Abstract
Objectives
To examine the association of employment and work schedule with shorter DNA telomeres, a marker of cellular ageing and disease risk factor, and consider whether differences were related to health, behaviours and sociodemographic factors, or varied by stress levels or menopausal status.
Methods
This cross-sectional analysis of 608 women aged 35–74 in the Sister Study examined determinants of relative telomere length (rTL) measured by quantitative PCR in leucocyte DNA. Age-adjusted regression models estimated base pair (bp) rTL differences for current and lifetime schedule characteristics (ie, part-time, full-time or overtime hours; multiple jobs; irregular hours; shiftwork; work at night). Covariates included race, smoking, perceived stress, sleep, physical activity, health and menopausal status, education, marital status, live births, children under 18, measured body mass index and urinary stress hormones.
Results
Compared with non-employed women with moderate or substantial past work histories (n=190), those currently working full-time (n=247; median 40 h/week) had a shorter rTL, an age-adjusted difference of −329 bp (95% CI −110 to −548). Longer-duration full-time work was also associated with shorter rTL (age-adjusted difference of −472 bp, 95% CI −786 to −158 for 20+ vs 1–5 years). Findings were not explained by health and demographic covariates. However, rTL differences for working at least full-time were greater in women with higher stress and epinephrine levels.
Conclusions
Current and long-term full-time work were associated with shorter rTL, with differences of similar magnitude to smoking and history of heart disease or diabetes. Longitudinal data with specific stress measures are needed to further evaluate the impact of work schedule on rTL.
INTRODUCTION
Repetitive telomere DNA sequences cap and protect the ends of chromosomes; critically short telomeres may lead to cellular senescence or carcinogenic transformation.1 Average relative leucocyte telomere length (rTL) may provide a cumulative marker of cellular ageing integrating across multiple pathways, including DNA damage due to oxidative stress and the replication history of various populations of white blood cells.2 Shorter telomeres have been associated with mortality, chronic diseases and lifestyle risk factors, such as obesity, smoking and perceived stress.3–7 External circumstances and intrinsic factors experienced over time can be chronic stressors and have a cumulative effect on rTL. For example, duration of care-giving for chronically ill children was associated with shorter telomeres.4
Structural aspects of employment and work schedule are potential stressors experienced over the adult lifespan. Long hours or overtime, working multiple jobs, rotating shift-work or irregular hours, and work at night, may be related to stress, inadequate sleep, and other behavioural and physiological risk factors for chronic disease.8, 9 Stress may also arise from cumulative or competing demands of paid work and informal (eg, familial) care-giving.10
We explored the association of current and lifetime work schedule characteristics with rTL in women aged 35–74, and examined whether schedule-related rTL differences were explained by differences in demographics, health behaviours or health status. We also considered whether associations varied by menopausal status, a marker of ovarian ageing and pronounced hormonal differences, or by urinary stress hormones and current perceived stress measures associated with shorter rTL in this sample.11
METHODS
Study sample
The study sample comprised 647 women selected from the first 2086 enrollees of The Sister Study, a prospective cohort study of women aged 35–74 who have a sister with breast cancer. Originally sampled for a study on perceived stress and neuroendocrine hormones, the sample was enriched for higher perceived stress, non-white race and current smoking; 23 women (~1%) who reported current rotating shift-work were excluded to facilitate interpretation of urinary hormone data.11 Women who had recent major dental work/surgery, chemotherapy/radiation for cancer or new diagnosis of breast cancer between enrolment and sample selection were also excluded. The median age was 53 years, 83% were non-Hispanic white, 7% were non-Hispanic black, 2% were Hispanic and 7% were other race/ethnicity.11 Ethics approval was granted by the Institutional Review Board of the National Institute of Environmental Health Science, NIH, and participants gave written informed consent at enrolment.
Telomere assays
Whole blood was collected during a home visit, shipped and stored at −80°C until DNA was extracted for a PCR-based assay of rTL, with full assay details previously described.11, 12 Samples were amplified for telomeric DNA and a single copy gene using 100–200 ng template DNA. The assay determines the amount of telomeric DNA (T) relative to a single-copy control gene DNA (S) to obtain a relative T/S ratio. Each specimen was assayed in triplicate, replicated on two plates with three known controls to adjust sample T/S ratios for variation across plates. Across intraplate replicates, R2 values for telomere and single-copy genes were ≥0.98; slopes ranged from −3.201 to −3.626 for standard curves constructed as previously described.12 The coefficient of variation (CV) across adjusted interplate replicates (T/S ratios adjusting for plate variation) was 8.5%. Averaged T/S ratios of adjusted replicates used in present analyses were normally distributed (mean 1.32; SD 0.25). For ease of comparing effect size differences, rTL base pairs (bp) were estimated by multiplying T/S ratios by 4270 bp (mean 5618; SD 1069), a conversion formula derived in the same laboratory for a previous study.12
Urinary catecholamines and cortisol
First morning urine specimens were collected on the same day as the blood draw,11 shipped cold and stored at −80°C. Quantitative assessment of epinephrine and norepinephrine was conducted by enzyme immunoassay (Total CAT EIA, Alpco Diagnostics, Windham, New Hampshire). Each specimen was run once, and each plate included two known controls. CVs for low and high controls across 11 plates ranged from 12% to 19% for epinephrine and from 19% to 28% for norepinephrine. Controls performed within expected ranges based on kit specifications. Urine samples were aliquoted, refrozen, shipped overnight and maintained at −80°C prior to evaluation of urinary free cortisol by radioimmunoassay (in the laboratory of C. Kirschbaum). Each specimen was run once; high, medium and low controls were included on each plate with % CV across 16 plates ranging from 14% (high) to 31% (medium) and 29% (low) for the controls. Hormone concentrations were directly adjusted for creatinine values.11
Questionnaire and measured data
Questionnaire data were collected by telephone interview. Weight and height were measured during home visits.
