Abstract
The yellow fever 17D (YF-17D) vaccine is one of the most efficacious vaccines developed to date. Interestingly, vaccination with YF-17D induces IFN-γ production early after vaccination (d 5–7) before the development of classical antigen-specific CD8+ and CD4+ T cell responses. Here we investigated the cellular source of this early IFN-γ production. At days 5 and 7 post vaccination activated CD8+ gamma-delta TCR T cells produced IFN-γ and TNF-α. Activated CD4+ T cells produced IFN-γ and TNF-α at day 7 post vaccination. This early IFN-γ production was also induced after vaccination with recombinant YF-17D (rYF-17D), but was not observed after recombinant Adenovirus type 5 (rAd5) vaccination. Early IFN-γ production, therefore, might be an important aspect of yellow fever vaccination.
Keywords: Yellow Fever vaccine, IFN-γ, Rhesus model
Introduction
The live-attenuated yellow fever vaccine 17D (YF-17D) was derived by serial passages of the Asibi yellow fever wild-type virus. This vaccine is one of the safest and most effective vaccines currently available, with a single injection conferring protection for at least 10 years [1].
Early events after YF-17D vaccination in humans may be crucial to the development of acquired immune responses against the virus [2, 3]. YF-17D stimulates several innate immune responses [4–7], which lead to the induction of efficient acquired immunity, with high titers of neutralizing antibodies and robust CD8+ T cell responses. YF-17D vaccination stimulates both arms of the acquired immune response, inducing lymphocytes that produce TH1/TH2 mixed cytokines [8, 9].
Since the yellow fever vaccine induces such efficacious immune responses, interest in the potential use of the YF-17D virus as a vector for the development of new vaccines is increasing. However, the cellular and molecular mechanisms by which this virus elicits such broad immunity remains unclear, both in humans or in animal models used to test the new recombinant vaccines.
We had previously found that YF-17D vaccination of Indian rhesus macaques induced massive IFN-γ expression at 7 days post vaccination, before the induction of classical YF-specific T cell responses [10]. In the present study, we have characterized two cellular sources of this IFN-γ production early after 17D vaccination of Indian rhesus macaques.
Material and Methods
Study groups and Blood samples
For the initial studies we used frozen, Ficoll-purified peripheral blood mononuclear cell (PBMC) samples from six rhesus macaques (Macaca mulatta) of Indian origin immunized subcutaneously with recombinant YF-17D (rYF-17D) virus. The rYF-17D viruses were constructed as described previously [10, 11] and expressed inserts from Gag, Vif and Nef proteins of the SIVmac239 virus.
In the kinetics studies, we used freshly isolated PBMC from six rhesus monkeys subcutaneously vaccinated with YF-17D virus. Additionally, we used fresh samples from ten macaques immunized intramuscularly with recombinant Adenovirus serotype 5 (Ad5) expressing SIV proteins. All of the animals were assigned to protocols approved by the University of Wisconsin Institutional Animal Care and Use Committee and cared for according to the NIH “Guide to the Care and Use of Laboratory Animals.”
Monoclonal Antibodies
We used mouse anti-human monoclonal antibodies cross-reacting with rhesus macaque cell surface markers for six- or seven-color flow cytometric assays. We purchased anti-CD8 alpha-Pacific Blue (Clone RPA-T8), anti-CD16 PE (Clone 3G.8), anti-CD16 PerCP-Cy5.5 (Clone 3G.8), anti-IL-4 PE (Clone 8D4-8), anti-CD4 PerCP-Cy5.5 (Clone L-200), anti-IFN-γ-FITC (Clone 4S.B3), anti-HLA-DR APC-Cy7 (Clone L243), anti-CD20 PerCP-Cy5.5 (Clone L27), anti-CD3 PE-Cy7 (Clone SP34-2), anti-TNF-α APC (Clone MAb11), anti-CD11c (Clone S-HCL-3), anti-CD14 Alexa700 (Clone M5E2) from BD Biosciences (San Diego, CA, USA), anti-CD4 APC (Clone M-T466) from Miltenyi Biotec Inc. (Auburn, CA), anti-CD69 PE-TexasRed (Clone TP-1-55-3) from Beckman Coulter (Fullerton, CA, USA) and anti-gamma-delta TCR PE (Clone 5A6.E9) from Caltag (Carlsbad, CA).
