Abstract
Background: The International Breast Cancer Study Group Trial VIII compared long-term efficacy of endocrine therapy (goserelin), chemotherapy [cyclophosphamide, methotrexate and fluorouracil (CMF)], and chemoendocrine therapy (CMF followed by goserelin) for pre/perimenopausal women with lymph-node-negative breast cancer.
Patients and methods: From 1990 to 1999, 1063 patients were randomized to receive (i) goserelin for 24 months (n = 346), (ii) six courses of ‘classical’ CMF (cyclophosphamide, methotrexate, 5-fluorouracil) chemotherapy (n = 360), or (iii) six courses of CMF plus 18 months goserelin (CMF→ goserelin; n = 357). Tumors were classified as estrogen receptor (ER) negative (19%), ER positive (80%), or ER unknown (1%); 19% of patients were younger than 40. Median follow-up was 12.1 years.
Results: For the ER-positive cohort, sequential therapy provided a statistically significant benefit in disease-free survival (DFS) (12-year DFS = 77%) compared with CMF alone (69%) and goserelin alone (68%) (P = 0.04 for each comparison), due largely to the effect in younger patients. Patients with ER-negative tumors whose treatment included CMF had similar DFS (12-year DFS CMF = 67%; 12-year DFS CMF→ goserelin = 69%) compared with goserelin alone (12-year DFS = 61%, P= NS).
Conclusions: For pre/perimenopausal women with lymph-node-negative ER-positive breast cancer, CMF followed by goserelin improved DFS in comparison with either modality alone. The improvement was the most pronounced in those aged below 40, suggesting an endocrine effect of prolonged CMF-induced amenorrhea.
Keywords: amenorrhea; breast cancer; chemotherapy; goserelin; hormonal therapy, node negative
introduction
A recent survey among researchers in the breast cancer field [1] found that the identification of hormone receptor-positive breast cancer patients who might derive equal benefit from endocrine treatment alone without the addition of adjuvant chemotherapy is one of the most important current clinical research topics. High levels of estrogen receptor expression predict efficacy of endocrine treatment [2], while patients with tumors having lower quantitative estrogen receptor expression seem to benefit most from the addition of chemotherapy to endocrine treatment alone [3, 4]. Some studies have found higher levels of Ki-67-labeling index to be predictive for chemotherapy benefit in the neoadjuvant setting, while others have not [5]. Studies in the adjuvant setting indicate a prognostic effect of Ki-67 but no clear predictive value for chemotherapy efficacy [6]. Various new multigene assays are being developed. Studies comparing different assays indicate similarities between them insofar as the genes selected mainly represent three domains: the steroid hormone expression pathways, the epidermal growth factor system, and proliferation markers [7]. The two former are useful for treatment prediction, while proliferation markers seem to be more prognostic. Although multigene assays are promising, high-quality immunohistochemistry and breast pathology remain the cornerstones for adjuvant treatment selection in most places. The International Breast Cancer Study Group (IBCSG) previously published results of Trial VIII at a median follow-up of 7 years, comparing chemotherapy plus ovarian function suppression in sequence to each modality alone in premenopausal patients with node-negative breast cancer [8]. Since a substantial proportion of the breast cancer events in the hormone receptor-positive group occur many years after diagnosis, it is important to explore the treatment efficacy pattern with long-term follow-up. The aim of the present study was to update the results of IBCSG Trial VIII with 12.1 years median follow-up comparing treatment efficacy pattern according to centrally determined ER expression and age at start of treatment.
patients and methods
Details about IBCSG Trial VIII have been previously published [8]. In short, Trial VIII is a randomized trial designed to evaluate the role of the adjuvant therapy with the sequential combination of chemotherapy and ovarian function suppression, compared with each modality alone, for pre- and perimenopausal patients with lymph-node-negative breast cancer. From March 1990 to October 1999, 1063 pre- and perimenopausal women were randomly assigned to receive either (i) 24 monthly subcutaneous implants of goserelin every 28 days, (ii) six 28-day courses of ‘classical’ CMF (cyclophosphamide, methotrexate, 5-fluorouracil) chemotherapy, or (iii) six 28-day courses of classical CMF followed by 18 monthly implants of goserelin. Initially the trial included a no adjuvant treatment group, which was discontinued in 1992, on the basis of results from other trials, after 46 patients had been randomized. Systemic adjuvant therapy was to begin within 6 weeks of primary surgery. For the sequential treatment arm, the first goserelin implant was scheduled to be given on day 28 of the sixth courses of CMF. Pre- or perimenopausal status was defined as having one of the following sets of characteristics: (i) aged older than 52 years with last normal menstrual period within 1 year, (ii) aged 52 years or younger with last normal menstrual period within 3 years, (iii) aged 55 years or younger with hysterectomy but no bilateral oophorectomy (for patients aged older than 45 years, biochemical confirmation for ovarian function was requested), or (iv) biochemical evidence of continuing ovarian function (for doubtful cases). The randomization was stratified according to locally determined estrogen receptor (ER) status (negative, positive, or unknown), whether radiotherapy was planned after breast-conserving surgical procedure (yes or no), and by participating institution.
In 1998, a protocol amendment restricted enrollment to patients with ER-positive tumors, based on evidence from other trials that ovarian ablation might not be effective for patients with ER-negative tumors. The intention to perform separate analyses according to ER status was specified in the original protocol. A retrospective collection of tumor blocks led to the central evaluation of ER expression by immunohistochemical (IHC) assay for 867 patients. Centrally determined ER status was considered negative if <1% of the cells were stained. For the remaining 196 patients without centrally reviewed tumors, the locally evaluated ER status was used [ER status was determined hierarchically from quantitative IHC (none versus 1%–100%); biochemical assay (0 versus >0 fmol/mg cytosol protein); or qualitative IHC (negative versus borderline/positive/strongly positive) results]. The locally obtained ER results were in concordance with the central IHC review for 81.2% of the patients [9].
statistical considerations
The end points of interest were disease-free survival (DFS), overall survival (OS), and breast cancer-free interval (BCFI). DFS was defined as the length of time from the date of randomization to any invasive breast cancer relapse (including ipsilateral or contralateral breast recurrence), the appearance of a second (nonbreast) malignancy, or death, whichever occurred first or was censored at date of last follow-up. OS was defined as the length of time from the date of randomization to death from any cause. BCFI was defined as the length of time from the date of randomization to any invasive breast cancer relapse (including ipsilateral or contralateral breast recurrence), according to the standardized definitions of efficacy end points (STEEP) criteria [10] and was censored at date of last follow-up or at date of death without relapse.
DFS, OS, and BCFI percentages, standard errors, and treatment effect comparisons were estimated using the Kaplan–Meier method, Greenwood's formula, and log-rank tests, respectively. Cox proportional hazards regression models were used to control for prognostic features and to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the treatment comparisons.