Work schedule
A lifetime job history included total number of current jobs of at least 10 h per week, and for each job, number of hours per week worked and whether the schedule was characterised by irregular hours or rotating shifts. For rotating shifts or irregular hours, participants were asked frequency of work at night (at least 1 h between 00:00 and 02:00). For women working regular hours, work at night was assessed through reported stop and start times. For non-employed participants, status was specified as homemaker, student, unemployed, retired or other. Past history included all jobs of at least 10 h per week held since age 18 for at least 12 months. Job-specific details included start and stop dates, work in rotating shifts or irregular hours, and at night.
Other variables
Current psychological stress was evaluated using the four-item Perceived Stress Scale.13 Other variables included age, body mass index from measured weight and height, smoking status (current, former, never), regular leisure time physical activity (in the past 12 months), average number of hours of sleep (in the past 6 weeks). Sociodemographic factors included race/ethnicity (white, black, Hispanic/other), education, household income, marital status and children under the age of 18 in the household. The number of children born and age at first birth were derived from the lifetime reproductive history. Menopausal status was based on self-report. Health status included self-reported doctor-diagnosed cardiovascular disease, diabetes and self-rated health.
Statistical analyses
Analyses were conducted using SAS Version 9.2. Work hours were categorised in equivalents of 8 h days per week: part-time was defined as less than or equal to 3 days or 24 h per week (median=20), full-time as more than 3 up to 5 days or 25 to 40 h per week (median=40) and overtime as more than 5 days or 40 h per week (median=50). The total number of years of each schedule characteristic (hours, irregular schedule, shift-work and work at night) were summed across the work history, taking into account multiple jobs overlapping 1 year or longer and creating a summary variable for years worked in multiple jobs. Consistency checks and cleaning during collection and processing minimised missing data on start/stop dates; the most common missing data were ‘month,’ which was estimated based on other reported dates or assigned to midyear. The job duration data (start/stop year) was missing on only seven of 3403 jobs (0.2%).
Currently non-employed women were categorised based on proportion of total years worked between age 18 and current age or age 65; past employment history was defined as minimal ≤0.25, moderate=0.25–0.75 or substantial ≥0.75. Because reasons for not working were unavailable, we excluded women with a minimal past work history (n=39) from the primary analysis sample (n=608) to increase comparability with respect to baseline work ability. Two sets of sensitivity analyses were also conducted: (1) including the 39 women with minimal work histories (total n=647) and (2) limiting to a more homogenous subset of women past childbearing but before typical retirement age (ages 45–64) who did not report heart disease, diabetes or poor health (total n=339).
Linear regression was used to model associations of current and past work schedule characteristics with rTL; negative β coefficients indicate an association with shorter telomeres. We estimated rTL differences for current work hours (part-time, full-time or overtime) and other schedule characteristics (irregular hours, multiple jobs or work at night) compared with non-employed women, adjusting for age and total years worked. Models were then limited to women currently working (referent=part-time). Multivariate models were run also, adjusting for sample selection variables (perceived stress, race and smoking status), health behaviours (body mass index, sleep and physical activity), health status (self-reported health, cardiovascular disease or diabetes), demographic factors (education, marital status, children at home under age 18, number of live births) and total years worked (any schedule type). Covariates considered were previously associated with rTL in the sample or other studies, or based on prespecified hypotheses on mediating effects or potential contribution to schedule related stress (eg, number of children). We explicitly examined the effect of adjusting for factors that might be considered pathway intermediates. We also examined rTL differences across categories of lifetime years of full-time work and other schedule characteristics (overtime, irregular hours, rotating shifts, work at night) adjusting for age and total years worked and in multivariate models. Among women who worked full-time at least 1 year, we tested the linear association per year of full-time work adjusting for age. Finally, we estimated mean rTL for current work hours, lifetime years of full-time work, and 10 or more years working multiple jobs/shiftwork/at night, overall and stratified by menopausal status.
We examined rTL differences stratified by the median cut-points for perceived stress and epinephrine, norepinephrine and cortisol levels.11 Models were run including two- and three-way product terms to explore potential interactions of current work schedule, epinephrine and perceived stress. We also explored work-related rTL differences in analyses stratified by socioeconomic factors (education and income) and childbearing history (age at first birth and number of children born).
RESULTS
Most women were currently employed (n=419; 69%), and most non-employed women with a previous moderate or substantial work history were retired (n=112; 59% of 190) or homemakers (n=58; 30%); a small number reported being unemployed (n =12), students or having other reasons for non-employment (n=7). Compared with non-employed women, employed women tended to be younger (46% vs 19% aged 45–54 years, and 4% vs 38% >65 years), have children at home (30% vs 18%) and have less cardiovascular disease or diabetes (16% vs 23%); however, they were more likely to smoke (28% vs 17%) and report no regular physical activity (23% vs 13%). Employed women were less likely to have a highschool education or less (12% vs 22%) or have higher household incomes (25% vs 46% over $100 000). Employed and non-employed women had a similar total number of years worked (average 25.6 vs 24.5 years), while employed women were more likely to report having held multiple jobs (24% vs 14%) or working at night (28% vs 19%). Most employed worked at least full-time (n=247 full-time, 121 overtime and 50 part-time; table 1). Part-time workers tended to be older (54 years old) and were less likely to have children at home (16% vs 35% full-time and 26% overtime workers).
