Abstract
Background
Most HIV infections are transmitted across mucosal epithelium. Understanding the role of innate and specific mucosal immunity in susceptibility or protection against HIV infection, as well as the effect of HIV infection on mucosal immunity, are of fundamental importance. HLA-G is a powerful modulator of the immune response. The aim of this study was to investigate whether soluble HLA-G (sHLA-G) expression in the female genital tract is associated with HIV-1 infection.
Methods and Findings
Genital levels of sHLA-G were determined in 52 HIV-1-uninfected and 44 antiretroviral naïve HIV-1-infected female commercial sex workers (CSWs), as well as 71 HIV-1-uninfected non-CSW women at low risk of exposure, recruited in Cotonou, Benin. HIV-1-infected CSWs had higher genital levels of sHLA-G compared with those in both the HIV-1-uninfected CSW (P = 0.009) and non-CSW groups (P = 0.0006). The presence of bacterial vaginosis (P = 0.008), and HLA-G*01:01:02 genotype (P = 0.002) were associated with higher genital levels of sHLA-G in the HIV-1-infected CSWs, whereas the HLA-G*01:04:04 genotype was also associated with higher genital level of sHLA-G in the overall population (P = 0.038). When adjustment was made for all significant variables, the increased expression of sHLA-G in the genital mucosa remained significantly associated with both HIV-1 infection (P = 0.02) and bacterial vaginosis (P = 0.03).
Conclusion
This study demonstrates that high level of sHLA-G in the genital mucosa is independently associated with both HIV-1 infection and bacterial vaginosis.
Introduction
HIV vaccines and microbicides hold promise for preventing the acquisition of HIV-1 infection [1], [2] but successful design of such agents requires a clear understanding of the mechanisms of HIV-1 transmission at the initial site of infection [3]. Most HIV-1 infections occur during heterosexual intercourse, and women are more likely to become infected than men [4]. Initial exposure to HIV-1 during sexual transmission occurs in the genital tract; however, little is known about HIV-1-specific immune responses at this site, as well as the effect of HIV-1 on mucosal immunity.
Human leukocyte antigen (HLA)-G is a non-classical major histocompatibility class I protein, characterised by limited polymorphism and tissue-restricted distribution. HLA-G is expressed as membrane-bound (HLA-G1, -G2, -G3 and -G4) and soluble (HLA-G5, -G6, -G7) isoforms as a result of alternative splicing [5]. The major isoforms present in the plasma are soluble HLA-G (sHLA-G)-1 and -G5 which are generated by shedding or proteolytic cleavage of membrane-bound HLA-G1 isoform and by secretion of a soluble form, respectively. Under physiological conditions, sHLA-G levels correlate with gender and HLA-G genetic polymorphisms. The level of sHLA-G is higher in women than in men [6]. Healthy individuals carrying the HLA-G*01:01:03 and HLA-G*0105N alleles have lower plasma sHLA-G levels than subjects carrying the more frequent HLA-G*01:01:01 allele. In addition, individuals with the latter allele have lower plasma sHLA-G levels than those with the HLA-G*01:04 allele. Polymorphisms in the 3′-untranslated region (3′UTR) can also affect the production of HLA-G molecules. The presence of a 14-bp sequence insertion in HLA-G 3′UTR has been associated with lower levels of sHLA-G in serum of healthy subjects [7]–[9]. HLA-G expression can be induced during pregnancy [10], antiretroviral (ART) therapy [11], [12] and in pathological conditions such as autoimmune diseases, cancers, transplantations, and viral infections [13]. HLA-G molecules inhibit the activity and mediate apoptosis of natural killer (NK) cells and cytotoxic CD8+ T cells [14]–[17], as well as CD4+ T cell proliferation [18] and induce tolerogenic dendritic cells (DC) and regulatory T cells [19]–[22].
The immunosuppressive properties of HLA-G might contribute to the susceptibility to HIV-1 infection. Recent studies have shown that HLA-G polymorphisms are associated with altered risks of heterosexual acquisition [23]–[25] and vertical transmission [26], [27] of HIV-1. Plasma sHLA-G expression, at the protein level, was recently associated with increased risk of HIV-1 infection and more rapid disease progression [19], [28], [29]. However, initial exposure to HIV-1 during sexual transmission occurs in the female genital tract and no data are available on the possible association between genital HLA-G expression and susceptibility to HIV-1 infection. We have therefore measured the genital levels of sHLA-G in HIV-1-infected and HIV-1-uninfected female commercial sex workers (CSWs), as well as HIV-1-uninfected non-CSW women at low risk for exposure to investigate whether sHLA-G expression is associated with HIV-1 infection.
