Abstract
Background: On average, aromatase inhibitors are better than tamoxifen when used as initial or sequential therapy for postmenopausal women with endocrine-responsive early breast cancer. Because there may be contraindications to their use based on side-effects or cost, we investigated subgroups in which aromatase inhibitors may be more or less important.
Patients and methods: Breast International Group 1-98 trial randomized 6182 women among four groups comparing letrozole and tamoxifen with sequences of each agent; 5177 (84%) had centrally confirmed estrogen receptor (ER) positivity. We assessed whether centrally determined ER, progesterone receptor (PgR), human epidermal growth factor receptor 2, and Ki-67 labeling index, alone or in combination with other prognostic features, predicted the magnitude of letrozole effectiveness compared with either sequence or tamoxifen monotherapy.
Results: Individually, none of the markers significantly predicted differential treatment effects. Subpopulation treatment effect pattern plot analysis of a composite measure of prognostic risk revealed three patterns. Estimated 5-year disease-free survival for letrozole monotherapy, letrozole→tamoxifen, tamoxifen→letrozole, and tamoxifen monotherapy were 96%, 94%, 93%, and 94%, respectively, for patients at lowest risk; 90%, 91%, 93%, and 86%, respectively, for patients at intermediate risk; and 80%, 76%, 74%, and 69%, respectively, for patients at highest risk.
Conclusion: A composite measure of risk informs treatment selection better than individual biomarkers and supports the choice of 5 years of letrozole for patients at highest risk for recurrence.
Keywords: aromatase inhibitor, breast cancer, prognostic factor, tamoxifen
introduction
On average, aromatase inhibitors improve disease control over tamoxifen in the adjuvant setting [1]. However, the magnitude of benefit is relatively small and in some patients the adverse event profile of the aromatase inhibitors and their cost may make it difficult to include or persist with such therapy. We sought to review our experience with the Breast International Group (BIG) 1-98 trial in an attempt to define groups of patients in whom it was more or less important to include an aromatase inhibitor as opposed to tamoxifen as part or all of the adjuvant therapy program.
The BIG 1-98 trial is a large international intergroup study comparing 5 years of adjuvant endocrine therapy with tamoxifen, letrozole or the sequence of 2 years tamoxifen followed by 3 years letrozole (tamoxifen→letrozole) or 2 years letrozole followed by 3 years tamoxifen (letrozole→tamoxifen) for postmenopausal women with hormone receptor-positive early breast cancer [2]. Previous analyses have described the superiority of letrozole over tamoxifen monotherapy [3, 4]. Centrally reviewed estrogen receptor (ER) and progesterone receptor (PgR) levels showed prognostic value but among patients with at least some ER expression, PgR levels were not redictive for a relative benefit of letrozole over tamoxifen monotherapy [5]. Human epidermal growth factor receptor 2 (HER2) overexpression or amplification was an adverse prognostic factor but did not predict relative benefit of letrozole over tamoxifen [6]. Higher levels of Ki-67 labeling index (LI) also was an adverse prognostic factor and while there was suggestive evidence that higher levels predicted a greater benefit of letrozole over tamoxifen monotherapy, this effect was not statistically significant and requires confirmation [7].
Clinical results comparing monotherapy with the sequences have recently been published, showing that neither sequence, tamoxifen→letrozole nor letrozole→tamoxifen, improved outcomes over those observed with letrozole monotherapy [8]. The present study took advantage of centrally reviewed ER, PgR, HER2 and Ki-67 LI biomarkers individually and jointly in conjunction with other clinico-pathological features [9] to explore which patients derived most benefit from letrozole monotherapy for 5 years as compared with a sequential treatment strategy or tamoxifen alone.