Table 1.
Telomere-length differences comparing various work-schedule characteristics: (1) comparing current workers versus non-employed women and (2) among current workers
| Age-adjusted* beta (Δ bp) | 95% CI | Adjusted† beta (Δ bp) | 95% CI | ||
|---|---|---|---|---|---|
| Current work schedule versus non-employed‡ | N | N=608 | N=604 | ||
| Non-employed | 190 | Referent | Referent | ||
| Hours | |||||
| Part-time | 50 | 167 | −178 to 512 | 197 | −169 to 562 |
| Full-time | 247 | −329 | −110 to −548 | −353 | −595 to −112 |
| Overtime | 121 | −202 | −417 to 66 | −234 | −530 to 62 |
| Other characteristics | |||||
| Irregular hours | 95 | −36 | −304 to 232 | −25 | −297 to 248 |
| Work at night | 17 | −201 | −719 to 317 | −196 | −726 to 334 |
| Multiple jobs | 27 | 155 | −304 to 615 | 88 | −371 to 548 |
| Current workers only | N=418 | N=415 | |||
| Hours | |||||
| Part-time | 50 | Referent | Referent | ||
| Full-time | 247 | −526 | −852 to −199 | −584 | −919 to −249 |
| Overtime | 121 | −404 | −767 to −41 | −458 | −830 to −86 |
| Other characteristics | |||||
| Irregular hours | 95 | −42 | −311 to 226 | −23 | −298 to 251 |
| Work at night | 17 | −203 | −721 to 316 | −196 | −734 to 342 |
| Multiple jobs | 27 | 171 | −289 to 631 | 97 | −363 to 557 |
Difference in base pairs (Δ bp) relative telomere length, adjusted for age. Bold indicates that 95% CI excludes the null.
Adjusted model included age, sampling variables (race, current smoking status, perceived stress), health behaviours and status (BMI, sleep, physical activity, health status, and cardiovascular disease or diabetes), demographic factors (marital status, education, number of children and having a child in the household) and total number of years worked.
Referent: all past workers with a history of substantial (>75% potential; n=50) or moderate (26–75%; n=140) employment; referent categories for other characteristics also include current regular hours, not working at night or working a single job.
Estimated rTL was shorter in currently employed women (age-adjusted rTL=5559 bp, 95% CI 5456 to 5663) than in non-employed women (5785 bp; 95% CI 5629 to 5942). Current full-time schedule was significantly associated with shorter rTL compared with non-employment (table 1); other factors significantly associated with shorter rTL in the fully adjusted model included current smoking (−280 bp, vs never smoking; 95% CI −504 to −56), higher perceived stress (−277 bp; 95% CI −552 to −4) and diagnosis of heart disease or diabetes (−236 bp; 95% CI −470 to −2). Compared with part-time, shorter rTL was seen for both full-time and overtime work. We saw no substantial changes in results adjusted for covariates, or restricted by age and health (not shown); differences comparing full-time and overtime with part-time were diminished, but still significant in analyses including women with a minimal work history.
Women who worked more than 20 years full-time had shorter rTL overall compared with women who worked full-time for only 1–5 years (table 2), with a trend (p=0.02) of −14 bp shorter rTL (95% CI −5 to −23) per year worked full-time in addition to −22 bp per year of age (95% CI −12 to −31). Women who had never worked ≥1 year full-time had shorter rTL, though most (n=23 of 30) had a history of at least 10 years overtime work. Overall, however, longer-duration overtime work was associated with somewhat longer rTL (trend p=0.13), while having more years of part-time work was associated with significantly longer rTL (trend p=0.013). Working multiple jobs simultaneously for 10 or more years was significantly associated with shorter rTL. In a mutually adjusted model (figure 1), rTL differences persisted for currently working at least full-time and increasing duration of full-time work, and were most apparent among postmenopausal women. Differences were not substantially changed with covariate adjustment (not shown), except in premenopausal women currently working at least full-time (fully adjusted β=−458 bp; 95% CI −805 to −110).
Table 2.