Detection of IFN-γ and IL-4 cellular immune responses by flow cytometry
We incubated 5.0 × 105 PBMC with 10 μg of brefeldin A (Sigma-Aldrich, ST. Louis, MO) per ml for 6 hours at 37°C in 100 μl of R10 [RPMI 1640 containing 10% fetal calf serum, 2 mM L-Glutamine, 10 U/ml Penicillin G, 10 μg/ml Streptomycin, and 0.025 μg/ml Amphotericin B (HYClone, Logan, UT)] to prevent protein transport from the Golgi apparatus. After the incubation period we washed and stained the cells for selected surface markers (CD3, CD8, CD4, CD16, CD20, HLA-DR, CD11c, CD14 or gamma-delta TCR) and fixed overnight in 2% paraformaldehyde at 4°C. On the following day, we permeabilized the cells with 0.1% saponin in PBS containing 10 % FCS (HyClone, Logan UT) and stained them for intracellular expression of CD69 and the cytokines gamma-interferon (IFN-γ) or interleukin-4 (IL-4) and TNF- After staining, we washed the cells with PBS containing 10 % FCS and fixed in 2% paraformaldehyde for at least 30 minutes at 4°C. We collected 1 – 1.5 × 105 events within the lymphocyte gate with FASCDiva 6.0 software on a custom made BD LSR II flow cytometer (Becton Dickinson, San Jose, Calif.) We analyzed the data with FlowJo v.8.7.3. (Treestar Inc., Ashland, OR).
Statistical analyses
We performed statistical analyses using paired non-parametric ANOVA (Friedman test) and Dunns post-test with 95% confidence interval for multiple comparisons between days 0, 3, 5 and 7 after vaccination or non-parametric ANOVA (Kruskall-Wallis) and Dunns post-test with 95% confidence interval, for group comparisons, using the GraphPad Prism 5.0 software package (GraphPad Software Inc, San Diego, CA USA). In all cases, the differences were considered significant when p-values were ≤ 0.05.
Results
Intracellular IFN-γ and IL-4 detection after vaccination with YF-17D
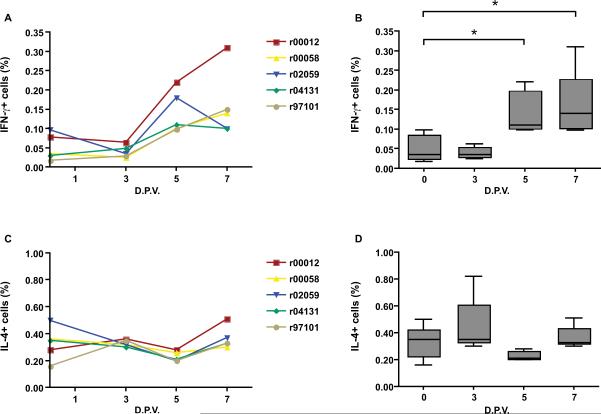
During previous IFN-γ ELISPOT assays to assess YF-specific cellular immune responses in rhesus macaques, we detected massive early IFN-γ production by lymphocytes at day 7 in wells that had not been stimulated by YF-derived peptides. We wished, therefore, to determine the cellular source of this early IFN-γ production. YF-17D vaccination in humans induces polyvalent immune responses [4, 8, 9]. We, therefore, investigated the kinetics of production of the two major TH1/TH2 cytokines, IFN-γ and IL-4 respectively, in rhesus peripheral blood mononuclear cells (PBMC). In concordance with our IFN-γ ELISPOT results, we detected IFN-γ producing cells in the lymphocyte gate at 5 and 7 days post-vaccination in all vaccinated monkeys (Fig.1A). IFN-γ production increased significantly from day 0 (Fig.1B). By contrast, we saw no differences in IL-4 production during the first week post infection (Fig.1C&D).