Treatment–covariate interactions were studied using nonparametric Subpopulation Treatment Effect Pattern Plot (STEPP) methodology [11]. STEPP involves defining several overlapping subgroups of patients on the basis of a covariate of interest and studying the resulting pattern of the treatment effects estimated within each subgroup. In this report, age was the covariate of interest, and treatment effects estimated within each age subpopulation were measured in terms of 5-year and 12-year DFS percentages. Probability values for the interaction test of treatment and age were provided on the basis of simulations.
results
The median follow-up for this analysis was 12.1 years (range 0.6–18.4). Patients’ tumors were classified as ER positive among 80% (n = 851) of patients, ER negative among 19% (n = 205), and unknown among 1% (n = 7) patients. The baseline patients’ characteristics according to ER status are shown in Table 1. The treatment groups were well balanced.
Table 1.
Patient and tumor characteristics for pre- and perimenopausal patients with lymph-node-negative breast cancer and cohorts defined according to estrogen receptor (ER) status
| Goserelin × 24, n (%) | CMF × 6, n (%) | CMF × 6→goserelin × 18, n (%) | Total, n (%) | |
| ER-positive cohort | n = 278 | n = 291 | n = 282 | n = 851 |
| Age (years) | ||||
| ≤34 | 11 (4) | 16 (6) | 16 (6) | 43 (5) |
| 35–39 | 36 (13) | 36 (12) | 37 (13) | 109 (13) |
| 40–44 | 65 (23) | 88 (30) | 69 (24) | 222 (26) |
| 45–49 | 115 (41) | 105 (36) | 105 (37) | 325 (38) |
| ≥50 | 51 (18) | 46 (16) | 55 (20) | 152 (18) |
| Primary surgical treatment | ||||
| Total mastectomy | 119 (43) | 126 (43) | 121 (43) | 366 (43) |
| Breast conservation | ||||
| With RT planned | 148 (53) | 151 (52) | 146 (52) | 445 (52) |
| With no RT planned | 11 (4) | 14 (5) | 15 (5) | 40 (5) |
| Tumor size (cm) | ||||
| ≤1.0 | 27 (10) | 37 (13) | 44 (16) | 108 (13) |
| 1.1–2.0 | 149 (54) | 151 (52) | 146 (52) | 446 (52) |
| >2 | 100 (36) | 98 (34) | 90 (32) | 288 (34) |
| Unknown | 2 (1) | 5 (2) | 2 (1) | 9 (1) |
| Tumor grade | ||||
| 1 | 52 (19) | 46 (16) | 77 (27) | 175 (21) |
| 2 | 148 (53) | 146 (50) | 129 (46) | 423 (50) |
| 3 | 76 (27) | 93 (32) | 74 (26) | 243 (29) |
| Unknown | 2 (1) | 6 (2) | 2 (1) | 10 (1) |
| ER-negative cohort | n = 65 | n = 68 | n = 72 | n = 205 |
| Age (years) | ||||
| ≤34 | 5 (8) | 6 (9) | 4 (6) | 15 (7) |
| 35–39 | 15 (23) | 10 (15) | 16 (22) | 41 (20) |
| 40–44 | 15 (23) | 18 (26) | 22 (31) | 55 (27) |
| 45–49 | 24 (37) | 24 (35) | 20 (28) | 68 (33) |
| ≥50 | 6 (9) | 10 (15) | 10 (14) | 26 (13) |
| Primary surgical treatment | ||||
| Total mastectomy | 34 (52) | 31 (46) | 35 (49) | 100 (49) |
| Breast conservation | ||||
| With RT planned | 27 (42) | 30 (44) | 33 (46) | 90 (44) |
| With no RT planned | 4 (6) | 7 (10) | 4 (6) | 15 (7) |
| Tumor size (cm) | ||||
| ≤1.0 | 5 (8) | 5 (7) | 5 (7) | 15 (7) |
| 1.1–2.0 | 31 (48) | 21 (31) | 28 (39) | 80 (39) |
| >2 | 29 (45) | 42 (62) | 37 (51) | 108 (53) |
| Unknown | 0 (0) | 0 (0) | 2 (3) | 2 (1) |
| Tumor grade | ||||
| 1 | 1 (2) | 2 (3) | 3 (4) | 6 (3) |
| 2 | 12 (18) | 16 (24) | 16 (22) | 44 (21) |
| 3 | 52 (80) | 49 (72) | 51 (71) | 152 (74) |
| Unknown | 0 (0) | 1 (1) | 2 (3) | 3 (1) |
CMF, cyclophosphamide, methotrexate and fluorouracil; RT, radiation therapy.
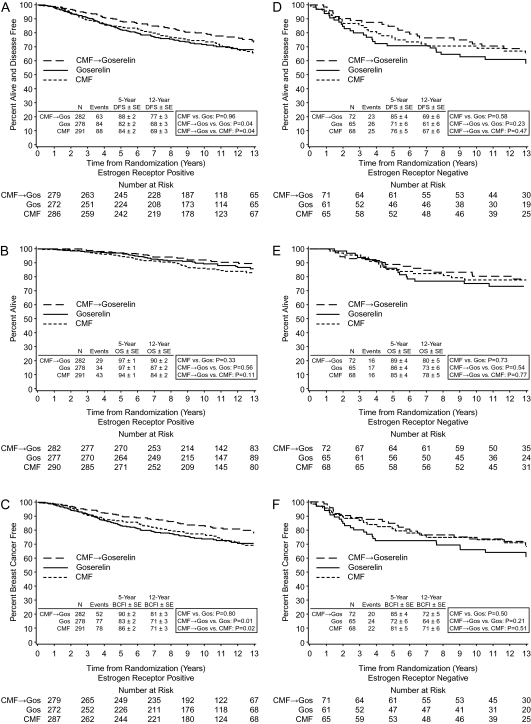
Table 2 summarizes the results for cohorts defined according to ER status and age for the three end points: DFS, OS, and BCFI. There were no statistically significant differences in overall survival between any of the treatment groups (Figures 1B and E and 2B and E).
Table 2.
