scholar]
- 9.Chakrapani H, Kalathur RC, Maciag AE, Citro ML, Ji X, Keefer LK, Saavedra JE. Synthesis, mechanistic studies, and anti-proliferative activity of glutathione/glutathione S-transferase-activated nitric oxide prodrugs. Bioorg Med Chem. 2008;16:9764–71. doi: 10.1016/j.bmc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Li C, Qu W, Leslie E, Bonifant CL, Buzard GS, Saavedra JE, Keefer LK, Waalkes MP. Nitric oxide prodrugs and metallochemotherapeutics: JS-K and CB-3-100 enhance arsenic and cisplatin cytolethality by increasing cellular accumulation. Mol Cancer Ther. 2004;3:709–14. [PubMed] [Google Scholar]
- 11.Shami PJ, Maciag AE, Eddington JK, Udupi V, Kosak KM, Saavedra JE, Keefer LK. JS-K, an arylating nitric oxide (NO) donor, has synergistic anti-leukemic activity with cytarabine (ARA-C). Leukemia Res. 2009;33:1525–9. doi: 10.1016/j.leukres.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiziltepe T, Anderson KC, Kutok JL, Jia L, Boucher KM, Saavedra JE, Keefer LK, Shami PJ. JS-K has potent anti-angiogenic activity in vitro and inhibits tumor angiogenesis in a multiple myeloma model in vivo. J Pharm Pharmacol. 2010;62:145–51. doi: 10.1211/jpp.62.01.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simeone AM, McMurtry V, Nieves-Alicea R, Saavedra JE, Keefer LK, Johnson MM, Tari AM. TIMP-2 mediates the anti-invasive effects of the nitric oxide-releasing prodrug JS-K in breast cancer cells. Breast Cancer Res. 2008;10(3):R44. doi: 10.1186/bcr2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieves-Alicea R, Saavedra J, Simeone AM, McMurtry V, Cortez V, Copper G, Kondo Y, Keefer L, Tari AM. Novel mechanism of cell death induction by nitric oxide pro-drugs (abstract); Proceedings of the American Association of Cancer Research; Washington, DC; Philadelphia (PA): AACR. April 1-5, 2006; 2006. Abstract 5489. [Google Scholar]
- 15.Kitagaki J, Yang Y, Saavedra JE, Colburn NH, Keefer LK, Perantoni AO. Nitric oxide prodrug JS-K inhibits ubiquitin E1 and kills tumor cells retaining wild-type p53. Oncogene. 2009;28:619–24. doi: 10.1038/onc.2008.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sargent JM, Williamson C, Hall AG, Elgie AW, Taylor CG. Evidence for the involvement of the glutathione pathway in drug resistance in AML. Adv Exp Med Biol. 1999;457:205–9. doi: 10.1007/978-1-4615-4811-9_22. [DOI] [PubMed] [Google Scholar]
- 17.Saavedra JE, Srinivasan A, Buzard GS, Davies KM, Waterhouse DJ, Inami K, Wilde TC, Citro ML, Cuellar M, Deschamps JR, Parrish D, Shami PJ, Findlay VJ, Townsend DM, Tew KD, Singh S, Jia L, Ji X, Keefer LK. PABA/NO as an anticancer lead: Analogue synthesis, structure revision, solution chemistry, reactivity toward glutathione, and in vitro activity. J Med Chem. 2006;49:1157–64. doi: 10.1021/jm050700k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Findlay VJ, Townsend DM, Saavedra JE, Buzard GS, Citro ML, Keefer LK, Ji X, Tew KD. Tumor cell responses to a novel glutathione S-transferase-activated nitric oxide-releasing prodrug. Mol Pharmacol. 2004;65:1070–9. doi: 10.1124/mol.65.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]