Figure 1.
Intracellular IFN-γ and IL-4 production by lymphocytes after YF-17D vaccination in rhesus macaques. PBMCs were analyzed by multi-color flow cytometry for IFN-γ and IL-4 production in the CD3+ cell gate. Panels A and C show the kinectics of IFN-γ and IL-4 production for each monkey, respectively. Medians for each day are represented in a box plot format (B and D). Boxes represent the 25th to the 75th percentile and the median is shown as a line across the box. D.P.V.- days post-vaccination. **P<0.01, *P<0.05.
CD8+ and CD4+ cells synthesize IFN-γ
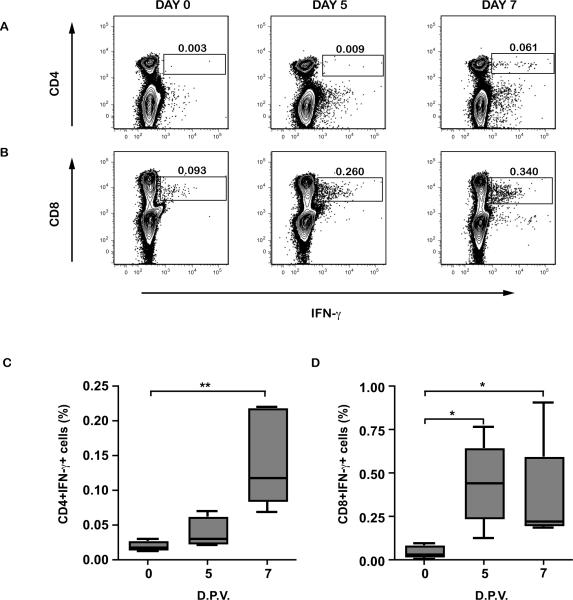
We then determined which cell type produced IFN-γ. CD8+ T cells appear to be the first IFN-γ source on day 5 after vaccination (Fig.2B&D). On day 7 both CD4+ and CD8+ T cell populations produced IFN-γ (Fig.2A, B, C & D). Surprisingly, we could not observe any IFN-γ coming from NK cells subset (data not shown).
Figure 2.
Intracellular IFN-γ production by CD4+ and CD8+ cells. Representative contour plots for increased IFN-γ detection in CD4+ (A) and CD8+ (B) cells at days 0, 5, and 7 after YF-17D vaccination. PBMCs from vaccinated rhesus macaques were incubated for 6 hours and analyzed for intracellular IFN-γ expression by flow cytometry within CD4+ (C) or CD8+ (D) gates. Box plots show medians for each day. D.P.V.- days post-vaccination. **P<0.01, *P<0.05.
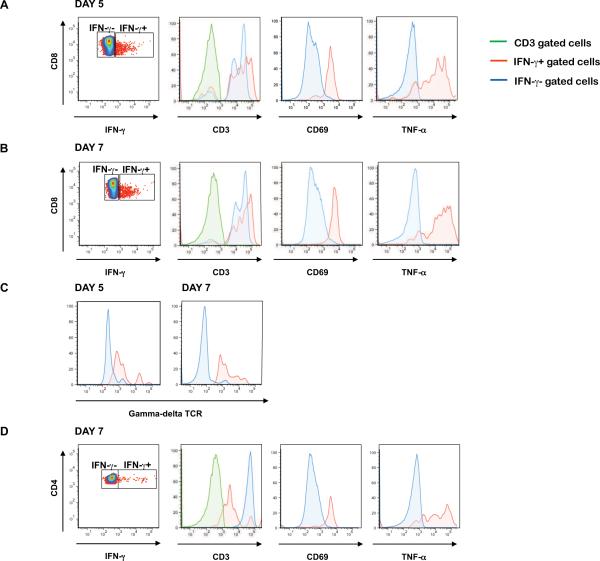
We further characterized these IFN-γ-producing cell populations for the expression of CD3 and the gamma-delta TCR, and the activation markers CD69 and HLA-DR. We also assessed the ability of these IFN-γ-producing lymphocytes to elaborate TNF-α and IL-6. The CD8+ and CD4+ IFN-γ-producing populations were also CD3+, indicating that they are indeed T cells (Fig. 3A, B & D). The CD8+, CD3+, IFN-γ-producing T cells also expressed the gamma-delta TCR (Fig. 3C), indicating that these innate immune cells were the first IFN-γ source. Additionally, the CD8+ and CD4+ populations were recently activated, as demonstrated by their CD69 expression (Fig. 3A & B). Despite the clear activation observed in these cell subsets, there was no increase in the percentages of CD3, CD4, CD8 and gamma-delta T cells (data not shown). The IFN-γ-producing CD8+ and CD4+ cells also synthesized TNF-α (Fig. 3A, B & D). These IFN-γ-producing cells did not express HLA-DR or produce IL-6 (data not shown). Thus, at days 5 and 7, the IFN-γ-producing CD8+ T cells are activated, produce TNF-α, and express the gamma-delta TCR. At day 7, CD4+ T cells also produce IFN-γ and these lymphocytes are activated and synthesize TNF-α.