Methods
Study population
Female CSWs were enrolled through a dedicated sex worker clinic in Cotonou, Benin and were divided into two groups: HIV-1-uninfected CSWs (n = 52) and ART-naïve HIV-1-infected CSWs (n = 44). The HIV-1-uninfected non-CSW control subjects at low risk for exposure (n = 71) were enrolled from a general health clinic in Cotonou. Women were invited to participate in the study as they attended clinics. Women were excluded from the study if <18 years old, menstruating, or pregnant. At enrolment, participants were asked to answer a questionnaire about demographic information, sexual behaviour, duration of prostitution, number of sex partners, condom use, vaginal douching practices, and reproductive history. Each participant underwent a genital examination by a physician. Vaginal specimens were obtained for diagnosis of candidiasis and bacterial vaginosis by microscopic examination. Endocervical swabs were obtained to test for Neisseria gonorrhoeae and Chlamydia trachomatis infection using BD ProbeTec ET system (Strand Displacement Assay, Becton Dickinson, Heidelberg, Germany). Peripheral blood was taken for HIV, HLA-G and CCR5 genotype analyses. Plasma and serum were kept frozen at - 80°C until use. HIV-1 positivity was defined by the presence of HIV-1 antibodies tested with Vironostika HIV Uni-Form II Ag/Ab (Organon Teknika, Boxtel, The Netherlands). Non-reactive samples were considered HIV-seronegative, whereas reactive samples were tested with Genie II HIV-1/HIV-2 (Bio-Rad, Hercules, CA). Genie II dually reactive samples (to HIV-1 and HIV-2) and discordant samples (Vironostika reactive/Genie II non-reactive) were further tested by INNO-LIA HIV I/II Score (Innogenetics NV, Technologiepark 6, Gent, Belgium). Viral loads were determined in the plasma of all HIV-1 infected CSWs using VERSANT HIV-1 RNA 3.0 Assay (bDNA) (Siemens Medical Solutions Diagnostics, Tarrytown NY). DNA samples were genotyped for the CCR5 32-bp deletion allele and all women were found to be homozygous for the wild-type allele.
Mucosal sample collection and preparation
Cervicovaginal lavage (CVL) samples were obtained from all study participants by a physician, using a 10-ml syringe filled with sterile phosphate-buffered solution and aimed directly into the cervical os. CVL fluids were then collected, transferred immediately into 20 ml of RPMI-1640, kept on ice, and processed within 1 hour. CVL samples were centrifuged at 1500 r.p.m. for 10 min to remove cells and debris, and supernatants were stored at −80°C until shipped on dry ice to Montréal, Canada. CVL samples were concentrated with Amicon Ultra-15 5 kDa (Millipore, Billerica MA) prior to sHLA-G measurement.
Soluble HLA-G measurements and HLA-G genotyping
sHLA-G CVL levels were measured using the Human sHLA-G Immunoassay kit (Alexis Biochemicals, San Diego, CA, USA), which allows simultaneous detection of HLA-G1 and -G5 soluble proteins without discrimination. The final concentration of sHLA-G in the CVL sample was determined as follows: concentration obtained with the sHLA-G Elisa assay (units per ml)/(CVL concentration factor)×total CVL volume prior to concentration. HLA-G alleles were determined by direct DNA sequencing analysis of the nucleotide regions encompassing HLA-G exons 2–4 and using purified DNA from blood samples as described previously [30]. HLA-G 3-UTR polymorphisms were determined according to the protocol previously described by [31].
Statistical analysis
Statistical analysis was performed using the GraphPad PRISM 5.0 for Windows (GraphPad Software, San Diego, CA). One-way analysis of variance and Chi-square tests were used to assess the significance of the associations between continuous and categorical variables across all study groups. Comparisons of continuous and categorical variables between two groups were assessed by the Mann-Whitney U and Chi-square or Fisher exact tests, respectively. Spearman's rank test was used to determine correlations between continuous variables. Multiple logistic regression analysis was used to define independent predictors identified as significant in the crude analysis. Odds ratio (OR) and 95% confidence interval (CI) were calculated with the exact method. Differences were considered significant at P≤0.05 or P≤0.015 when comparing two or three groups, respectively.