patients and methods
The design and conduct of the study have been described previously [2, 3]. BIG 1-98 is a randomized, phase III, four-arm double-blind trial comparing monotherapy with letrozole or with tamoxifen for 5 years, sequential administration of tamoxifen→letrozole, or the reverse sequence letrozole→tamoxifen for postmenopausal women with early breast cancer locally assessed as ER and/or PgR positive. From March 1998 to March 2000, patients were randomly assigned to one of the monotherapy arms (N = 1828), and from April 1999 to May 2003, to all four arms (N = 6182). After the initial trial results favoring letrozole were released in 2005, patients assigned to tamoxifen monotherapy were so informed and offered the chance to switch to letrozole for the remainder of their adjuvant therapy, and 612 of them (39% of those randomly assigned to tamoxifen monotherapy in the four-arm option) did so [8]. This analysis used results recently reported [8] at a median follow-up of 71 months.
central pathological review
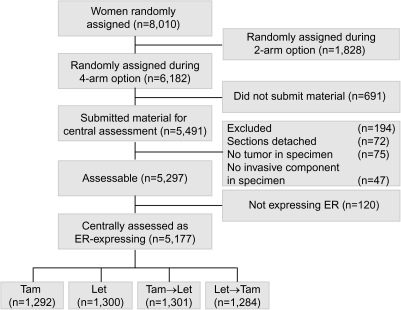
Retrospective tissue collection was carried out in accordance with institutional guidelines and national laws. Funding was provided to participating institutions by the trial's pharmaceutical partner, Novartis, to partially cover the associated costs. Of the 6182 patients enrolled during the four-arm randomization option, the International Breast Cancer Study Group (IBCSG) Central Pathology Laboratory in Milan, Italy, received material for 5491 patients (89%), of which 5297 (86%) were assessable, including 5177 (98% of 5297 assessable) whose tumor was confirmed to express some ER (Figure 1). These 5177 patients represent the analytic cohort. The material was reviewed for histopathological features and expression of tumor markers without the knowledge of patients' treatment assignment or outcome. A comparison of patients with and without central pathological review has been described previously [5].
Figure 1.
Patients from the BIG 1-98 trial included and excluded in this study according to treatment group and availability of tumor material. Tam, tamoxifen; Let, letrozole; ER, estrogen receptor.
ER, PgR and Ki-67 LI were determined by immunohistochemistry (IHC) as previously described [5, 7] and recorded as the percentage of immunostained cells. HER2 was assayed by IHC and FISH using standard reagents and procedures [6] and was considered positive if FISH showed a ratio of HER2-to-chromosome 17 of ≥2.0 or based only on IHC score of 3+ (in 0.5% of cases).
statistical methods
The primary trial end point was disease-free survival (DFS), defined as the time from random assignment to the earliest time of invasive recurrence, a new invasive breast cancer in the contralateral breast, any second (non-breast) malignancy, or death from any cause [2, 3]. Trial patients assigned to 5 years of tamoxifen were offered the opportunity to switch to letrozole after the initial publication of the results of the monotherapy treatments showed the superiority of letrozole [3], and 39% in the four-arm study design did so. An inverse probability of censoring weighted (IPCW) analysis estimated the relative benefit of letrozole versus tamoxifen monotherapy that would have been observed in the absence of such selective crossover [10]. This analysis was preferred to the intent-to-treat analysis, which was clearly biased as 39% of patients in the tamoxifen arm received a treatment (sequential tamoxifen followed by letrozole) shown to be superior to tamoxifen alone [1]. The IPCW analysis was reasonably approximated by an unweighted censored analysis with censoring at the time of selective crossover to letrozole [8, 10]. Because IPCW was intractable for the subpopulation treatment effect pattern plot (STEPP) analyses, we used the unweighted censored analysis as a close approximation in this report. There were 861 DFS events among the 5177 patients in the analytic cohort at a median follow-up of 71 months.
The distributions of DFS and 5-year DFS were estimated using the Kaplan–Meier method. Cox proportional hazards regression (stratified for chemotherapy use) estimated hazard ratios (HRs), 95% confidence intervals (CIs) and assessed interactions of the treatment effect with a marker considering P < 0.05 (two sided) as statistically significant without adjustment for multiple treatment comparisons.