Figure 3.
Characterization of the IFN-γ-producing cell populations after YF-17D vaccination in rhesus monkeys. Expression of the T cell marker CD3, the very early activation marker CD69 and TNF-α cytokine by IFN-γ+ (red) and IFN-γ−-(blue) populations within the CD8+ lymphocyte subset five (A) and seven (B) days after vaccination. The gated CD8+ cells were also analyzed for gamma-delta TCR expression (C). Expression of CD3, CD69 and TNF-α by IFN-γ+ (red) and IFN-γ− (blue) populations within the CD4+ lymphocyte subset seven (D) days after vaccination.
Early IFN-γ production by the CD4+ and CD8+ T cell population is a feature of YF-17D vaccination
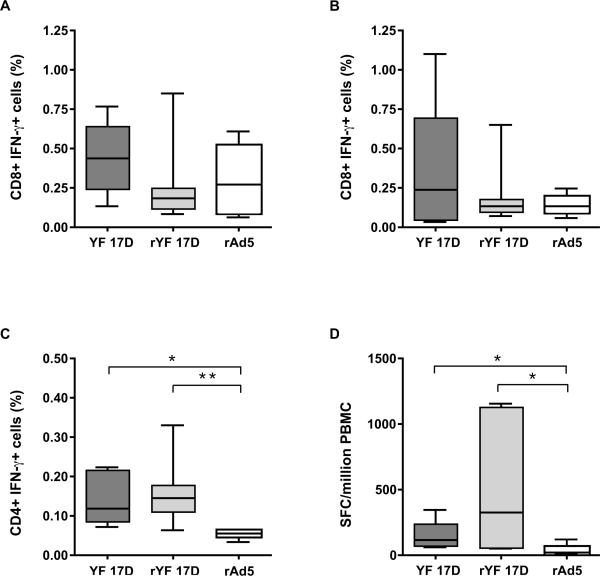
We next investigated whether this unusual early IFN-γ production was a feature of yellow fever vaccination or was common also to other viral infections. For this purpose, we analyzed PBMC samples from monkeys vaccinated with YF-17D, rYF-17D and rAd5. As shown in figure 4, there were no significant differences in intracellular IFN-γ detection in the CD8+ population between the YF-17D, rYF-17D, and rAd5 vaccinees, either on day 5 (A) or on day 7 (B) after vaccination. There was, however, a trend towards early IFN-γ production in the YF-17D and rYF-17D-vaccinated animals. Furthermore, as shown in table 1, there was an increase in the percentage of IFN-γ gamma-delta+ T cells in YF-17D vaccinated animals. This was not observed for Ad-5 vaccinated animals. We also observed a significant increase in IFN-γ detection in the CD4+ T cell population seven days after YF-17D or rYF-17D vaccination compared to rAd-5 vaccination (Fig.4 C&D). These cells were already producing IFN-γ on day 7 after vaccination, as shown by the significant number of spot-forming cells in unstimulated wells during ELISPOT assays in both the YF-17D and the rYF-17D vaccinees but not in the rAd5 vaccinees (Fig.4D).