Telomere-length differences in relation to lifetime history of work-schedule characteristics
| N (%) | Age-adjusted* beta (Δ bp) | 95% CI | Covariate-adjusted† beta (Δ bp) | 95% CI | |
|---|---|---|---|---|---|
| N=608 | |||||
| Hours | |||||
| Full-time | |||||
| None/<1 year | 30 (5) | −389 | −848 to 71 | −528 | −991 to −66 |
| 1–5 years | 57 (9) | Referent | Referent | ||
| 6–10 years | 85 (14) | −168 | −518 to 182 | −155 | −510 to 200 |
| 11–20 years | 196 (32) | −202 | −508 to 106 | −274 | −587 to 37 |
| 20+ years | 240 (39) | −472 | −786 to−158 | −525 | −847 to−204 |
| p-trend‡ | 0.0017 | 0.0003 | |||
| Overtime | |||||
| None/<1 year | 284 (47) | Referent | Referent | ||
| 1–5 years | 95 (16) | −151 | −396 to 94 | −147 | −396 to 102 |
| 6–10 years | 82 (13) | 75 | −183 to 333 | 144 | −118 to 405 |
| 11–20 years | 86 (14) | 156 | −98 to 411 | 164 | −100 to 427 |
| >20 years | 61 (10) | 147 | −150 to 444 | 178 | −126 to 483 |
| p-trend | 0.13 | 0.08 | |||
| Part-time | |||||
| None/<1 year | 410 (67) | Referent | Referent | ||
| 1–5 years | 123 (20) | 4 | −207 to 215 | −4 | −220 to 212 |
| 6–10 years | 37 (6) | 317 | −38 to 627 | 332 | −27 to 690 |
| >10 years | 38 (6) | 401 | 55 to 749 | 356 | 1 to 711 |
| p-trend | 0.013 | 0.024 | |||
| Other characteristics | |||||
| Irregular hours | |||||
| None/<1 year | 420 (69) | Referent | Referent | ||
| 1–5 years | 69 (11) | 98 | −172 to 368 | 122 | −151 to 396 |
| 6–10 years | 38 (6) | 20 | −330 to 370 | 81 | −276 to 437 |
| 11–20 years | 47 (8) | 93 | −224 to 409 | 116 | −206 to 438 |
| >20 years | 34 (6) | 243 | −128 to 614 | 275 | −99 to 649 |
| p-trend | 0.20 | 0.11 | |||
| Rotating shifts | |||||
| None/<1 year | 514 (85) | Referent | Referent | ||
| 1–5 years | 55 (9) | 1 | −291 to 294 | −27 | −320 to 267 |
| 6–10 years | 15 (2) | 14 | −526 to 555 | 40 | −201 to 585 |
| >10 years | 24 (4) | −233 | −663 to 197 | −209 | −642 to 223 |
| p-trend | 0.40 | 0.44 | |||
| Nights (22:00–02:00) | |||||
| None/<1 year | 456 (75) | Referent | Referent | ||
| 1–5 years | 102 (17) | 84 | −141 to 310 | 112 | −116 to 341 |
| 6–10 years | 27 (4) | 30 | −380 to 440 | −58 | −489 to 373 |
| >10 years | 23 (4) | −120 | −563 to 322 | −10 | −448 to 440 |
| p-trend | 0.98 | 0.83 | |||
| Multiple jobs | |||||
| None/<1 year | 484 (80) | Referent | Referent | ||
| 1–5 years | 77 (13) | −81 | −334 to 171 | −25 | −281 to 230 |
| 6–10 years | 30 (5) | −8 | −394 to 378 | 42 | −358 to 442 |
| >10 years | 17 (3) | −513 | −1024 to −3 | −542 | −1054 to −31 |
| p-trend | 0.12 | 0.18 | |||
Difference in base pairs (Δ bp) relative telomere length, adjusted for age and total years worked. Not mutually adjusted for other variables listed in table. Bold indicates when 95% CI excludes the null.
Adjusted for age, sampling variables (race, current smoking status, perceived stress), health behaviours and status (BMI, sleep, physical activity, health status, and cardiovascular disease or diabetes), demographic factors (marital status, education, number of children and having a child in the household) and total number of years worked; N=3 missing covariate data.
Trend test across categories of increasing years for each schedule characteristic; for full-time limited to those working at least 1 year full-time.
Figure 1.
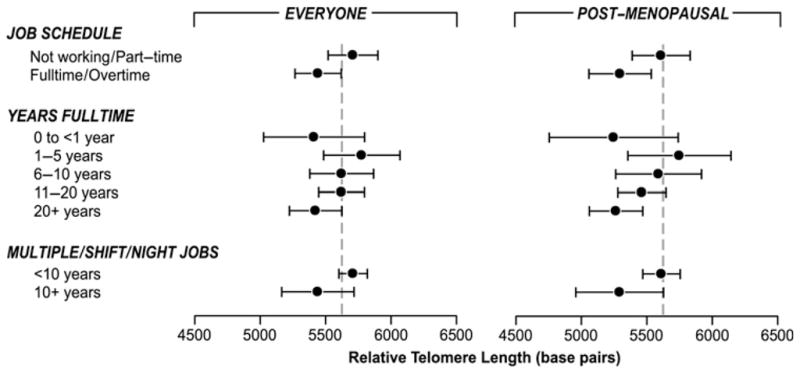
Estimated mean telomere length for current work schedule, years of full-time work and long-term work in multiple jobs, shiftwork or work at night in the entire sample (n=608) and postmenopausal women only (n=361). The mean telomere length base pairs and 95% CIs were estimated from a mutually adjusted regression including age and variables shown (current work schedule, years full-time work and long-term work multiple jobs, shiftwork or work at night). The sample includes everyone currently working or those with a moderate or substantial past employment history and a subanalysis of postmenopausal women. The dashed line represents the mean unadjusted relative telomere length in the sample.
Stratified analyses suggested that rTL differences for currently working full-time or longer hours were more apparent in women with higher-than-average epinephrine levels, with less apparent differences for norepinephrine and no differences seen in cortisol-stratified models (not shown). Joint effects of schedule, perceived stress and epinephrine levels are shown in figure 2; schedule-related rTL differences were most apparent in women with higher epinephrine levels, and the shortest rTL was seen in women with both higher epinephrine and perceived stress. This represented an interaction of epinephrine and current schedule (p=0.08) in addition to the previously described interaction of perceived stress and epinephrine (p=0.0012).11
Figure 2.