In the initial assessment of markers individually, subgroups defined by categorizing ER, PgR, and Ki-67 LI were defined a priori: ER < 80% versus ER ≥ 80% as highly endocrine responsive, rather than ≥50% as suggested by St Gallen Consensus [11], because only 3.8% of tumors expressed ER < 50%; PgR 0% versus >0% as previously published [5]; Ki-67 LI < 14% versus ≥14% as suggested to distinguish luminal B from luminal A tumors using IHC [12]. Models included the individual marker-by-treatment interaction and were adjusted for the other centrally assessed tumor-related variables, tumor grade, tumor size, lymph node, presence of peritumoral vascular invasion, and patient age. The nonparametric STEPP methodology [13, 14] investigated trends in treatment effect differences across the continuum of expression for each marker.
In clinical practice, multiple factors are integrated to arrive at a treatment decision. A majority of the 2009 St Gallen panelists preferred initial aromatase inhibitor ‘particularly for patients at higher risk’ [11]. Thus, we aimed to replicate the clinical approach of a composite assessment of risk of an individual patient in our clinical trial cohort. A composite measure of prognostic risk was calculated for each patient by developing a Cox proportional hazards model, stratified by planned chemotherapy use and treatment assignment, for the entire analytic cohort. The hope was that the prognostic measure obtained would prove predictive of the comparative benefit of the various letrozole-containing therapies. Factors included in the model were selected based on the 2007 St Gallen Consensus [15] (number of involved lymph nodes, tumor grade, tumor size, presence of peritumoral vascular invasion as determined by local pathology), plus age and the four centrally assessed markers. Details of the model as developed are displayed in supplemental Table S1 (available at Annals of Oncology online) and the distribution of scores in supplemental Figure S1 (available at Annals of Oncology online).
role of coordinating group, trial steering committee, and funding sources
The IBCSG was responsible for study design and coordination, data collection and management, tissue management and central pathology assessment, data analysis, and reporting (including the decision to publish). Novartis, the manufacturer of letrozole, provided financial support for study conduct and pathology material collection but imposed no restrictions on the investigators with respect to trial data, writing the report, and decision to submit the publication. Susan G. Komen for the Cure provided funds for the data analysis and reporting and imposed no restrictions. The manuscript was prepared by the authors, who had full access to the data and who made final decisions on content, while the steering committee (including a minority membership of Novartis employees) reviewed the paper and offered changes.
ethics committees' approvals
The ethics committees and required health authorities of each participating center approved the study protocol, and all patients gave written informed consent.
results
patient characteristics
The 5177 patients in the analytic cohort are described in Table 1. All recorded prognostic factors were well balanced among the treatment groups. In this cohort of patients with tumors expressing at least some ER, only 6% of patients had HER2-positive tumors. Most patients (85%) had tumors expressing ER in ≥80% of cells. Similarly, 90% of patients had tumors expressing PgR. Ki-67 LI was expressed in <14% of cells in 54% of patients' tumors.
Table 1.