Figure 4.
IFN-γ-producing cells after vaccination with YF-17D, recombinant YF-17D, and recombinant Ad5 virus. Panels A and B show the frequency of CD8+ IFN-γ+ cells on days 5 and 7 after YF-17D, recombinant YF-17D (rYF-17D), or recombinant Adenovirus type 5 (rAd5) vaccination in rhesus monkeys. The percentage of CD4+IFN-γ+ cells measured seven days after vaccination with each virus is shown in panel C. (D) Number of spot-forming cells in unstimulated wells detected seven days after vaccination (IFN-γ ELISpot assay). The median is shown as a line across the box. **P<0.01, *P<0.05.
Table 1.
Proportion of IFN-γ+ cells in the γδ T cell subset
| Days post-vaccine |
||||
|---|---|---|---|---|
| Vaccine | Animal | 0 | 5 | 7 |
| YF | ||||
| r04109 | 1.23 | 2.30 | 5.74 | |
| r04136 | 0.63 | 1.45 | 1.47 | |
|
| ||||
| Ad5 | ||||
| r03105 | 1.56 | 1.00 | 0.14 | |
| r04149 | 9.82 | 12.00 | 9.94 | |
| r05073 | 1.65 | 1.35 | 0.56 | |
| r05086 | 9.00 | 4.71 | 2.12 | |
Data are expressed in percentages.
Discussion
It has been suggested that early events after yellow fever vaccination are crucial to the development of adequate acquired immunity [2, 3, 12]. Unfortunately, it is still unclear which early events are important in the development of efficient YF-specific memory responses. Here, we focused on IFN-γ production during the first days after yellow fever vaccination in Indian rhesus macaques, a relevant animal model for pre-clinical trials of vaccine development.
IFN-γ is one of the most important molecules in the immune system with many different functions [13]. In our study, we detected intracellular IFN-γ in the lymphocyte gate, which peaked at days 5 and 7, indicating a possible role for this cytokine early after YF-17D vaccination. Similarly, an increase in IFN-γ levels in human serum after YF-17D vaccination has already been demonstrated [7]. IFN-γ is recognized as a hallmark of the TH1 subset of helper T cells and activates macrophages, NK and CD8+ T cells. It is noteworthy that all of these cell types are activated after yellow fever vaccination in humans and this activation has been implicated in vaccine viral clearance. This may limit viral spread, which may help to prevent serious viscerotropic adverse events [6–8, 14]. On the other hand, it has also been shown that IFN-γ may drive humoral immune responses, redirecting B cells from proliferation towards differentiation, promoting isotype switching to certain IgG subclasses [reviewed in 13]. In this regard, only IgG2a antibodies (classically elicited by IFN-γ in mice) directed against the yellow fever Envelope (E) and Non-structural 1 (NS-1) proteins, but not IgG1, were able to protect mice against encephalitis after intracerebral challenge with YF-17D [15, 16].