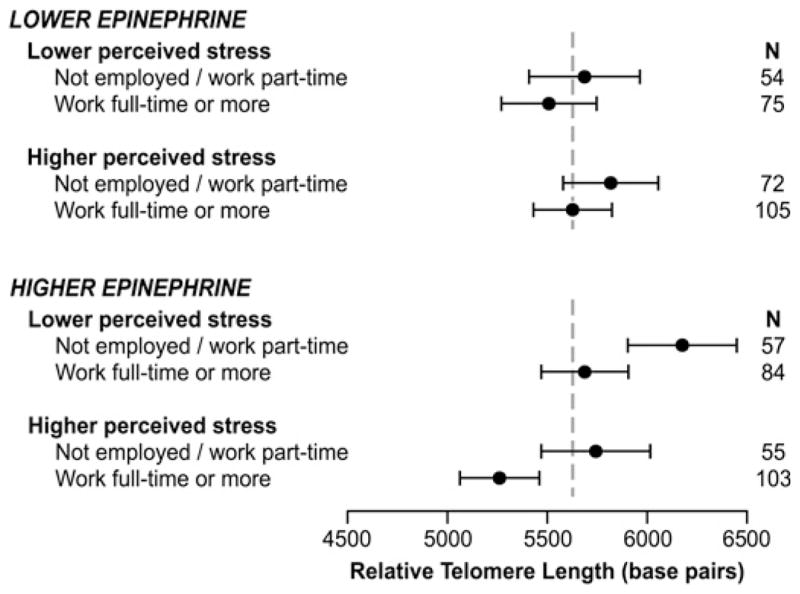
Estimated mean telomere length for current full-time/overtime versus part-time/non-employed in women characterised by low and high urinary epinephrine and perceived stress. Lower epinephrine, at or below the median of creatinine-adjusted urinary epinephrine; lower perceived stress, at or below the median perceived stress score of 2.11 The mean telomere-length base pairs and 95% CI were estimated from a regression model adjusted for age. The dashed line represents the mean unadjusted relative telomere length in the sample.
Table 3 shows that current schedule-related rTL differences were most apparent in women with a moderate education (some college; 9% shorter rTL for working at least full-time), moderate incomes ($50 000–100 000; 9% shorter), three or more children (9% shorter) or younger age at first birth (less than the median, 23 years; 9% shorter). For long-term full-time schedules (20 or more vs 1–5 years), rTL differences were most apparent for women with an Associate or Bachelor’s degree (14% shorter for 20+ years), moderate incomes (9% shorter), having two or three or more children (10 and 13% shorter) and younger age at first birth (11% shorter). Covariate adjustment had a limited impact on these differences, except for increasing the magnitude of schedule-related rTL differences in women with a high-school education or less (fully adjusted β −382 bp, 95% CI −1037 to 272; 7% shorter for current full-time work, and −648 bp, 95% CI −1587 to 290; 12% shorter for 20+ years). No interaction tests were performed on these exploratory analyses.
Table 3.
Age-adjusted differences in telomere length comparing (1) currently working at least full-time versus part-time/not working and (2) long-term (≥20 years) versus short-term (1–5 years) full-time employment
| N | Current
|
Percentage difference† | Lifetime
|
Percentage difference‡ | |||
|---|---|---|---|---|---|---|---|
| Mean relative telomere length (95% CI)* not working/part-time | Difference for working ≥full-time base pairs (95% CI)* | Mean relative telomere length (95% CI)* worked 1–5 years FT | Difference for working ≥20 years full-time base pairs (95% CI)* | ||||
| Education | |||||||
| ≤High school | 93 | 5365 (5041 to 5689) | −85 (−578 to 408) | 2 | 5262 (4499 to 6024) | 79 (−912 to 753) | +2 |
| Some college | 156 | 5964 (5701 to 6227) | −530 (−876 to −184) | 9 | 5760 (5054 to 6468) | −343 (−1109 to 423) | 6 |
| Associate degree | 91 | 5933 (5576 to 6292) | −483 (−947 to 7) | 8 | 6397 (5616 to 7177) | −926 (−1762 to 91) | 14 |
| Bachelor’s degree | 156 | 5902 (5624 to 6181) | −296 (−656 to 64) | 5 | 6277 (5787 to 6767) | −846 (−1413 to −279) | 14 |
| Graduate degree | 112 | 5994 (5598 to 6392) | −298 (−793 to 196) | 5 | 5729 (5224 to 6234) | 163 (−448 to 774) | +3 |
| Household income | |||||||
| <$50 000/year | 183 | 5745 (5526 to 5965) | −340 (−680 to 1) | 6 | 5602 (5028 to 6177) | −91 (−715 to 532) | 2 |
| $50–100 000/year | 259 | 5993 (5766 to 6219) | −467 (−748 to −185) | 8 | 6070 (5637 to 6503) | −573 (−1044 to 101) | 9 |
| >$100 000/year | 143 | 5714 (5393 to 6037) | −95 (−498 to 308) | 2 | 5903 (5400 to 6405) | −360 (−986 to 263) | 6 |
| Children (live births) | |||||||
| 3 or more | 170 | 5784 (5523 to 6045) | −541 (−921 to −160) | 9 | 6028 (5507 to 6550) | −765 (−1354 to −176) | 13 |
| 2 | 209 | 5849 (5626 to 6072) | −266 (−558 to 26) | 5 | 6207 (5749 to 6667) | −631 (−1145 to −118) | 10 |
| 0–1 | 228 | 5872 (5626 to 6118) | −259 (−572 to 54) | 4 | 5540 (5101 to 5890) | 54 (−435 to 543) | +1 |
| Age at first birth | |||||||
| ≤23 years | 246 | 5820 (5607 to 6034) | −515 (−817 to −213) | 9 | 6117 (5677 to 6557) | −698 (−1182 to −215) | 11 |
| >23 years | 227 | 5792 (5560 to 6024) | −177 (−478 to 124) | 3 | 5834 (5417 to 6252) | −312 (−796 to 171) | 5 |
Mean and difference in telomere length base pairs estimated in regression adjusted for age. Bold indicates when 95% CI excludes the null.