Characteristics of the analytic cohort of patients with ER-expressing tumors
| Treatment assignment |
All |
|||||||||
| Tam (N = 1292) |
Let (N = 1300) |
Tam→Let (N = 1301) |
Let→Tam (N = 1284) |
|||||||
| M | IQR | M | IQR | M | IQR | M | IQR | M | IQR | |
| Age at randomization | 61 | 56–67 | 61 | 56–67 | 60 | 56–67 | 61 | 56–67 | 61 | 56–67 |
| ER (centrally assessed) | 95 | 90–99 | 90 | 85–99 | 90 | 87–99 | 90 | 90–99 | 90 | 90–99 |
| PgR (centrally assessed) | 70 | 10–90 | 70 | 20–90 | 70 | 15–90 | 70 | 15–90 | 70 | 15–90 |
| Ki-67 LI (centrally assessed) | 12 | 6–18 | 12 | 7–19 | 12 | 7–18 | 12 | 7–18 | 12 | 7–18 |
| n | % | n | % | n | % | n | % | n | % | |
| Prior chemotherapy | 305 | 24 | 303 | 23 | 309 | 24 | 312 | 24 | 1229 | 24 |
| HER2 positive (centrally assessed) | 75 | 6 | 93 | 7 | 63 | 5 | 84 | 7 | 315 | 6 |
| Number of positive lymph nodes | ||||||||||
| Unknown | 12 | 1 | 11 | 1 | 14 | 1 | 7 | 1 | 44 | 1 |
| 0 | 749 | 58 | 748 | 58 | 748 | 57 | 747 | 58 | 2992 | 58 |
| 1–3 | 384 | 30 | 383 | 29 | 357 | 27 | 365 | 28 | 1489 | 29 |
| 4–9 | 103 | 8 | 103 | 8 | 122 | 9 | 98 | 8 | 426 | 8 |
| 10+ | 44 | 3 | 55 | 4 | 60 | 5 | 67 | 5 | 226 | 4 |
| Tumor size (cm) | ||||||||||
| Unknown | 10 | 1 | 4 | 0 | 7 | 1 | 18 | 1 | 39 | 1 |
| ≤2 | 827 | 64 | 838 | 64 | 803 | 62 | 802 | 62 | 3270 | 63 |
| >2 to <5 | 408 | 32 | 411 | 32 | 440 | 34 | 407 | 32 | 1666 | 32 |
| ≥5 | 47 | 4 | 47 | 4 | 51 | 4 | 57 | 4 | 202 | 4 |
| Tumor grade | ||||||||||
| Unknown | 62 | 5 | 70 | 5 | 61 | 5 | 56 | 4 | 249 | 5 |
| 1 | 384 | 30 | 345 | 27 | 357 | 27 | 369 | 29 | 1455 | 28 |
| 2 | 652 | 50 | 662 | 51 | 669 | 51 | 675 | 53 | 2658 | 51 |
| 3 | 194 | 15 | 223 | 17 | 214 | 16 | 184 | 14 | 815 | 16 |
| Peritumoral vascular invasion | ||||||||||
| Not assessed | 43 | 3 | 59 | 5 | 61 | 5 | 57 | 4 | 220 | 4 |
| Not present | 1012 | 78 | 1010 | 78 | 994 | 76 | 1015 | 79 | 4031 | 78 |
| Present | 237 | 18 | 231 | 18 | 246 | 19 | 212 | 17 | 926 | 18 |
ER, estrogen receptor; Tam, tamoxifen; Let, letrozole; M, median; IQR, interquartile range; PgR, progesterone receptor; LI, labeling index.
predictive value of individual markers
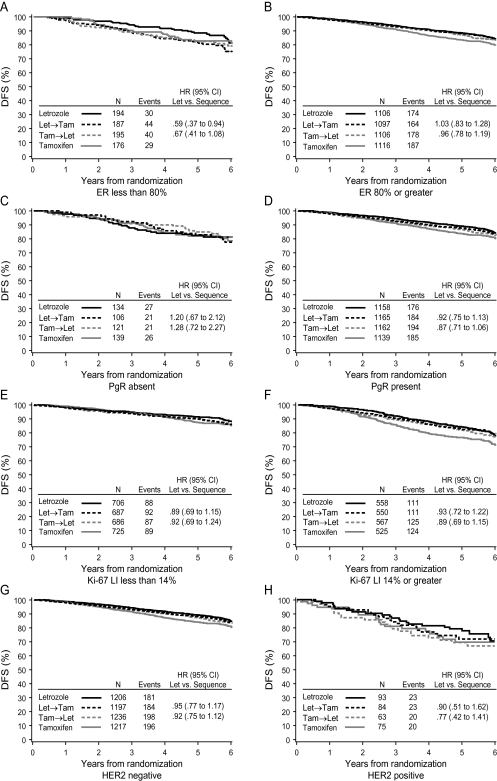
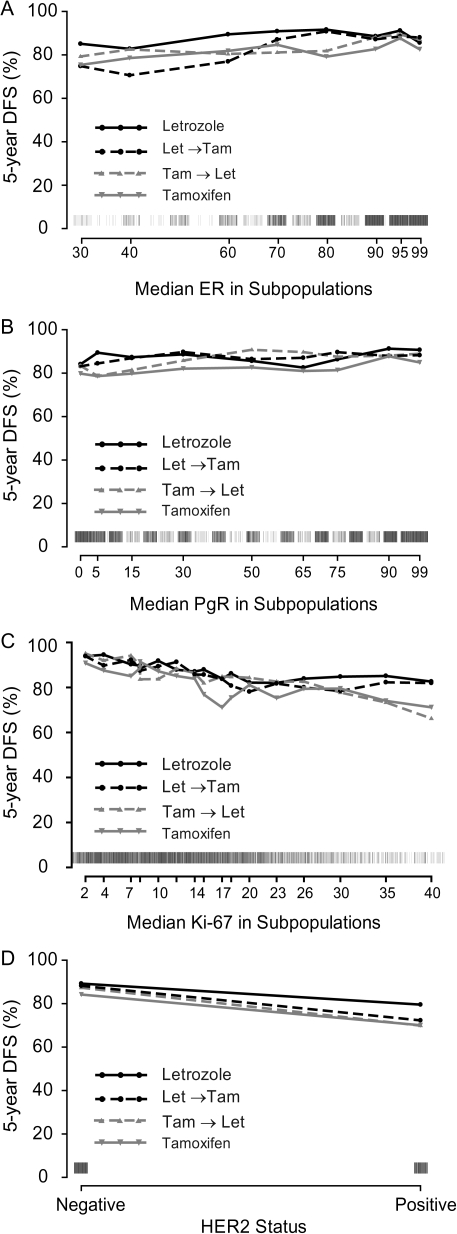