IFN-γ is produced by several cell types, within the innate and adaptive immune response, after microbial stimulation [13, 17]. We, therefore, investigated which cell types were responsible for early IFN-γ production during the first week after yellow fever vaccination. We demonstrated that gamma-delta TCR+ CD8+ T cells were likely the first source of IFN-γ in response to YF-17D. Surprisingly, we could not see any IFN-γ production in the NK cells subset, despite the clear activation (CD69 expression) of this population within the first week after monkey vaccination (data not shown), as has been observed previously in human cells [7]. Gamma-delta TCR+ CD8+ T cells which produced IFN-γ were recently activated, as demonstrated by CD69 expression, and also elaborated TNF-α. In line with our findings, another group has shown that human gamma-delta TCR+ T cells cultured with YF-17D-infected dendritic cells (DCs) expressed CD69 and released IFN-γ [18]. Activation of gamma-delta TCR+ T cell subsets has also been observed in other infections caused by flaviviruses, in humans and animal models, such as Hepatitis C virus (HCV), GB-type C virus (GBC-V) and West Nile virus (WNV) [19]. During acute WNV infections in mice, gamma-delta TCR+ T cells released IFN-γ early following infection. Early IFN-γ production, in this model, was responsible for protection against encephalitis caused by WNV [20]. It was proposed that, in this WNV model of protection, gamma-delta TCR+ T cells regulate DC maturation, initiating CD4+ T cell priming, in large part due to release of IFN-γ and TNF-α [21]. Indeed, in our study, IFN-γand TNF-α production by gamma-delta TCR+ CD8+ T cells was followed by IFN-γ detection in the CD4+ T cell population two days later. CD4+ IFN-γ-producing cells were activated and also produced TNF-α, indicating that they were probably derived from the TH1 lineage. These CD4+ TH1 cells induced by YF vaccination may be responsible for the CD8+ T cell activation and differentiation observed both in humans or in rhesus monkeys [10, 14].
We have shown that early IFN-γ production by gamma-delta T cells and CD4 T cells was also seen after vaccination with the recombinant YF-17D virus. This was not observed with recombinant Ad5, suggesting that this early activation might be an aspect of the yellow fever vaccine, which is a replication-competent vector. In this regard, previous work has shown that human gamma-delta T cell activation and IFN-γ production induced by the YF-17D virus was mediated by the cross-talk between these cells and plasmacytoid DCs. This involved replicative nucleic acids engagement of TLRs 7/8 and 9 and type I IFN production, indicating that the replication capacity of this virus may be crucial for triggering this early response. A role of Toll receptors for nucleic acids sensing in innate immune cells activation after YF-17D stimulation/vaccination has been also demonstrated for human NK cells and DCs [5,7,18]. The early IFN-γ release in the context of yellow fever vaccination probably results in induction of CD4 T cells committed to TH1 responses observed for yellow fever vaccinated animals, but not in Ad-5 vaccinated animals in the present study. In fact, results obtained in mice immunized with two different rYF-17D carrying HIV-p24 antigen have shown that YF-17D is able to induce CD4 as well as CD8 T cell specific responses, comparing to rAd-5, which induced largely CD8 T cell responses [22].
Taken together, these data demonstrate that IFN-γ is produced early after yellow fever vaccination in rhesus monkeys by gamma-delta T cells and CD4 T cells. This early IFN-γ production is also induced after vaccination with recombinant YF, but was not observed after recombinant Ad5 vaccination, indicating it may be dependent on virus replication. Therefore, IFN-γ production within the first week after vaccination might be an important aspect of the yellow fever vaccine and probably plays an important role in helping the development of classical CD8+ T cell responses and the production of neutralizing antibodies.
Acknowledgments
This work was supported by National Institutes of Health grant R01 AI076114 (to D.I.W.) and Grant P51 RR000167 from the National Center for Research Resources, a component of the National Institutes of Health (to the Wisconsin National Primate Research Center). This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grants RR15459-01 and RR020141-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].WHO Assessment of yellow fever epidemic risk- a decision- making tool for preventive imunization campaigns. Weekly epidemiological record. 2007;18(82):153–60. [PubMed] [Google Scholar]