Estimated percentage difference (decrease) in telomere base pairs worked full-time or longer compared with not working or working part-time.
Estimated percentage difference (decrease) in telomere base pairs for long-term versus short-term full-time work.
DISCUSSION
Our findings suggest that shorter leucocyte rTL, a putative marker of biological ageing, is associated with current employment and some characteristics of work schedule, specifically current and long-term full-time hours. Observed differences were not explained by a variety of covariates, including potential intermediates in pathways mediating schedule effects on health, such as obesity, smoking, perceived stress and other factors associated with rTL in this sample and other studies.6, 11, 14, 15 Although we did not directly evaluate schedule-related stress, the impact of current full-time or longer work hours on rTL was most apparent in women with higher epinephrine and perceived stress, implying a potential role of neuroendocrine stress responses. As the first to describe cross-sectional associations of current and past work schedule with a leucocyte rTL, these findings should be interpreted with caution but support further investigation of work and schedule-related stress in relation to rTL, especially in the context of prospective data and more specific information on work and schedule-related stress exposures.
Work schedule is a measure of chronic stress distinct from job strain, which relates to demands and control within the work-place. Work schedule may be an intrinsic stressor, or act indirectly through behaviours or confiicts relating to schedule control and demands of work and other roles. We saw no evidence of behavioural factors mediating schedule-related rTL differences; rather, our findings may suggest more direct schedule-related differences or something about the balance of work and other roles. Women currently working part-time and those with more years of part-time work had a significantly longer rTL. In a national sample, part-time work was related to lower job–family interference and job stress compared with full-time.16 The impact of full-time work on rTL differences was also most apparent in women with more children and earlier age at first birth, perhaps pointing towards stress arising from the need to balance work and other roles. More direct information on role strain was not available, and we had no data on demands of informal (unpaid) caregiving for children and adult relatives, common experiences among mid-life women.
In these cross-sectional data, we cannot account for self-selection out of different schedule types depending on work ability and career trajectory; a healthy survivor effect may be expected among women who maintain or start overtime positions over their worklife course. We saw no additional impact of current or more total years of overtime schedule. We had limited opportunities to disentangle the effects of overtime from full-time and part-time work. Stress from longer workhours may depend on predictability or control, compensation and personal fulfilment. Despite associations with job stress and perceived ‘overwork,’ a national survey found that having an overtime schedule was related to greater opportunities for developing abilities, participation in decision-making and job satisfaction.16 In some professions, longer hours are related to self-directed, fiexible work hours,17 and the impact of overtime may be buffered by resources enabling rest and recovery, or lower job strain.18 In this sample, women reporting current overtime schedules were more likely to have a graduate or professional education (27% vs 18% of those who worked full-time) and higher job control scores in a later job-strain questionnaire (not shown).
Although current employment was associated with shorter telomeres, women with a minimal work history (employed less than 25% of their potential workspan) had a significantly shorter rTL. This may reflect a positive impact of employment on health, or selection of unhealthy individuals out of the workforce.19 Post-hoc analyses showed that retired women had a significantly longer rTL than homemakers (age-adjusted rTL=5972 vs 5567 bp, p=0.023), which suggests resiliency from schedule-related stress after retirement. A recent study described improved self-reported health with retirement,20 though this may depend on conditions at work. Homemakers may differ from both employed and retired women, and have a unique set of stressors. Being non-employed has been associated with increased mortality and coronary heart disease in women,21, 22 but findings comparing homemakers with employed women are not consistent.23 Gender roles may influence the impact of work schedule-related stresses. Consistent with our findings, one study suggested somewhat longer telomeres in retired compared with currently employed men and also described significantly shorter telomeres in those who were unemployed.24 We had too few unemployed women to replicate this second finding. Future research should also consider rTL in relation to underemployment and precarious employment in both men and women.
Menopause is associated with female (ovarian) ageing, though published findings on telomere length, menopause and post-menopausal hormone use are inconclusive.25, 26 In the present study sample, menopausal status was not independently associated with rTL overall or in women aged 45–54 years (not shown). We observed differences by menopausal status in schedule-associations with rTL, but these were sometimes diminished in multivariate models. The meaning of work may vary by age, and work schedule-related stress might increase in older women if retirement or part-time work is not an option, especially in the context of other age-related changes in personal health or caregiving demands.
Interpreting cross-sectional data on work and health is challenging, owing to the cumulative impact of work on health, including development of chronic diseases that may lead to disability or early retirement. We considered current health status and behaviours, but did not account for past behaviours or changes over time. Longitudinal findings in a large occupational cohort showed that socio-economic status effects on mortality were substantially attenuated after adjusting for health behaviours measured several times over two decades.27 In the present study, similar patterns of work-related rTL differences were seen in analyses excluding those with heart disease, diabetes or poor health, which may be related to past health behaviours. We cannot rule out selection biases; women with the greatest work/life demands or chronic health problems may not have participated in the study, owing to the time and effort required.