DFS according to treatment for each of the four centrally assessed tumor features is summarized in Figure 2. There was weak evidence of differential effects of the three letrozole-containing treatments depending on level of ER expression (Figure 2A and B). Patients whose tumors expressed lower levels of ER (<80%) may have received greater benefit of letrozole monotherapy over either sequence relative to those with ER levels of ≥80% (interaction P = 0.17 letrozole versus tamoxifen→letrozole and P = 0.03 letrozole versus letrozole→tamoxifen). In the STEPP analysis examining the relationship of ER expression levels with 5-year DFS (Figure 3A), greater heterogeneity among treatments in the 5-year DFS estimates was also observed across lower levels of ER expression. Letrozole showed the highest DFS, especially at lower ER levels, while the sequence of letrozole→tamoxifen appeared to be less effective at lower ER levels.
Figure 2.
Kaplan–Meier estimates of DFS according to treatment group and (A, B) ER status, (C, D) PgR status, (E, F) Ki-67 LI status, and (G, H) HER2 status. DFS, disease-free survival; ER, estrogen receptor; PgR, progesterone receptor; LI, labeling index.
Figure 3.
STEPP analysis of 5-year DFS according to level of centrally assessed (A) ER expression (%), (B) PgR expression (%), (C) Ki-67 LI (%), and (D) HER2 status. Rug plots along the x-axis display the distribution of individual values. STEPP, subpopulation treatment effect patternplot; DFS, disease-free survival; ER, estrogen receptor; PgR, progesterone receptor; LI, labeling index.
Similarly, according to the Ki-67 LI (Figure 3C), letrozole showed the highest DFS, especially at the highest Ki-67 LI level, although after dichotomizing Ki-67 LI expression at 14% it did not affect the relative treatment comparisons (interaction P = 0.85 letrozole versus tamoxifen→letrozole and P = 0.96 letrozole versus letrozole→tamoxifen; Figure 2E and F). PgR did not show evidence of differential effects among the three treatments whether grouped as absent or present (interaction P = 0.21 letrozole versus tamoxifen→letrozole and P = 0.39 letrozole versus letrozole→tamoxifen; Figure 2C and D) or examined across the continuum of expression levels (Figure 3B).