Ethics statement
Written informed consent was obtained from all subjects who participated in the study and the investigation reported in this paper was approved by the Comité National Provisoire d'Éthique de la Recherche en Santé in Cotonou and the CHUM Research Ethics Committee.
Results
Sociodemographic and clinical characteristics of the study population are described in Table 1. These data were collected to address the issue of confounding variables for risk of HIV-1 infection. The three groups were similar with respect to age, days from last menses, vaginal douching, and the presence of vaginal candidiasis. The HIV-1-infected CSWs were more likely to have a bacterial vaginosis (P = 0.003) than the HIV-1-uninfected non-CSWs. The HIV-1-unifected non-CSWs, were less likely to have Chlamydia trachomatis or Neisseria gonorrhoeae genital infections than the HIV-1-uninfected (P = 0.027) and HIV-1-infected (P = 0.022) CSW groups. The average number of clients was higher in HIV-1-uninfected CSWs than in HIV-1-infected CSWs (P = 0.044), whereas the duration of sex work, and condom use were equivalent between the two CSW groups.
Table 1. Distribution of demographic, sexual behaviour and genital tract infection characteristics in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1-uninfected non-CSW women.
| HIV-1-uninfected | HIV-1-infected | HIV-1-uninfected | p | |
| CSWs | CSWs | non-CSWs | valuea | |
| N = 52 | N = 44 | N = 71 | ||
| Age, mean (SD), years | 34.4 (12.3) | 34 (8.7) | 32.6 (9.4) | NS |
| Duration of sex work, mean (SD), years | 4.3 (3.2) | 4.1 (2.6) | NA | NS |
| Number of clients last week, mean (SD) | 17.1 (14.0) | 11.3 (11.0) | NA | 0.044 |
| Days since last menses, mean (SD) | 17.1 (13.0) | 18.2 (15.4) | 19.1 (12.3) | NS |
| Vaginal douching | 50/52 (96%) | 42/43 (98%) | 65/71 (93%) | NS |
| Condom always used with clients past month | 39/52 (75%) | 22/39 (56%) | NA | NS |
| Bacterial vaginosis | 33/51 (65%) | 34/43 (79%) | 36/71 (51%) | 0.009b |
| Candidiasis | 4/51 (8%) | 5/44 (11%) | 15/71 (21%) | NS |
| NG and/or CT infections | 7/46 (15%) | 6/39 (15%) | 2/72 (3%) | 0.029c |
CSW, commercial sex worker; HIV-1, human immunodeficiency virus type 1; N: number of participants; NA: non applicable; NG/CT: Neisseria gonorrhoeae/Chlamydia trachomatis, NS: nonsignificant; SD, standard deviation.
P-values for the comparison across all groups were calculated with one-way ANOVA analysis of variance for the age and days since last menses; Mann-Whitney U test for the duration of sex work and average number of clients; Chi-square test for vaginal douching, condom use, bacterial vaginosis, candidiasis, and NG/CT infections.
P = 0.125 for the comparison between HIV-1-uninfected CSWs and HIV-1-infected CSWs, P = 0.105 for the comparison between HIV-1-uninfected CSWs and HIV-1-uninfected non-CSWs, and P = 0.003 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected non-CSWs as determined by Chi-square test.
P = 0.987 for the comparison between HIV-1-uninfected CSWs and HIV-1-infected CSWs, P = 0.027 for the comparison between HIV-1-uninfected CSWs and HIV-1-uninfected non-CSWs, and P = 0.022 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected non-CSWs as determined by Fisher exact test.