Disease-free survival (DFS), overall survival (OS), and breast cancer-free interval (BCFI) for 1063 pre- and perimenopausal women with lymph-node-negative breast cancer according to randomized treatment group at a median follow-up for 12.1 years in cohorts defined by ER status and age at study entry
| Total patients | Total events | 5-year DFS % ± SE | 12-year DFS % ± SE | Comparison | HR | Lower 95% CI | Upper 95% CI | Log-rank P-value | ||
| DFS | ||||||||||
| ER+ | Gos | 278 | 84 | 82 ± 2 | 68 ± 3 | C versus G | 0.99 | 0.74 | 1.34 | 0.96 |
| CMF | 291 | 88 | 84 ± 2 | 69 ± 3 | C→G versus G | 0.71 | 0.51 | 0.98 | 0.04 | |
| CMF→Gos | 282 | 63 | 88 ± 2 | 77 ± 3 | C→G versus C | 0.71 | 0.52 | 0.99 | 0.04 | |
| ER+ age <40 | Gos | 47 | 22 | 72 ± 7 | 51 ± 8 | C versus G | 1.01 | 0.56 | 1.82 | 0.98 |
| CMF | 52 | 22 | 67 ± 7 | 55 ± 7 | C→G versus G | 0.41 | 0.20 | 0.83 | 0.01 | |
| CMF→Gos | 53 | 12 | 89 ± 4 | 77 ± 6 | C→G versus C | 0.42 | 0.21 | 0.85 | 0.02 | |
| ER+ age ≥40 | Gos | 231 | 62 | 84 ± 2 | 72 ± 3 | C versus G | 1.00 | 0.71 | 1.42 | 0.99 |
| CMF | 239 | 66 | 88 ± 2 | 72 ± 3 | C→G versus G | 0.82 | 0.56 | 1.18 | 0.28 | |
| CMF→Gos | 229 | 51 | 88 ± 2 | 77 ± 3 | C→G versus C | 0.82 | 0.57 | 1.18 | 0.29 | |
| ER− | Gos | 65 | 26 | 71 ± 6 | 61 ± 6 | C versus G | 0.86 | 0.49 | 1.49 | 0.58 |
| CMF | 68 | 25 | 76 ± 5 | 67 ± 6 | C→G versus G | 0.71 | 0.40 | 1.24 | 0.23 | |
| CMF→Gos | 72 | 23 | 85 ± 4 | 69 ± 6 | C→G versus C | 0.81 | 0.46 | 1.43 | 0.47 | |
| ER− age <40 | Gos | 20 | 7 | 75 ± 10 | 64 ± 11 | C versus G | 0.93 | 0.31 | 2.79 | 0.90 |
| CMF | 16 | 6 | 81 ± 10 | 74 ± 11 | C→G versus G | 1.03 | 0.36 | 2.94 | 0.96 | |
| CMF→Gos | 20 | 7 | 80 ± 9 | 65 ± 11 | C→G versus C | 1.02 | 0.34 | 3.04 | 0.98 | |
| ER− age ≥40 | Gos | 45 | 19 | 69 ± 7 | 60 ± 7 | C versus G | 0.81 | 0.43 | 1.53 | 0.52 |
| CMF | 52 | 19 | 75 ± 6 | 65 ± 7 | C→G versus G | 0.60 | 0.31 | 1.18 | 0.14 | |
| CMF→Gos | 52 | 16 | 87 ± 5 | 70 ± 7 | C→G versus C | 0.74 | 0.38 | 1.44 | 0.38 |
| Total patients | Total events | 5-year OS % ± SE | 12-year OS % ± SE | HR | Lower95% CI | Upper95% CI | P-value | |||
| OS | ||||||||||
| ER+ | Gos | 278 | 34 | 97 ± 1 | 87 ± 2 | C versus G | 1.25 | 0.80 | 1.96 | 0.33 |
| CMF | 291 | 43 | 94 ± 1 | 84 ± 2 | C→G versus G | 0.86 | 0.53 | 1.42 | 0.56 | |
| CMF→Gos | 282 | 29 | 97 ± 1 | 90 ± 2 | C→G versus C | 0.68 | 0.42 | 1.09 | 0.11 | |
| ER+ age <40 | Gos | 47 | 10 | 96 ± 3 | 76 ± 8 | C versus G | 1.11 | 0.47 | 2.62 | 0.81 |
| CMF | 52 | 11 | 92 ± 4 | 78 ± 6 | C→G versus G | 0.41 | 0.14 | 1.21 | 0.11 | |
| CMF→Gos | 53 | 5 | 98 ± 2 | 92 ± 4 | C→G versus C | 0.40 | 0.14 | 1.14 | 0.09 | |
| ER+ age ≥40 | Gos | 231 | 24 | 97 ± 1 | 89 ± 2 | C versus G | 1.32 | 0.78 | 2.23 | 0.31 |
| CMF | 239 | 32 | 95 ± 1 | 85 ± 3 | C→G versus G | 1.04 | 0.59 | 1.83 | 0.89 | |
| CMF→Gos | 229 | 24 | 97 ± 1 | 90 ± 2 | C→G versus C | 0.78 | 0.46 | 1.32 | 0.36 | |
| ER− | Gos | 65 | 17 | 86 ± 4 | 73 ± 6 | C versus G | 0.88 | 0.45 | 1.75 | 0.73 |
| CMF | 68 | 16 | 85 ± 4 | 78 ± 5 | C→G versus G | 0.81 | 0.41 | 1.60 | 0.54 | |
| CMF→Gos | 72 | 16 | 89 ± 4 | 80 ± 5 | C→G versus C | 0.90 | 0.45 | 1.80 | 0.77 | |
| ER− age <40 | Gos | 20 | 5 | 85 ± 8 | 75 ± 10 | C versus G | 0.51 | 0.10 | 2.65 | 0.43 |
| CMF | 16 | 2 | 88 ± 8 | 88 ± 8 | C→G versus G | 0.83 | 0.22 | 3.08 | 0.78 | |
| CMF→Gos | 20 | 4 | 85 ± 8 | 80 ± 9 | C→G versus C | 1.53 | 0.28 | 8.35 | 0.62 | |
| ER− age ≥40 | Gos | 45 | 12 | 87 ± 5 | 72 ± 7 | C versus G | 1.00 | 0.46 | 2.15 | 0.99 |
| CMF | 52 | 14 | 85 ± 5 | 75 ± 6 | C→G versus G | 0.80 | 0.36 | 1.79 | 0.59 | |
| CMF→Gos | 52 | 12 | 90 ± 4 | 80 ± 6 | C→G versus C | 0.78 | 0.36 | 1.70 | 0.54 |
| Total patients | Total events | 5-year BCFI % ± SE | 12-year BCFI % ± SE | HR | Lower95% CI | Upper95% CI | P-value | |||
| BCFI | ||||||||||
| ER+ | Gos | 278 | 77 | 83 ± 2 | 71 ± 3 | C versus G | 0.96 | 0.70 | 1.32 | 0.80 |
| CMF | 291 | 78 | 86 ± 2 | 71 ± 3 | C→G versus G | 0.63 | 0.45 | 0.90 | 0.01 | |
| CMF→Gos | 282 | 52 | 90 ± 2 | 81 ± 3 | C→G versus C | 0.66 | 0.46 | 0.94 | 0.02 | |
| ER+ age <40 | Gos | 47 | 21 | 72 ± 7 | 54 ± 8 | C versus G | 1.05 | 0.58 | 1.92 | 0.86 |
| CMF | 52 | 22 | 67 ± 7 | 55 ± 7 | C→G versus G | 0.40 | 0.19 | 0.83 | 0.01 | |