Among the patients with HER2-negative disease, DFS was similar for all letrozole-containing treatment groups, while DFS for patients assigned letrozole in the small cohort of patients with HER2-positive disease appears somewhat better than the two sequences (Figure 2G and H). However, the evidence of differential treatment benefit according to HER2 status was not statistically significant (interaction P = 0.58 letrozole versus tamoxifen→letrozole and P = 0.87 letrozole versus letrozole→tamoxifen).
Recent papers have suggested that a combination of conventional markers [12, 16] can identify a set of breast cancers closely similar to that identified as luminal A or luminal B by gene array analysis [17–19]. Using this approach, 54% had tumors classified as luminal A (HER2 negative and low Ki-67 LI) and 46% as luminal B (HER2 positive or high Ki-67 LI). There was no evidence that the relative efficacy of letrozole and the two sequences differed among these subtypes (data not shown).
Thus, each of the four tumor markers showed a trend favoring letrozole monotherapy at the ‘higher risk’ end of its spectrum, but none of the individual markers were statistically significant predictive factors to guide treatment selection.
predictive value of a composite measure of prognostic risk
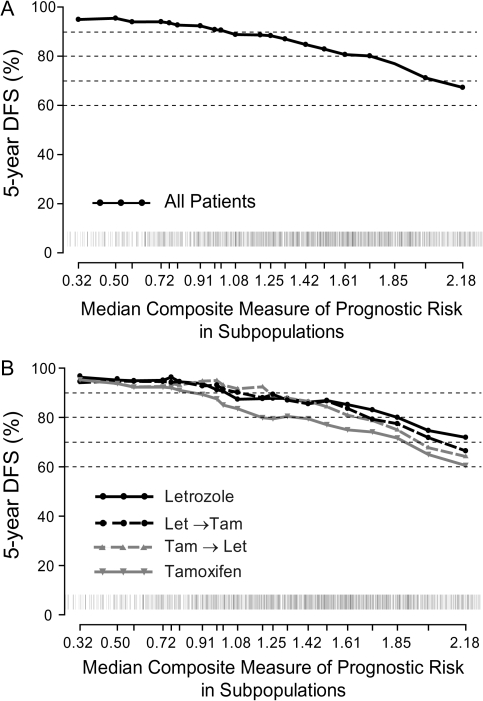
The prognostic value of the composite measure is illustrated by STEPP analysis in Figure 4A. Patients with the lowest values had 5-year DFS exceeding 90%, while those with the highest values had a 5-year DFS <70%. More importantly, the composite measure appeared to discriminate between the treatment arms (Figure 4B). Patients with low values had similar 5-year DFS regardless of treatment allocation; those with intermediate values showed better DFS with any of the three arms containing letrozole as compared with tamoxifen; and only in the highest range of values was there separation favoring 5 years of letrozole over each of the sequences and tamoxifen. Formal tests of treatment-by-composite risk interaction in a Cox model supported the observed pattern, particularly the heterogeneity of responsiveness between letrozole and the sequence of tamoxifen→letrozole or tamoxifen alone (P = 0.06 letrozole versus tamoxifen→letrozole; P = 0.39 letrozole versus letrozole→tamoxifen; P = 0.08 letrozole versus tamoxifen). Dividing the composite measure of prognostic risk into thirds of its distribution (i.e. tertiles) for convenience, the estimated 5-year DFS percents for letrozole monotherapy, letrozole→tamoxifen, tamoxifen→letrozole, and tamoxifen monotherapy were 96%, 94%, 93%, and 94%, respectively, among patients at lowest risk; 90%, 91%, 93%, and 86%, respectively, among patients at intermediate risk; and 80%, 76%, 74%, and 69%, respectively, among those at highest risk.
Figure 4.