A strength of this study is that lifetime job history and schedules were collected separately from stress or health-behaviour data. Errors in data recall are expected to be non-differential with respect to rTL but could be affected by age or education. Although our findings are limited by the lack of direct work-related stress measures, differences were most clearly seen at higher epinephrine levels in the presence of higher perceived stress. This suggests that physiological stress response may play a role. Although transiently influenced by acute exposures, overnight urinary catecholamine levels are an integrative measure of sympathetic nervous system activation spanning hours to days.28 We cannot explain why we saw limited differences stratifying by norepinephrine or cortisol, as these analytes are biologically related and correlated in these data.11 Cortisol has been studied extensively in relation to work-related stress.29 Typically characterised through circadian patterns and response to acute stress, interpretation of cortisol levels in first morning urines may be complicated by long-term stress-related changes in cortisol response. Our findings are also limited by a wide variation in assay performance, especially for norepinephrine and cortisol, and low specimen volume prohibited assay replication. This non-differential misclassification may have limited our ability to see potential underlying differences.
Other explanations may underlie our findings, including reasons for working (eg, personal fulfilment, economic necessity) or not working (eg, health, social and economic opportunity), schedule control and resources for balancing non-work demands (eg, household help and childcare).30 Though not adjusted for multiple comparisons, exploratory analyses suggest that the impact of work schedule on the rTL might be buffered by higher education or income, pointing towards the possible role of the type of work or socio-economic resources. Lower socio-economic status has been associated with mortality risk,31, 32 but associations with shorter rTL have been inconsistently observed.24, 33 Long-term work in multiple jobs was also associated with a shorter rTL, suggesting that economic necessity or strain in managing complex schedules might be important. The sample was limited to women not currently working rotating shifts; however, several aspects of shiftwork may act as schedule-related stressors (eg, work at night, rotating shifts). We saw a somewhat shorter rTL for long-term (>10 years) work at night or rotating shiftwork. Together, long-term work in multiple jobs, shift-work or work at night was associated with a shorter rTL in postmenopausal women, though this was attenuated with covariate adjustment (not shown).
Our findings of independent rTL differences for both current and past full-time work may reflect a short-term impact on leucocyte rTL and more lasting effects operating through different mechanisms. Interpretation of rTL associations typically implies unidirectional shortening with age and other exposures, as most somatic tissues do not normally express the telomere maintenance enzyme, telomerase. Some blood cells require telomerase as part of a normal immune response34; there may be short-term and, to some extent, reversible effects of stress on leucocyte rTL, owing to changes in telomerase expression,35 as well as longer-term effects of cellular replication, oxidative stress and impact on the haematopoetic stem cell rTL. Longitudinal studies describe the maintenance or extension of leucocyte telomeres in up to a third of individuals sampled over time (eg, 8–10 years),36, 37 suggesting potential recovery from acute or chronic telomere shortening. An intensive, 3-month intervention in patients with prostate cancer showed an improvement in psychological distress associated with increased telomerase expression.38 Thus, both short- and long-term changes in stressors and resiliency may influence telomere length across the lifespan. Further research is needed to understand the public health implications of these findings. Longer work hours have been infrequently studied in women,9 though one study did show significantly elevated mortality associated with five overtime hours per week after 24 years of follow-up.39 Our study sample, drawn from a volunteer cohort of women at increased risk of breast cancer, was enriched for higher perceived stress, non-white race and smoking; replication is needed in other samples. However, the magnitude of schedule-related rTL differences in the present study was similar to the differences observed for established risk factors and consistent with other telomere research. Though cautious interpretation is warranted, findings could have widespread implications, as employment is a common and often extended lifetime experience, with related stresses arising at the interface of resources and demands, and possible links to socio-economic gradients in health.
What this paper adds.
Perceived and chronic stress have been associated with shorter DNA telomeres, a marker of cellular ageing and risk for ageing-related diseases, but studies have not considered the impact of work schedule, a common potential stressor affecting health and behaviours.
Current full-time work was associated with shorter telomeres compared with not working, and both full-time and overtime schedules were associated with shorter telomeres compared with working part-time.
Having more years of a past full-time work schedule was associated with shorter telomeres, as was having at least 10 years’ work in multiple jobs.
Schedule-related associations with shorter telomeres were not explained by health, demographic or behavioural covariates, but were more apparent in women with higher perceived stress and higher urinary epinephrine levels.
Further research is needed, including longitudinal data on telomere length, more specific measures of stress in relation to work schedule and links to health outcomes.
Acknowledgments
Funding This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES044005), and by Department of Defense Breast Cancer Research Concept Award (BC045286).
Footnotes
Competing interests None.