| CMF→Gos | 53 | 11 | 89 ± 4 | 77 ± 6 | C→G versus C | 0.39 | 0.19 | 0.81 | 0.01 | |
| ER+ age ≥40 | Gos | 231 | 56 | 86 ± 2 | 74 ± 3 | C versus G | 0.94 | 0.65 | 1.36 | 0.75 |
| CMF | 239 | 56 | 91 ± 2 | 75 ± 3 | C→G versus G | 0.72 | 0.48 | 1.08 | 0.11 | |
| CMF→Gos | 229 | 41 | 90 ± 2 | 82 ± 3 | C→G versus C | 0.77 | 0.52 | 1.16 | 0.21 | |
| ER− | Gos | 65 | 24 | 72 ± 6 | 64 ± 6 | C versus G | 0.82 | 0.46 | 1.46 | 0.50 |
| CMF | 68 | 22 | 81 ± 5 | 71 ± 6 | C→G versus G | 0.68 | 0.38 | 1.24 | 0.21 | |
| CMF→Gos | 72 | 20 | 85 ± 4 | 72 ± 5 | C→G versus C | 0.81 | 0.44 | 1.49 | 0.51 | |
| ER− age <40 | Gos | 20 | 7 | 75 ± 10 | 64 ± 11 | C versus G | 0.93 | 0.31 | 2.79 | 0.90 |
| CMF | 16 | 6 | 81 ± 10 | 74 ± 11 | C→G versus G | 1.03 | 0.36 | 2.94 | 0.96 | |
| CMF→Gos | 20 | 7 | 80 ± 9 | 65 ± 11 | C→G versus C | 1.02 | 0.34 | 3.04 | 0.98 | |
| ER− age ≥40 | Gos | 45 | 17 | 71 ± 7 | 64 ± 7 | C versus G | 0.77 | 0.39 | 1.52 | 0.45 |
| CMF | 52 | 16 | 81 ± 5 | 70 ± 6 | C→G versus G | 0.58 | 0.28 | 1.19 | 0.14 | |
| CMF→Gos | 52 | 13 | 87 ± 5 | 74 ± 6 | C→G versus C | 0.74 | 0.35 | 1.53 | 0.41 |
CMF, cyclophosphamide, methotrexate and fluorouracil; CI, confidence interval; ER, estrogen receptor; Gos, goserelin; HR, hazard ratio; SE, standard error.
Figure 1.
Kaplan–Meier estimates of disease-free survival (A,D), overall survival (B,E), and breast cancer-free interval (C,F) by treatment according to cohorts defined as estrogen receptor positive (A–C) and estrogen receptor negative (D–F) in the International Breast Cancer Study Group Trial VIII.
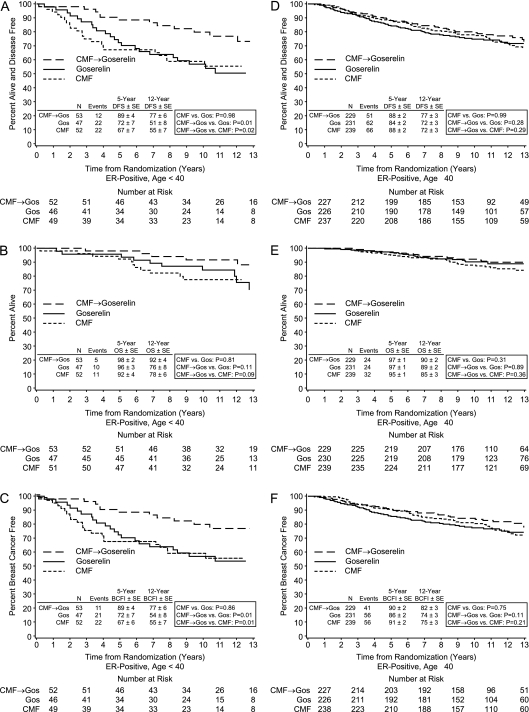
Figure 2.
Kaplan–Meier estimates of disease-free survival (A,D), overall survival (B,E), and breast cancer free interval (C,F) by treatment according to age cohorts within the estrogen receptor-positive cohort in IBCSG Trial VIII. The younger (<40 years) cohort is on the left (A–C).
For patients with ER-positive disease, the 12-year DFS was 68% in the goserelin alone group, 69% in the CMF alone group, and 77% in the CMF followed by goserelin (CMF→ goserelin) group; the 12-year BCFI was 71% in the goserelin alone group, 71% in the CMF alone group, and 81% in the CMF→ goserelin group. There were no statistically significant differences between CMF alone versus goserelin alone in DFS and BCFI [HR 0.99 (95% CI 0.74–1.34) for DFS, and HR 0.96 (95% CI 0.70–1.32) for BCFI]. CMF→ goserelin significantly improved DFS and BCFI compared with goserelin alone [HR 0.71 (95% CI 0.51–0.98, P= 0.04) for DFS, and HR 0.63 (95% CI 0.45–0.90, P = 0.01) for BCFI]. CMF→ goserelin also significantly improved DFS and BCFI compared with CMF alone [HR 0.71 (95% CI 0.52–0.99, P= 0.04) for DFS, and HR 0.66 (95% CI 0.46–0.94, P= 0.02) for BCFI] (Table 2, Figure 1A and C). The superiority in treatment efficacy of CMF→ goserelin compared with goserelin alone or with CMF alone was most pronounced for patients with ER-positive tumors who were younger than 40 years of age [compared with goserelin alone, HR 0.41 (95% CI 0.20–0.83, P = 0.01) for DFS, and HR 0.40 (95% CI 0.19–0.83, P= 0.01) for BCFI; compared with CMF alone, HR 0.42 (95% CI 0.21–0.85, P= 0.02) for DFS, and HR 0.39 (95% CI 0.19–0.81, P= 0.01) for BCFI] (Table 2, Figure 2A and C). The interactions between the treatments and age groups (age <40 versus age ≥40) were assessed using Cox proportional hazard models and the interaction Pvalues were 0.16 and 0.21 for DFS and BCFI, respectively.
In the small ER-negative cohort, there were no significant differences between the treatment groups. However, patients with ER-negative tumors who received treatments that included CMF appeared to have better DFS and BCFI (12-year DFS for CMF = 67% and 12-year DFS for CMF→ goserelin = 69%; 12-year BCFI for CMF = 71% and 12-year BCFI for CMF→ goserelin = 72%) than if they received goserelin alone (12-year DFS = 61%; 12-year BCFI = 64%) (Table 2, Figure 1D and F).
Multivariate Cox regression analyses on DFS in the ER-positive cohort indicated that age <40, breast-conserving surgery without radiotherapy and grade 2 or 3 tumors were significant risk factors. None of the risk factors were significant in the ER-negative cohort (Table 3).