STEPP analysis of 5-year DFS according to level of composite measure of prognostic risk (A) among all patients and (B) according to treatment assignment. Rug plots along the x-axis display the distribution of individual values. STEPP, subpopulation treatment effect pattern plot; DFS, disease-free survival.
discussion
Although aromatase inhibitors provide, on average, superior outcomes to tamoxifen in the adjuvant therapy for endocrine-responsive early breast cancer in postmenopausal women [1], their optimal use has yet to be defined. Cost may be an issue in some settings, and some patients may experience adverse events that make continuation of aromatase inhibitor therapy clinically difficult. We have therefore attempted to examine whether clinical and pathological features can identify patient groups in which it is more or less important to include or persist with aromatase inhibitor therapy.
The primary analysis presented here examined whether the level of ER, PgR, Ki-67 LI expression or HER2 positivity was predictive of the efficacy of letrozole monotherapy as compared with the two sequences, tamoxifen→letrozole and letrozole→tamoxifen. We did not see strong evidence that any of these markers, when considered individually, was clearly useful for treatment selection. By contrast, a composite measure of prognostic risk including these markers as well as recognized clinical prognostic factors appeared to have predictive value. Patients at highest risk did best when treated with 5 years of letrozole; any of the three letrozole-containing regimens was acceptable for those patients in an intermediate risk range; whereas patients at lowest risk did similarly well with letrozole monotherapy, a sequence of letrozole and tamoxifen, or tamoxifen monotherapy. For example, a typical low-risk BIG 1-98 patient for whom any of the four treatments may be considered was aged between 55 and 69 years at randomization who had negative lymph nodes with a <2 cm, grade 2, HER2-negative tumor without peritumoral vascular invasion and with strongly positive receptors and low Ki-67 (e.g. ER = 90%, PgR = 80%, Ki-67 < 14%) (composite value = 0.50). By contrast, a typical high-risk BIG 1-98 patient who should receive letrozole for 5 years was aged between 55 and 69 years who had 12 positive lymph nodes, a 2.5 cm, grade 2, HER2-negative tumor with peritumoral vascular invasion, strongly positive receptors and Ki-67 = 20% (composite value = 2.49).
A strength of this study was its basis in a prospective randomized clinical trial with pathological material centrally assessed for a large majority of patients [5177 of 6182 patients (84%) enrolled in the four-arm study design]. Our composite measure was developed based on the outcomes of patients in this single study and may not be directly applicable to other trials or patient cohorts. Nevertheless, our results clearly raise the general hypothesis that a composite assessment of risk may be useful in selecting from among patients with endocrine-responsive early breast cancer those who might particularly benefit from adjuvant aromatase inhibitor therapy either as monotherapy or as part of a sequence with tamoxifen. This hypothesis could be tested in other individual aromatase inhibitor trials, especially those with pathological material available from a substantial proportion of participating patients, or in the context of a multi-trial meta-analysis. Translational arimedex, tamoxifen alone or in combination (ATAC) trial investigators recently reported in abstract form [20] the development of a prognostic score based on quantitative immunohistochemical assessments of ER, PgR, Ki-67 and HER2 expressed as positive/negative in 1125 women. Although this score proved prognostically useful, data on its ability to predict the difference in outcome between anastrozole and tamoxifen in the ATAC trial have not been reported.
When investigating the interaction of a prognostic factor—or here the composite measure of prognostic risk—and treatment effects, the absolute differences in outcomes between treatment regimens, rather than the relative treatment effect, may be more useful for assessing the trade-off between treatment effectiveness (i.e. gains in DFS percent) and incidence of side-effects. Absolute differences are more discernable among patients at the higher end of the risk spectrum than at the lower end. Small but statistically undetectable differences may exist between treatment regimens at the lower end of the risk spectrum. None the less, our analysis supports the St Gallen panelists' preference for initiating treatment with aromatase inhibitors particularly in patients at higher risk of early relapse [11].