Ethics approval Ethics approval was provided by the Institutional Review Board of the National Institute of Environmental Health Science, National Institutes of Health.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–8. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 2.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–79. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 3.Cawthon RM, Smith KR, O’Brien E, et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 4.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–15. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGrath M, Wong JY, Michaud D, et al. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16:815–19. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 6.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 7.Tentolouris N, Nzietchueng R, Cattan V, et al. White blood cells telomere length is shorter in males with type 2 diabetes and microalbuminuria. Diabetes Care. 2007;30:2909–15. doi: 10.2337/dc07-0633. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama S, Morimoto K. Effects of long workhours on life-style, stress and quality of life among intermediate Japanese managers. Scand J Work Environ Health. 1996;22:353–9. doi: 10.5271/sjweh.153. [DOI] [PubMed] [Google Scholar]
- 9.van der Hulst M. Long workhours and health. Scand J Work Environ Health. 2003;29:171–88. doi: 10.5271/sjweh.720. [DOI] [PubMed] [Google Scholar]
- 10.Artazcoz L, Borrell C, Cortes I, et al. Occupational epidemiology and work related inequalities in health: a gender perspective for two complementary approaches to work and health research. J Epidemiol Community Health. 2007;61(Suppl 2):II39–45. doi: 10.1136/jech.2007.059774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parks CG, Miller DB, McCanlies EC, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18:551–60. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 14.Kim S, Parks CG, DeRoo LA, et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009;18:816–20. doi: 10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Q, Parks CG, DeRoo LA, et al. Multivitamin use and telomere length in women. Am J Clin Nutr. 2009;89:1857–63. doi: 10.3945/ajcn.2008.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosch JW, Caruso CC, Rosa RR, et al. Long hours of work in the US: associations with demographic and organizational characteristics, psychosocial working conditions, and health. Am J Ind Med. 2006;49:943–52. doi: 10.1002/ajim.20388. [DOI] [PubMed] [Google Scholar]
- 17.Beckers DG, van Hooff ML, van der Linden D, et al. A diary study to open up the black box of overtime work among university faculty members. Scand J Work Environ Health. 2008;34:213–23. doi: 10.5271/sjweh.1226. [DOI] [PubMed] [Google Scholar]
- 18.van der Hulst M, van Veldhoven M, Beckers D. Overtime and need for recovery in relation to job demands and job control. J Occup Health. 2006;48:11–19. doi: 10.1539/joh.48.11. [DOI] [PubMed] [Google Scholar]
- 19.McMunn A, Bartley M, Hardy R, et al. Life course social roles and women’s health in mid-life: causation or selection? J Epidemiol Community Health. 2006;60:484–9. doi: 10.1136/jech.2005.042473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westerlund H, Kivimaki M, Singh-Manoux A, et al. Self-rated health before and after retirement in France (GAZEL): a cohort study. Lancet. 2009;374:1889–96. doi: 10.1016/S0140-6736(09)61570-1. [DOI] [PubMed] [Google Scholar]
- 21.Carson AP, Rose KM, Catellier DJ, et al. Employment status, coronary heart disease, and stroke among women. Ann Epidemiol. 2009;19:630–6. doi: 10.1016/j.annepidem.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose KM, Carson AP, Catellier D, et al. Women’s employment status and mortality: the atherosclerosis risk in communities study. J Womens Health (Larchmt) 2004;13:1108–18. doi: 10.1089/jwh.2004.13.1108. [DOI] [PubMed] [Google Scholar]
- 23.Haynes SG, Feinleib M. Women, work and coronary heart disease: prospective findings from the Framingham heart study. Am J Public Health. 1980;70:133–41. doi: 10.2105/ajph.70.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batty GD, Wang Y, Brouilette SW, et al. Socioeconomic status and telomere length: the West of Scotland Primary Prevention Study. J Epidemiol Community Health. 2009;63:839–41. doi: 10.1136/jech.2009.088427. [DOI] [PubMed] [Google Scholar]
- 25.Lee DC, Im JA, Kim JH, et al. Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei Med J. 2005;46:471–9. doi: 10.3349/ymj.2005.46.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassidy A, De Vivo I, Liu Y, et al. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr. 2010;91:1273–80. doi: 10.3945/ajcn.2009.28947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010;303:1159–66. doi: 10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown DE. Measuring hormonal variation in the sympathetic nervous system: catecholamines. In: Ice G, editor. Measuring stress in humans; a practical guide for the field. New York: Cambridge University Press; 2007. [Google Scholar]
- 29.Sluiter JK, Frings-Dresen MH, Meijman TF, et al. Reactivity and recovery from different types of work measured by catecholamines and cortisol: a systematic literature overview. Occup Environ Med. 2000;57:298–315. doi: 10.1136/oem.57.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caruso CC, Bushnell T, Eggerth D, et al. Long working hours, safety, and health: toward a National Research Agenda. Am J Ind Med. 2006;49:930–42. doi: 10.1002/ajim.20373. [DOI] [PubMed] [Google Scholar]
- 31.Shishehbor MH, Litaker D, Pothier CE, et al. Association of socioeconomic status with functional capacity, heart rate recovery, and all-cause mortality. JAMA. 2006;295:784–92. doi: 10.1001/jama.295.7.784. [DOI] [PubMed] [Google Scholar]
- 32.Lantz PM, House JS, Lepkowski JM, et al. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA. 1998;279:1703–8. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 33.Cherkas LF, Aviv A, Valdes AM, et al. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–5. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 34.Hathcock KS, Jeffrey Chiang Y, Hodes RJ. In vivo regulation of telomerase activity and telomere length. Immunol Rev. 2005;205:104–13. doi: 10.1111/j.0105-2896.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 35.Epel ES, Lin J, Dhabhar FS, et al. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun. 2010;24:531–9. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aviv A, Chen W, Gardner JP, et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–9. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nordfjall K, Svenson U, Norrback KF, et al. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009;5:e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048–57. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 39.Nylen L, Voss M, Floderus B. Mortality among women and men relative to unemployment, part time work, overtime work, and extra work: a study based on data from the Swedish twin registry. Occup Environ Med. 2001;58:52–7. doi: 10.1136/oem.58.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]