Table 3.
Multivariable Cox proportional hazards regression analyses on disease-free survival for estrogen receptor (ER)-positive and -negative cohorts
| Cohort | Hazard ratio (95% CI) | Pvalue |
| ER-positive cohort (n = 851) | ||
| CMF versus goserelin | 1.00 (0.74–1.36) | 0.99 |
| CMF→goserelin versus goserelin | 0.71 (0.51–1.00) | 0.05 |
| CMF→goserelin versus CMF | 0.72 (0.51–1.00) | 0.05 |
| Age <40 versus ≥40 | 1.50 (1.10–2.06) | 0.01 |
| Primary surgical treatment | 0.0002 | |
| BCS without RT versus Mast | 2.39 (1.45–3.94) | |
| BCS with RT versus Mast | 0.83 (0.63–1.11) | |
| Tumor size (cm) | 0.19 | |
| 1.1–2.0 versus ≤1 | 1.14 (0.73–1.79) | |
| >2.1 versus ≤1 | 1.43 (0.90–2.27) | |
| Grade | 0.0001 | |
| 2 versus 1 | 1.75 (1.14–2.70) | |
| 3 versus 1 | 2.54 (1.63–3.97) | |
| ER-negative cohort (n = 205) | ||
| CMF versus goserelin | 0.81 (0.46–1.42) | 0.46 |
| CMF→goserelin versus goserelin | 0.71 (0.40–1.24) | 0.23 |
| CMF→goserelin versus CMF | 0.87 (0.49–1.55) | 0.64 |
| Age <40 versus ≥40 | 0.95 (0.56–1.61) | 0.84 |
| Primary surgical treatment | 0.69 | |
| BCS without RT versus Mast | 1.46 (0.61–3.52) | |
| BCS with RT versus Mast | 1.04 (0.63–1.73) | |
| Tumor size (cm) | 0.31 | |
| 1.1–2.0 versus ≤1 | 0.55 (0.26–1.19) | |
| >2.1 versus ≤1 | 0.63 (0.29–1.38) | |
| Grade | 0.24 | |
| 2 versus 1 | 1.32 (0.30–5.72) | |
| 3 versus 1 | 0.82 (0.20–3.39) |
CMF, cyclophosphamide, methotrexate and fluorouracil; CI, confidence interval; RT, radiation therapy; BCS, breast-conserving surgery; Mast, mastectomy.
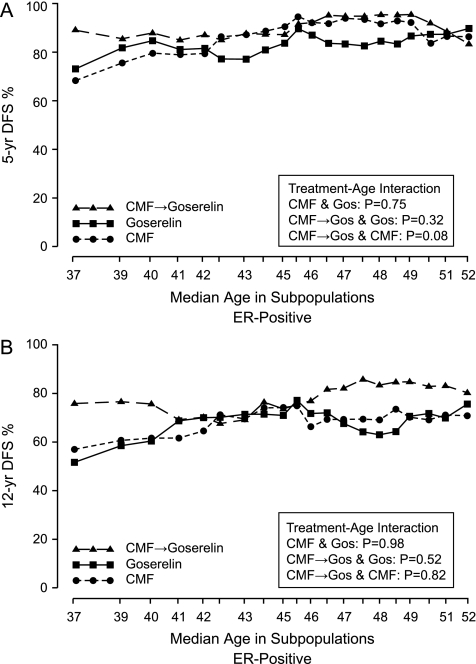
STEPP analyses were used to explore the pattern of treatment effect differences in terms of 5- and 12-year DFS percentages according to the age at diagnosis in the ER-positive cohort. For this sliding window STEPP analysis, each subpopulation contained 165 patients, and each subsequent subpopulation was formed moving from left to right by dropping ∼30 patients with the lowest age and adding ∼30 patients with the next higher age. The x coordinate indicates the median age for the patients in each subpopulation. The y coordinate indicates the 5- or 12-year DFS percentages estimated using the Kaplan–Meier method on data from patients in each subpopulation. These plots indicated a pattern with a clear additional effect of CMF→ goserelin versus goserelin alone or CMF alone predominantly in the younger ages (∼<40 years) in the ER-positive cohort (Figure 3).
Figure 3.
Subpopulation Treatment Effect Patten Plot showing 5-year (A) and 12-year (B) DFS percentages by treatment group according to overlapping subpopulations of age at randomization for 851 patients in the estrogen receptor-positive cohort.
discussion
With 12.1 years of median follow-up in this trial of pre- and perimenopausal women with node-negative breast cancer, CMF followed by goserelin significantly improved both 12-year DFS and BCFI compared with either modality alone among patients with ER-positive disease. These results are in line with the previous report from this study at 7 years of follow-up [8], though at that time the superiority of CMF followed by goserelin versus either modality alone was not statistically significant in the ER-positive cohort.
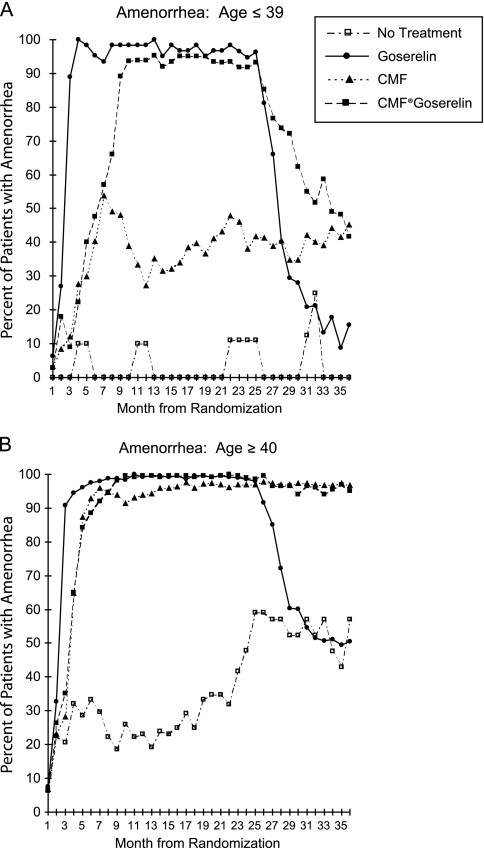
The superiority of adding CMF to goserelin in this study is also consistent with results from the Early Breast Cancer Trialists’ Collaborative Group overview that showed additional benefit of chemoendocrine therapy compared with endocrine therapy alone in hormone receptor-positive breast cancer [2]. However, the biological reason for the additional effect of chemotherapy could not be addressed in the overview. In the present study, the STEPP analysis with subpopulations of different ages at diagnosis clearly shows a pattern with additional treatment efficacy of CMF followed by goserelin in patients at younger ages (<40 years of age) with ER-positive disease. Amenorrhea rate was meticulously recorded. CMF followed by goserelin clearly prolonged amenorrhea compared with goserelin alone among patients <40 years [8] (Figure 4). This suggests that the treatment efficacy may reflect prolonged ovarian function suppression induced by CMF.