Overall, individual markers provided limited ability to inform selection among adjuvant letrozole-containing regimens or tamoxifen alone for postmenopausal women with early breast cancer. In the BIG 1-98 trial, a composite measure of ‘prognostic’ risk incorporating clinico-pathological data and biological markers was better able to ‘predict’ the relative treatment benefit of endocrine therapy regimens. Although the specific composite measure of prognostic risk derived here applies only in this trial, the principle that a composite assessment of risk is able to better inform treatment selection can be generalized to help physicians and patients when deciding on the best adjuvant endocrine treatment for the individual patient.
funding
Susan G. Komen for the Cure (Promise Grant KG080081); Novartis; National Cancer Institute at National Institutes of Health (CA075362); IBCSG (funded by Swedish Cancer Society; Cancer Council Australia; Australian New Zealand Breast Cancer Trials Group; Frontier Science and Technology Research Foundation; Swiss Group for Clinical Cancer Research; Foundation for Clinical Cancer Research of Eastern Switzerland).
disclosure
GV, EM and HM have received honoraria from Novartis. BT owns stock in Novartis. JFF has received honoraria from Astra Zeneca. None of the other authors have conflicts to declare.
Supplementary Material
Acknowledgments
We thank the many pathologists who submitted tumor blocks and slides, Rosita Kammler, and Stefania Andrighetto and the patients, physicians, nurses, and data managers who participated in the BIG 1-98 clinical trial. Presented in part elsewhere—San Antonio Breast Cancer Symposium, December, 2009.
References
- 1.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 2.Giobbie-Hurder A, Price KN, Gelber RD. Design, conduct, and analyses of Breast International Group (BIG) 1-98: a randomized, double-blind, phase-III study comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive, early breast cancer. Clin Trials. 2009;6:272–287. doi: 10.1177/1740774509105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breast International Group 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 4.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 5.Viale G, Regan MM, Maiorano E, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol. 2007;25:3846–3852. doi: 10.1200/JCO.2007.11.9453. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen BB, Regan MM, Lykkesfeldt AE, et al. Centrally assessed HER2 status in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal women with early breast cancer: results from study BIG 1-98. Lancet Oncol. 2008;9:23–28. doi: 10.1016/S1470-2045(07)70386-8. [DOI] [PubMed] [Google Scholar]
- 7.Viale G, Giobbie-Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–5575. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breast International Group 1-98 Collaborative Group. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361:22–32. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khleif SN, Doroshow JH, Hait WN AACR-FDA-NCI Cancer Biomarkers Collaborative. AACR-FDA-NCI Cancer Biomarkers Collaborative consensus report: advancing the use of biomarkers in cancer drug development. Clin Cancer Res. 2010;16:3299–3318. doi: 10.1158/1078-0432.CCR-10-0880. [DOI] [PubMed] [Google Scholar]
- 10.Regan MM, Colleoni M, Giobbie-Hurder A, et al. Adjusting for selective crossover in analyses of letrozole (Let) versus tamoxifen (Tam) in the BIG 1-98 trial. Cancer Res. 2009;69(24 Suppl 3):488s. (Abstr 16) [Google Scholar]
- 11.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheang MCU, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonetti M, Gelber RD. A graphical method to assess treatment-covariate interactions using the Cox model on subsets of the data. Stat Med. 2000;19:2595–2609. doi: 10.1002/1097-0258(20001015)19:19<2595::aid-sim562>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Lazar AA, Cole BF, Bonetti M, et al. Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: subpopulation treatment effect pattern plot. J Clin Oncol. 2010;28:4539–4544. doi: 10.1200/JCO.2009.27.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldhirsch A, Wood WC, Gelber RD, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 16.Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 Trial. J Clin Oncol. 2009;27:1168–1176. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 19.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuzick J, Dowsett M, Wale C, et al. Prognostic value of a combined ER, PgR, Ki67, HER2 immunohistochemical (IHC4) score and comparison with the GHI recurrence score—results from TransATAC. Cancer Res. 2010;69:74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.