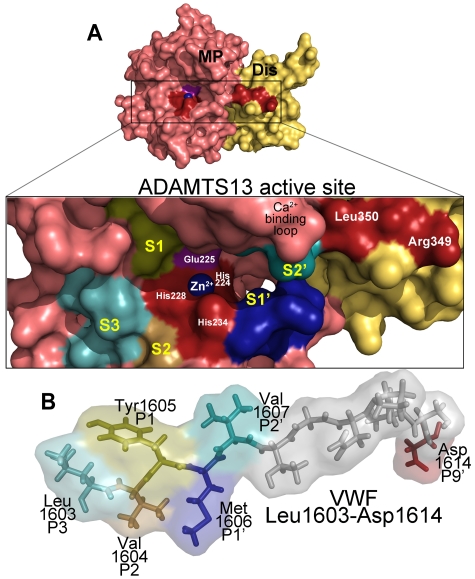
Figure 3.
ADAMTS13 recognition of the VWF cleavage site. (A) Molecular model of the ADAMTS13 metalloprotease (MP; light red) and disintegrin-like (Dis; yellow) domains. (Inset) The active site cleft. Active site residues (Zn2+ ion, its 3 coordinating His residues His224, His228, and His234, as well as the catalytic Glu225 residue) and disintegrin-like domain exosite residues are labeled. The high-affinity functional Ca2+-binding loop is also marked. Regions of the metalloprotease domain that are predicted to harbor the S3, S2, S1, S1′, and S2′ subsites are labeled marked in different colors. (B) Below the active site cleft, VWF A2 domain residues Leu1603-Asp1614 are depicted as sticks and transparent spacefill. The P3, P2, P1, P1′, P2′, and P9′ residues in VWF that are important for proteolysis are labeled and colored according to their predicted complementary subsites (S) in the metalloprotease domain.