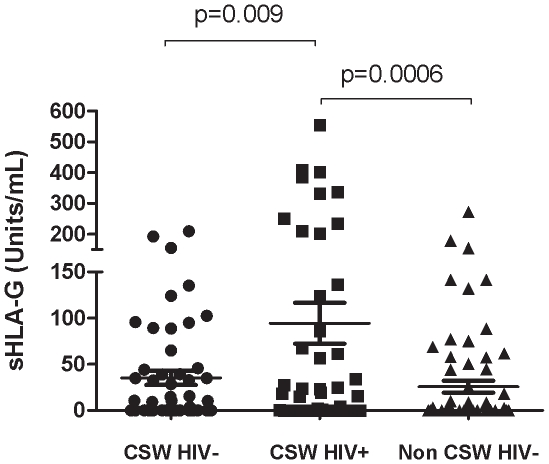
HIV-1-infected CSWs had significantly higher levels of sHLA-G in their CVL samples (94±145 units/ml) than did the HIV-1-uninfected CSWs (35±53 units/ml; P = 0.009) and the HIV-1-uninfected non-CSW women (26±53 units/ml; P = 0.0006) (Figure 1). There was no significant correlation between the HIV-1 plasma viral load and the sHLA-G level in the CVLs of the HIV-1-infected CSWs (r2 = −0.162, P = 0.344).
Figure 1. Mean genital soluble HLA-G levels according to the study groups.
Statistical significance of differences in the genital levels were evaluated with the Mann-Whitney U test. CSW, commercial sex worker; HIV-1, human immunodeficiency virus type 1.
Since sHLA-G expression has been associated with HLA-G polymorphism [7]–[9], we looked at the distribution of sHLA-G levels, either between study groups or in the total population, according to the HLA-G genetic variants (Table 2). The HLA-G*01:01:02 genotype, in the heterozygous or homozygous states, was associated with increased expression of genital sHLA-G in HIV-1-infected CSWs compared with those in both the HIV-1-uninfected CSW (P = 0.051) and non-CSW (P = 0.002) groups. In the overall population, women carrying the HLA-G*01:04:04 heterozygous or homozygous genotypes expressed the highest levels of genital sHLA-G molecules when compared with those expressed by women harbouring other genotypes (P = 0.038). However, there was no significant association between HLA-G alleles and sHLA-G levels within the three groups taken separately. Because HLA-G polymorphism can also be associated with HIV-1 infection [23]–[27], we looked at the distribution of the HLA-G genetic variants among the study groups (Table 2) and found no significant association between HLA-G alleles and HIV-1 infection (data not shown). The presence of bacterial vaginosis could potentially affect the genital level of sHLA-G molecules and since the rate of bacterial vaginosis was significantly higher in the HIV-1-infected CSWs (Table 1), we investigated the possible correlation between sHLA-G levels and the presence of bacterial vaginosis (Table S1). We found that the expression of sHLA-G in genital samples was significantly associated with bacterial vaginosis among the HIV-1-infected CSWs (P = 0.035).
Table 2. Genital soluble HLA-G levels in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1- uninfected non-CSW women according to the HLA-G gene polymorphism.
| HLA-G | HIV-1- uninfected | HIV-1-infected | HIV-1 uninfected | P-valuea | Total population | P-valueb | ||||
| CSWs | CSWs | non-CSWs | ||||||||
| allelec | N | Levels | N | Levels | N | Levels | N | Levels | ||
| 01:01:01 | 34 | 59.4 (148) | 20 | 113 (230) | 33 | 48.0 (86) | NS | 87 | 67.0 (152) | NS |
| 01:01:02 | 15 | 64.5 (203) | 14 | 191 (259) | 15 | 3.9 (12) | 0.034d | 44 | 84.0 (200) | NS |
| 01:03 | 11 | 47.8 (73) | 11 | 59.4 (109) | 8 | 12.2 (27) | NS | 30 | 42.6 (81) | NS |
| 01:04:01 | 4 | 48.2 (96) | 7 | 171 (228) | 14 | 18.0 (49) | NS | 25 | 65.6 (142) | NS |
| 01:04:04 | 20 | 118 (248) | 19 | 118 (165) | 12 | 31.8 (59) | NS | 51 | 97.6 (188) | 0.038 |
| 0105N | 8 | 30.7 (32) | 5 | 67.4 (151) | 10 | 14.1 (42) | NS | 23 | 31.5 (75) | NS |
| 3′UTR SNPe | ||||||||||
| 3777 (c/c) | 12 | 81.8 (228) | 5 | 61.6 (107) | 13 | 6.5 (21) | NS | 30 | 45.8 (151) | NS |
| 3952 (a/a) | 27 | 86.7 (217) | 11 | 64.6 (134) | 27 | 39.1 (77) | NS | 65 | 64.0 (158) | NS |
| 14-bp (I/I) | 6 | 16.4 (36) | 7 | 97.7 (135) | 6 | 0.45 (1.1) | NS | 19 | 41.3 (92) | NS |
CSW, commercial sex worker; HIV-1, human immunodeficiency virus type 1; I, insertion,
N, number of participants; NS, nonsignificant; SD, standard deviation; SNP, single nucleotide polymorphism; UTR, untranslated region.