Figure 4.
Percentage of patients enrolled in the IBCSG Trial VIII with amenorrhea during each month from randomization according to treatment. (A) Results for patients aged 39 years or younger; (B) the results for patients aged 40 years or older. Reprinted by permission from Journal of the National Cancer Institute [8]. Gos, goserelin; SE, standard error.
Ovarian function suppression as manifest by chemotherapy-induced amenorrhea was associated with improved outcome in patients with ER-positive node-positive breast cancer participating in IBCSG Trial 13–93 [DFS HR (amenorrhea versus no amenorrhea) = 0.59; 95% CI 0.35–1.00 based on a 1.5 year landmark analysis for patients randomized to tamoxifen] [12]. By contrast, no association between amenorrhea and outcome was seen in the ER-negative cohort in Trial 13–93 (DFS HR =1.58; 95% CI 0.72–3.45). These findings have recently been confirmed with data from NSABP B-30 [13, 14]. While NSABP B-30 originally reported a positive association between amenorrhea and outcome in the ER-negative cohort [13], no association was seen in a subsequently published landmark analysis for this cohort [14]. Similarly, in the present study, ovarian function suppression alone was less effective than regimens containing chemotherapy among patients with ER-negative disease.
If the benefit of chemotherapy in hormone receptor-positive breast cancer in young patients in the present study is mediated mainly through more prolonged ovarian function suppression, this information would be highly clinically relevant since suppression can be achieved without the toxic effects of chemotherapy, which might not be needed for such patients. Trials designed to address this question have proved unpopular and have failed to meet their accrual goals. IBCSG Trial 11–93 randomized patients receiving ovarian function suppression and tamoxifen to the addition of four cycles of adriamycin-cyclophosphamide or to no chemotherapy, but only 174 patients were randomized. After a median follow-up of 10 years, no difference in outcome was seen between the two groups [15]. Similarly, the PERCHE trial [16] was closed after 3 years with only 29 patients randomized.
The quality of life in this trial has previously been reported [17]. The patients who received goserelin alone had less deterioration in quality-of-life measures during the first 6 months compared with patients on CMF. Regarding hot flushes, these symptoms closely followed the pattern of amenorrhea in the three groups with prolonged symptoms of hot flushes in the CMF–goserelin group compared with the other groups, especially among patients <40 years.
A limitation of the present study is that goserelin alone for 2 years is not considered optimal endocrine therapy by today's standards. Tamoxifen was not included in the endocrine therapy regimen because it was not routinely used in premenopausal women when the trial was initiated. Furthermore, the duration of ovarian function suppression achieved by 2 years of goserelin may be insufficient, especially for patients under age 40 with ER-positive node-negative disease, who experienced a high risk of recurrence in the goserelin alone group. Davidson et al. showed a significant benefit of adding tamoxifen (T) to CAF-Z (cyclophosphamide, adriamycin, fluorouracil–goserelin), with a 9-year DFS of 57% for CAF, 60% for CAF-Z, and 67% for CAF-ZT among premenopausal patients with hormone receptor-positive node-positive breast cancer [18]. Goserelin (Z) and T were administered for a duration of 5 years. Whether adding ovarian function suppression to tamoxifen improves outcome remains an open question, which is being addressed in the ongoing Suppression of Ovarian Function Trial study [19]. It is also possible that CMF, used in the current study, is slightly inferior to newer chemotherapy regimens that include anthracyclines or taxanes for some groups of patients. Since both the endocrine treatment and the chemotherapy in the present trial differ from the current optimal standard treatments, the results cannot directly be applied in the management of breast cancer patients today.
The ongoing Trial Assigning Individualized Options for Treatments Rx-trial addresses the value of adding chemotherapy to endocrine therapy alone [20]. Hormone receptor-positive breast cancers in both pre- and postmenopausal women are assessed with Oncotype-DX. Patients with intermediate recurrence scores are randomized to either chemoendocrine therapy or endocrine therapy alone. The Oncotype-DX recurrence score is complex and involves genes representing both human epidermal growth factor receptor 2 (HER2) and steroid hormone receptor pathways as well as proliferation genes. The group of patients with an intermediate recurrence score may be biologically heterogeneous with different expected additional effect of chemotherapy.
Viale et al. [21] have published STEPP analyses with 5-year DFS according to different overlapping ER levels in IBCSG Trials VIII and IX. Low levels of ER were predictive of the benefit of adding chemotherapy. For premenopausal women with ER-present disease, variability in the pattern of treatment effect differences between CMF→goserelin and CMF alone or goserelin alone was suggested. The pattern in this update was similar to that report (data not shown).
In a recent publication involving the central review of HER2 in IBCSG Trials VIII and IX, Colleoni et al. [22] showed no clear benefit of CMF in endocrine receptor present patients [HR (CMF versus no CMF) 0.90, 95% CI 0.74–1.11] but a significant benefit in the triple-negative group (HR 0.46, 95% CI 0.29–0.73). The magnitude of CMF efficacy seemed to be lower in the HER2-positive/endocrine receptor absent group (HR 0.58, 95% CI 0.29–1.17) and in the small group of HER2-positive/endocrine receptor present group (n = 220, HR 0.73, 95% CI 0.42–1.25).
In conclusion, at 12 years of median follow-up, CMF followed by goserelin resulted in significant improvement of DFS and BCFI for premenopausal patients with ER-positive node-negative breast cancer compared with either modality alone. The effect was most pronounced in patients below <40 years of age. This effect may be mediated by the prolonged ovarian function suppression induced by CMF. To sort out the additional effects of chemotherapy compared to endocrine therapy alone, a randomized comparison in a pure highly endocrine responsive patient population is still needed to ascertain whether such patients can safely be spared the toxic effects of chemotherapy.
funding
We acknowledge the initial support provided by the Ludwig Institute for Cancer Research and the Cancer League of Ticino and the continuing support for central coordination, data management, and statistics provided by the Swedish Cancer Society; The Cancer Council Australia; Australian New Zealand Breast Cancer Trials Group (NHMRC 890028, 920876, 950328, 980379, 141711); the Frontier Science and Technology Research Foundation; the Swiss Group for Clinical Cancer Research (SAKK); the Swiss Cancer League; and the United States National Institutes of Health (CA-75362). We also acknowledge support for the Cape Town participants from the Cancer Association of South Africa; for the St Gallen participants from the Foundation for Clinical Research of Eastern Switzerland (OSKK); and for the Gothenburg participants from the Swedish Society for Cancer Research (Cancerfonden). Astra-Zeneca provided the Zoladex® free of charge.
disclosure
None of the authors reported any conflicts of interest.