- [2].Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–25. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205(13):3119–31. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barba-Spaeth G, Longman RS, Albert ML, Rice CM. Live attenuated yellow fever 17D infects human DCs and allows for presentation of endogenous and recombinant T cell epitopes. J Exp Med. 2005;202(9):1179–84. doi: 10.1084/jem.20051352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203(2):413–24. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Martins MA, Silva ML, Eloi-Santos SM, Ribeiro JG, Peruhype-Magalhaes V, Marciano AP, et al. Innate immunity phenotypic features point toward simultaneous raise of activation and modulation events following 17DD live attenuated yellow fever first-time vaccination. Vaccine. 2008;26(9):1173–84. doi: 10.1016/j.vaccine.2007.12.035. [DOI] [PubMed] [Google Scholar]
- [7].Neves PC, Matos DC, Marcovistz R, Galler R. TLR expression and NK cell activation after human yellow fever vaccination. Vaccine. 2009;27(41):5543–9. doi: 10.1016/j.vaccine.2009.07.028. [DOI] [PubMed] [Google Scholar]
- [8].Martins MA, Silva ML, Marciano AP, Peruhype-Magalhaes V, Eloi-Santos SM, Ribeiro GL, et al. Activation/modulation of adaptive immunity emerges simultaneously after 17DD yellow fever first-time vaccination: is this the key to prevent severe adverse reactions following immunization? Clin Exp Immunol. 2007;148(1):90–100. doi: 10.1111/j.1365-2249.2006.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Santos AP, Matos DC, Bertho AL, Mendonca SC, Marcovistz R. Detection of Th1/Th2 cytokine signatures in yellow fever 17DD first-time vaccinees through ELISpot assay. Cytokine. 2008;42(2):152–5. doi: 10.1016/j.cyto.2008.02.007. [DOI] [PubMed] [Google Scholar]
- [10].Bonaldo MC, Martins MA, Rudersdorf R, Mudd PA, Sacha JB, Piaskowski SM, et al. Recombinant yellow fever vaccine virus 17D expressing simian immunodeficiency virus SIVmac239 gag induces SIV-specific CD8+ T-cell responses in rhesus macaques. J Virol. 84(7):3699–706. doi: 10.1128/JVI.02255-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bonaldo MC, Mello SM, Trindade GF, Rangel AA, Duarte AS, Oliveira PJ, et al. Construction and characterization of recombinant flaviviruses bearing insertions between E and NS1 genes. Virol J. 2007;4:115. doi: 10.1186/1743-422X-4-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009;9(10):741–7. doi: 10.1038/nri2629. [DOI] [PubMed] [Google Scholar]
- [13].Billiau A, Matthys P. Interferon-gamma: a historical perspective. Cytokine Growth Factor Rev. 2009;20(2):97–113. doi: 10.1016/j.cytogfr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- [14].Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- [15].Schlesinger JJ, Foltzer M, Chapman S. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology. 1993;192(1):132–41. doi: 10.1006/viro.1993.1015. [DOI] [PubMed] [Google Scholar]
- [16].Schlesinger JJ, Chapman S. Neutralizing F(ab')2 fragments of protective monoclonal antibodies to yellow fever virus (YF) envelope protein fail to protect mice against lethal YF encephalitis. J Gen Virol. 1995;76(Pt 1):217–20. doi: 10.1099/0022-1317-76-1-217. [DOI] [PubMed] [Google Scholar]
- [17].Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- [18].Devilder MC, Allain S, Dousset C, Bonneville M, Scotet E. Early triggering of exclusive IFN-gamma responses of human Vgamma9Vdelta2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J Immunol. 2009;183(6):3625–33. doi: 10.4049/jimmunol.0901571. [DOI] [PubMed] [Google Scholar]
- [19].Poccia F, Agrati C, Martini F, Capobianchi MR, Wallace M, Malkovsky M. Antiviral reactivities of gammadelta T cells. Microbes Infect. 2005;7(3):518–28. doi: 10.1016/j.micinf.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shrestha B, Wang T, Samuel MA, Whitby K, Craft J, Fikrig E, et al. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol. 2006;80(11):5338–48. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fang H, Welte T, Zheng X, Chang GJ, Holbrook MR, Soong L, et al. gammadelta T cells promote the maturation of dendritic cells during West Nile virus infection. FEMS Immunol Med Microbiol. 2010 Jun 1;59(1):71–80. doi: 10.1111/j.1574-695X.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Franco D, Li W, Qing F, Stoyanov CT, Moran T, Rice CM, Ho DD. Evaluation of yellow fever virus 17D strain as a new vector for HIV-1 vaccine development. Vaccine. 2010 Aug 9;28(35):5676–85. doi: 10.1016/j.vaccine.2010.06.052. [DOI] [PubMed] [Google Scholar]