Data are mean (SD).
P-values for the comparison between all groups were calculated with one-way analysis of variance test.
P-values were calculated with Mann-Whitney U test.
Presence of the allele in the homozygous or heterozygous states.
P = 0.051 for the comparison between HIV-1-uninfected CSWs and HIV-1-infected CSWs, P = 0.153 for the comparison between HIV-1-uninfected CSWs and HIV-1-uninfected non-CSWs and P = 0.002 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected non-CSWs as determined by Mann-Whitney U test.
Presence of the variants in the homozygous state.
When adjustment was made for all significant variables found in the crude analysis (HIV-1 infection, bacterial vaginosis, HLA-G*01:01:02 and HLA-G*01:04:04 genotypes), the expression of sHLA-G in the genital mucosa remained significantly associated with both HIV-1 infection (OR: 3.0, 95% CI = 1.17–7.53, P = 0.02) and bacterial vaginosis (OR 3.4, 95% CI = 1.10–10.5, P = 0.03).
Discussion
High level of sHLA-G in the genital mucosa is associated with HIV-1 infection in Beninese CSWs. In the present study, we have carefully controlled for potential confounding factors that could influence HLA-G expression such as gender [6], pregnancy [10], ART therapy [11], [12] and HLA-G polymorphism [7]–[9]. All study participants were ART-naïve nonpregnant women. The HLA-G*01:01:02 and HLA-G*01:04:04 genotypes were significantly associated with sHLA-G expression in the crude analysis but these associations disappeared after adjustment was done for HIV-1 infection. In contrast to previous studies [23]–[25], HLA-G polymorphism was not associated with risk of HIV-1 infection among the Beninese CSWs. The relatively small number of subjects analysed in each groups have limited the power of the present study to reproduce previous findings.
We have previously measured the level of sHLA-G in the blood of these women and found that HIV-infected CSWs had lower plasma levels when compared to HIV-uninfected CSWs and non-CSWs. This is in sharp contrast with that found in the genital mucosa of these women. The discordance in the production of sHLA-G between the two compartments may depend on local factors such as immune cells, micro-organisms and derived products that could affect sHLA-G expression. sHLA-G plays a crucial role in the regulation of both innate and adaptive immunity by modulating the function of DC, NK and T lymphocytes [14]–[22]. These effects depend on interactions of HLA-G molecules with inhibitory receptors expressed on myeloid cells (immunoglobuline-like transcripts (ILT)-4), on myeloid and lymphoid cells (ILT-2) and on NK cells (killing inhibitory receptor (KIR)-2DL4) [32]. The outcome of the immune response may therefore vary according to the specific interactions of sHLA-G with the different types of cells and receptors. Interaction of sHLA-G with ILT-2 receptor on DC and NK cells decreased the release of interferon (INF)-gamma and increased the production of interleukin (IL)-10 and transforming growth factor (TGF)-beta [33], [34]. IL-10 has been shown to induce HLA-G expression [35] and HLA-G can also stimulate IL-10 expression in peripheral blood monocytes [36]. Triggering ILT-4 by sHLA-G induces tolerogenic DC and T regulatory cells [20], [37]. On the other hand, interaction of sHLA-G with KIR2DL4 receptor on peripheral blood monocytes and NK cells promotes the production of pro-inflammatory cytokines and chemokines [36], [38]–[40]. We have previously measured the cytokine and chemokine expression patterns in the genital samples of our study subjects and found that HIV-1-infected CSWs had significantly higher levels of IFN-gamma tumor necrosis factor (TNF)-alpha, monocyte chemotactic protein (MCP-3/CCL7) and monokine induced by IFN-gamma (MIG/CXCL9) compared with those in both the HIV-1-uninfected CSW and non-CSW groups [41], [42]. The same observations were made for IL-1 beta and IL-8 (data unpublished). High level of IL-1 beta and TNF-alpha in the female genital tract has been associated with enhanced HIV-1 shedding at this site [43]. The inflammatory response observed in the genital mucosa of HIV-1-infected women may promote the recruitment, differentiation and activation of immune cells, which act as targets favouring viral replication and viral dissemination at the initial site of infection. As to whether sHLA-G is directly involved in the induction of such mucosal inflammation via its interaction with KIRD2L4 on monocytes and NK cells in the female genital tract remains to be confirmed. Although the genital mucosa levels of sHLA-G correlate significantly with those of the cytokines and chemokines in the HIV-1-uninfected groups, these correlations were not significant in the HIV-1-infected CSW group (Tables S2 and S3). Thus, in the absence of HIV-1, genital levels of the immunosuppressive sHLA-G molecules and pro-inflammatory cytokines and chemokines are low and correlate to maintain mucosal homeostasis. Conversely, in the presence of HIV-1, there is an aberrant and independent production of both factors in the female genital tract that may reflect a viral strategy of immune piracy, allowing for the simultaneous production of chemokines/cytokines to recruit and activate HIV-1 target cells and sHLA-G to induce immune tolerance towards HIV-1.