Acknowledgments
We thank the patients, physicians, nurses, and data managers who participate in the IBCSG trials. We thank Joie Celano for data management.
appendix. International Breast Cancer Study Group—trial VIII participants
Scientific Committee: A. Goldhirsch, A. S. Coates (Co-Chairs); Foundation Council: S. Aebi, A. S. Coates, M. Colleoni, J. P. Collins, H. Cortés Funes, R. D. Gelber, A. Goldhirsch, M. Green, A. Hiltbrunner, S. B. Holmberg, P. Karlsson, I Kössler, I. Láng, J. Lindtner, F Paganetti M. de Stoppani, C.-M. Rudenstam, H.-J. Senn, R. Stahel, B. Thürlimann, A. Veronesi; Coordinating Center, Bern, Switzerland: A. Hiltbrunner (Director), M. Castiglione-Gertsch (Study Chair), G. Egli, R. Maibach, M. Rabaglio, B. Ruepp, P. Sicher; Statistical Center, Harvard School of Public Health and Dana-Farber Cancer Institute, Boston, MA, USA: R. Gelber (Director), M.M. Regan (Group Statistician), Z. Sun (Trial Statistician), K.N. Price (Scientific Director), J. Aldridge, B.F. Cole, S. Gelber, A. Giobbie-Hurder, P.K. Ruan; Data Management Center, Frontier Science & Tech. Res. Found., Amherst, NY, USA: L. Blacher (Director), R. Hinkle, S. Lippert, J. Celano; Pathology Office: B. Gusterson, G. Viale, R. Bettelheim, R. Reed, E. Mallon; The Ontario Cancer Treatment and Research Foundation, Toronto Sunnybrook Regional Cancer Centre, Toronto, Canada: K. Pritchard, D. Sutherland, C. Sawka, G. Taylor, R. Choo, C. Catzavelos, K. Roche; National Institute of Oncology, Budapest, Hungary: I. Láng, E. Hitre, E. Juhos, I. Szamel; Centro di Riferimento Oncologico, Aviano, Italy: D. Crivellari, S. Monfardini, E. Galligioni, M.D. Magri, A. Veronesi, A. Buonadonna. S. Massarut, C. Rossi, E. Candiani, A. Carbone, R. Volpe, M. Roncadin, M. Arcicasa, F. Coran, S. Morassut; Spedali Civili & Fondazione Beretta, Brescia, Italy: E. Simoncini, G. Marini, P. Marpicati, M. Braga, P. Grigolato, L. Lucini; General Hospital, Gorizia, Italy: S. Foladore, L. Foghin, G. Pamich, C. Bianchi, B. Marino, A. Murgia, V. Milan; European Institute of Oncology, Milan, Italy: A. Goldhirsch, M. Colleoni, G. Martinelli, L. Orlando, F. Nolé, A. Luini, R. Orecchia, G. Viale, F. Peccatori, F. de Braud, A. Costa, S. Zurrida, P. Veronesi, V. Sacchini, V. Galimberti, M. Intra, U. Veronesi; Ospedale Infermi, Rimini, Italy: A. Ravaioli, L. Gianni, D. Tassinari, G. Oliverio, F. Barbanti, P. Rinaldi, G. Drudi; Ospedale S. Eugenio, Roma, Italy: M. Antimi, M. Minelli, V. Bellini, R. Porzio, E. Pernazza, G. Santeusanio, L.G. Spagnoli; Ospedale S. Bortolo, Vicenza, Italy: M. Magazu, V. Fosser, P. Morandi, G. Scalco, M. Balli, M. Gion, S. Meli, G. Torsello; The Institute of Oncology, Ljubljana, Slovenia: J. Lindtner, D. Erzen, E. Majdic, B. Stabuc, A. Plesnicar, R. Golouh, J. Lamovec, J. Jancar, I. Vrhoved, M. Kramberger; Groote Schuur Hospital and University of Cape Town, Cape Town, Republic of South Africa: D.M. Dent, A. Gudgeon, E. Murray, I.D. Werner, P. Steynor, J. Toop, E. McEvoy; Sandton Oncology Center, Johannesburg, Republic of South Africa: D. Vorobiof, M. Chasen, G. Fotheringham, G. de Muelenaere, B. Skudowitz, C. Mohammed, A. Rosengarten; Madrid Breast Cancer Group, Madrid, Spain: H. Cortés-Funes, C. Mendiola, J. Hornedo, R. Colomer, F. Cruz Vigo, P. Miranda, A. Sierra, F. Martinez-Tello, A. Garzon, S. Alonso, A. Ferrero; West Swedish Breast Cancer Study Group, Göteborg, Sweden: C.M. Rudenstam, A. Wallgren, S. Ottosson-Lönn, R. Hultborn, G. Colldahl-Jäderström, E. Cahlin, J. Mattsson, S.B. Holmberg, L. Ivarsson, O. Ruusvik, L.G. Niklasson, S. Dahlin, G. Karlsson, P. Karlsson, B. Lindberg, A. Sundbäck, S. Bergegårdh, H. Salander, C. Andersson, M. Heideman, Y. Hessman, O. Nelzén, G. Claes, T. Ramhult, J.H. Svensson, P. Liedberg, M. Suurküla, S. Persson; Swiss Group for Clinical Cancer Research (SAKK) member institutions—Inselspital, Bern, Switzerland: M.F. Fey, M. Castiglione-Gertsch, E. Dreher, H. Schneider, S. Aebi, J. Ludin, G. Beck, A. Haenel, J.M. Lüthi, H.J. Altermatt, M. Nandedkar, K. Buser; Kantonsspital, St. Gallen, Switzerland: H.J. Senn, B. Thürlimann, Ch. Oehlschlegel, G. Ries, M. Töpfer, U. Lorenz, O. Schiltknecht, B. Späti, A. Ehrsam, M. Bamert, W.F. Jungi; Istituto Oncologico della Svizzera Italiana, Bellinzona, Switzerland: F. Cavalli, O. Pagani, H. Neuenschwander, L. Bronz, C. Sessa, M. Ghielmini, T. Rusca, P. Rey, J. Bernier, E. Pedrinis, T. Gyr, L. Leidi, G. Pastorelli, G. Caccia, A. Goldhirsch; Kantonsspital, Basel, Switzerland: R. Herrmann, C.F. Rochlitz, J.F. Harder, S. Bartens, U. Eppenberger, J. Torhorst; Hôpital des Cadolles, Neuchâtel, Switzerland: D. Piguet, P. Siegenthaler, V. Barrelet, R.P. Baumann; University Hospital, Zürich, Switzerland: B. Pestalozzi, C. Sauter, D. Fink, M. Fehr, U. Haller, U. Metzger, P. Huguenin, R. Caduff; Centre Hospitalier Universitaire Vandois, Lausanne, Switzerland: K. Zaman, L. Perey, S. Leyvraz, P. Anani, F. Gomez, D. Wellman, G. Chapuis, P. De Grandi, P. Reymond, M. Gillet, J.F. Delaloye; Hôpital Cantonal, Geneva, Switzerland: P. Alberto, P. Schäfer, F. Krauer, M. Forni, S. Diebold; Kantonsspital Graubünden, Chur, Switzerland: F. Egli, P. Forrer, A. Willi, R. Steiner. J. Allemann, T. Rüedi, A. Leutenegger, U. Dalla Torre; Australian New Zealand Breast Cancer Trials Group (ANZ BCTG) member institutions—Operations Office, University of Newcastle: J.F. Forbes, D. Lindsay; Statistical Center, NHMRC CTC, University of Sydney: R. J. Simes, H. Dhillon; The Cancer Council Victoria (previously Anti-Cancer Council of Victoria), Clinical Trials Office, Melbourne: J. Collins, R. Snyder, E. Abdi, A. Barling, R. Basser, W.I. Burns, M. Chipman, J. Chirgwin, I. Davis, R. Holmes, M. Green, D. Hastrich, P. Gregory, R. McLennan, L. Mileshkin, P. Mitchell, G. Richardson, M. Schwarz, I. Russell, C. Underhill, D. Reading, A. Zimet; Auckland Breast Cancer Study Group, Auckland, New Zealand: V.J. Harvey, P. Thompson, D. Porter; Flinders Medical Centre, Bedford Park, South Australia: T. Malden; Newcastle Mater Misericordiae Hospital Waratah, Newcastle, Australia: J.F. Forbes, J. Stewart, D. Jackson, R. Gourlay, J. Bishop, S. Cox, S. Ackland, A. Bonaventura, C. Hamilton, J. Denham, P. O'Brien, M. Back, S. Brae, R. Muragasu; Prince of Wales, Randwick, NSW, Australia: M. Friedlander, B. Brigham, C. Lewis; Royal Adelaide Hospital, Adelaide, Australia: I.N. Olver, P.G. Gill, A. Taylor, D. Keefe; University of Sydney, Dubbo Base Hospital and Royal Prince Alfred Hospital, Sydney, Australia: J. Beith, M. Boyer, A.S. Coates, R. J. Simes, A. Sullivan, M.H.N. Tattersall; W.P. Holman Clinic, Launceston: D. Boadle, I. Byard, D. Byram.
References
- 1.Dowsett M, Goldhirsch A, Hayes DF, et al. International web-based consultation on priorities for translational breast cancer research. Breast Cancer Res. 2007;9:R81. doi: 10.1186/bcr1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Clinical Trialists' Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Viale G, Regan MM, Maiorano E, et al. Chemoendocrine compared with endocrine adjuvant therapies for node-negative breast cancer: predictive value of centrally reviewed expression of estrogen and progesterone receptors–International Breast Cancer Study Group. J Clin Oncol. 2008;26:1404–1410. doi: 10.1200/JCO.2007.10.6393. [DOI] [PubMed] [Google Scholar]
- 4.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colozza M, Azambuja E, Cardoso F, et al. Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol. 2005;16:1723–1739. doi: 10.1093/annonc/mdi352. [DOI] [PubMed] [Google Scholar]
- 6.Viale G, Regan MM, Mastropasqua MG, et al. Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst. 2008;100:207–212. doi: 10.1093/jnci/djm289. [DOI] [PubMed] [Google Scholar]
- 7.Wirapati P, Sotiriou C, Kunkel S, et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008;10:R65. doi: 10.1186/bcr2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Breast Cancer Study Group. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst. 2003;95:1833–1846. doi: 10.1093/jnci/djg119. [DOI] [PubMed] [Google Scholar]
- 9.Regan MM, Viale G, Mastropasqua MG, et al. Re-evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst. 2006;98:1571–1581. doi: 10.1093/jnci/djj415. [DOI] [PubMed] [Google Scholar]
- 10.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 11.Lazar AA, Cole BF, Bonetti M, Gelber RD. Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: Subpopulation Treatment Effect Pattern Plot. J Clin Oncol. 2010;28:4539–4544. doi: 10.1200/JCO.2009.27.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Breast Cancer Study Group. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13-93. J Clin Oncol. 2006;24:1332–1341. doi: 10.1200/JCO.2005.03.0783. [DOI] [PubMed] [Google Scholar]
- 13.Swain SM, Jeong JH, Geyer CE, Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swain SM, Jeong JH, Wolmark N. Amenorrhea from breast cancer therapy - not a matter of dose. N Engl J Med. 2010;363:2268–2270. doi: 10.1056/NEJMc1009616. [DOI] [PubMed] [Google Scholar]
- 15.Thürlimann B, Price KN, Gelber RD, et al. Is chemotherapy necessary for premenopausal women with lower-risk node-positive, endocrine responsive breast cancer? 10-year update of International Breast Cancer Study Group Trial 11-93. Breast Cancer Res Treat. 2009;113:137–144. doi: 10.1007/s10549-008-9912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regan MM, Pagani O, Walley B, et al. Premenopausal endocrine-responsive early breast cancer: who receives chemotherapy? Ann Oncol. 2008;19:1231–1241. doi: 10.1093/annonc/mdn037. [DOI] [PubMed] [Google Scholar]
- 17.Bernhard J, Zahrieh D, Castiglione-Gertsch M, et al. Adjuvant chemotherapy followed by goserelin compared with either modality alone: the impact on amenorrhea, hot flashes, and quality of life in premenopausal patients—the International Breast Cancer Study Group Trial VIII. J Clin Oncol. 2007;25:263–270. doi: 10.1200/JCO.2005.04.5393. [DOI] [PubMed] [Google Scholar]
- 18.Davidson NE, O'Neill AM, Vukov CK, et al. Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101 (E5188) J Clin Oncol. 2005;23:5973–5982. doi: 10.1200/JCO.2005.05.551. [DOI] [PubMed] [Google Scholar]
- 19.Price KN, Goldhirsch A. Clinical trial update: International Breast Cancer Study Group. Breast Cancer Res. 2005;7:252–254. doi: 10.1186/bcr1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute. The TAILORx breast cancer trial. http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=472066&protocolsearchid=7680044&version=healthprofessional#StudyIdInfo_CDR0000472066 (10 May 2010, date last accessed) [Google Scholar]
- 21.Viale G, Regan MM, Maiorano E, et al. Chemoendocrine compared with endocrine adjuvant therapies for node-negative breast cancer: predictive value of centrally reviewed expression of estrogen and progesterone receptors—International Breast Cancer Study Group. J Clin Oncol. 2008;26:1404–1410. doi: 10.1200/JCO.2007.10.6393. [DOI] [PubMed] [Google Scholar]
- 22.Colleoni M, Cole BF, Viale G, et al. Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Clin Oncol. 2010;28:2966–2973. doi: 10.1200/JCO.2009.25.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]