Interestingly, the increased level of sHLA-G in genital samples was also significantly associated with the presence of bacterial vaginosis. Although HIV-1-infected CSWs had higher levels of sHLA-G and were more likely to have a bacterial vaginosis than the HIV-1-uninfected non-CSWs, the association between sHLA-G levels and bacterial vaginosis remained significant after adjusting for HIV-infection. This suggests that genital sHLA-G level is independently associated with both bacterial vaginosis and HIV-1 infection. Bacterial vaginosis is an established risk factor for HIV infection [44], [45]. It has been suggested that bacterial vaginosis increases risk of HIV infection by inducing a clinical or subclinical mucosal inflammatory response, recruiting target cells and breaching of intact cervico-vaginal mucosa [46]. Indeed, bacterial vaginosis has been associated with increased levels of IL-1 beta, IL-6, IL-8, IL-10 and TNF-alpha, RANTES (CCL5), macrophage inflammatory protein (MIP-1 alpha/CCL3) and MIP-1 beta (CCL4) in genital samples [47]–[49]. However, bacterial vaginosis was not associated with the production of these cytokines and chemokines in the genital tract of the Beninese women (Tables S4 and S5).
Altogether, these results suggest that in the context of HIV-1 infection, sHLA-G expression in the female genital tract is a complex process modulated by many factors such as HIV-1, bacterial vaginosis HLA-G genotypes, and cytokine/chemokine expression patterns, which may all contribute to an immunological environment promoting viral replication and escape from the mucosal immune response.
Supporting Information
sHLA-G genital levels according to the presence or absence of vaginosis in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1- uninfected non-CSW control subjects.
(DOC)
Spearman's correlations between soluble HLA-G and cytokine genital levels in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1-uninfected non-CSW women.
(DOC)
Spearman's correlations between soluble HLA-G and chemokine genital levels in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1- uninfected non-CSW women.
(DOC)
Cytokine genital levels according to the presence or absence of bacterial vaginosis in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1- uninfected non-CSW women.
(DOC)
Chemokine genital levels according to the presence or absence of bacterial vaginosis in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1- uninfected non-CSW women.
(DOC)
Acknowledgments
We are indebted to N. Geraldo, A. Gabin, C. Assogba and C. Agossa-Gbenafa for their clinical expertise, to M. Massinga-Loembe, G. Ahotin, L. Djossou, and E. Goma for their technical assistance and to G. Batona and other field workers who helped with recruitment of commercial sex workers.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grant HOP-79213 from the Canadian Institutes of Health Research (CIHR) and by the Réseau SIDA from the Fonds de la Recherche en Santé du Québec (FRSQ) to MR. JL holds a Student Research award from the CIHR. MR is recipient of a Research Scholar award from the FRSQ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Padian NS, van der Straten A, Ramjee G, Chipato T, de Bruyn G, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet. 2007;370:251–261. doi: 10.1016/S0140-6736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes BF, Alam SM. HIV-1 hides an Achilles' heel in virion lipids. Immunity. 2008;28:10–12. doi: 10.1016/j.immuni.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hel Z, McGhee JR, Mestecky J. HIV infection: first battle decides the war. Trends Immunol. 2006;27:274–281. doi: 10.1016/j.it.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS report of the global AIDS epidemic 2010. http://www.unaids.org/globalreport/global_report.htm.
- 5.Ishitani A, Geraghty DE. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc Natl Acad Sci U S A. 1992;89:3947–3951. doi: 10.1073/pnas.89.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudstein-Svetlicky N, Loewenthal R, Horejsi V, Gazit E. HLA-G levels in serum and plasma. Tissue Antigens. 2007;69(Suppl 1):140–142. doi: 10.1111/j.1399-0039.2006.763_4.x. [DOI] [PubMed] [Google Scholar]
- 7.Hviid TV, Hylenius S, Lindhard A, Christiansen OB. Association between human leukocyte antigen-G genotype and success of in vitro fertilization and pregnancy outcome. Tissue Antigens. 2004;64:66–69. doi: 10.1111/j.1399-0039.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 8.Hviid TV, Rizzo R, Christiansen OB, Melchiorri L, Lindhard A, et al. HLA-G and IL-10 in serum in relation to HLA-G genotype and polymorphisms. Immunogenetics. 2004;56:135–141. doi: 10.1007/s00251-004-0673-2. [DOI] [PubMed] [Google Scholar]
- 9.Rebmann V, van der Ven K, Passler M, Pfeiffer K, Krebs D, et al. Association of soluble HLA-G plasma levels with HLA-G alleles. Tissue Antigens. 2001;57:15–21. doi: 10.1034/j.1399-0039.2001.057001015.x. [DOI] [PubMed] [Google Scholar]
- 10.Hackmon R, Hallak M, Krup M, Weitzman D, Sheiner E, et al. HLA-G antigen and parturition: maternal serum, fetal serum and amniotic fluid levels during pregnancy. Fetal Diagn Ther. 2004;19:404–409. doi: 10.1159/000078992. [DOI] [PubMed] [Google Scholar]
- 11.Cabello A, Rivero A, Garcia MJ, Lozano JM, Torre-Cisneros J, et al. HAART induces the expression of HLA-G on peripheral monocytes in HIV-1 infected individuals. Hum Immunol. 2003;64:1045–1049. doi: 10.1016/j.humimm.2003.08.353. [DOI] [PubMed] [Google Scholar]
- 12.Rivero A, Lozano JM, Gonzalez R, Garcia-Jurado G, Camacho A, et al. Nucleoside reverse transcriptase inhibitors are able and protease inhibitors unable to induce the tolerogenic molecule HLA-G1 on monocytes from HIV-1 infected patients. Hum Immunol. 2007;68:303–306. doi: 10.1016/j.humimm.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Pistoia V, Morandi F, Wang X, Ferrone S. Soluble HLA-G: Are they clinically relevant? Semin Cancer Biol. 2007;17:469–479. doi: 10.1016/j.semcancer.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riteau B, Menier C, Khalil-Daher I, Martinozzi S, Pla M, et al. HLA-G1 co-expression boosts the HLA class I-mediated NK lysis inhibition. Int Immunol. 2001;13:193–201. doi: 10.1093/intimm/13.2.193. [DOI] [PubMed] [Google Scholar]
- 15.Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, et al. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol. 2000;164:6100–6104. doi: 10.4049/jimmunol.164.12.6100. [DOI] [PubMed] [Google Scholar]
- 16.Le Gal FA, Riteau B, Sedlik C, Khalil-Daher I, Menier C, et al. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol. 1999;11:1351–1356. doi: 10.1093/intimm/11.8.1351. [DOI] [PubMed] [Google Scholar]
- 17.Park GM, Lee S, Park B, Kim E, Shin J, et al. Soluble HLA-G generated by proteolytic shedding inhibits NK-mediated cell lysis. Biochem Biophys Res Commun. 2004;313:606–611. doi: 10.1016/j.bbrc.2003.11.153. [DOI] [PubMed] [Google Scholar]
- 18.Lila N, Rouas-Freiss N, Dausset J, Carpentier A, Carosella ED. Soluble HLA-G protein secreted by allo-specific CD4+ T cells suppresses the allo-proliferative response: a CD4+ T cell regulatory mechanism. Proc Natl Acad Sci U S A. 2001;98:12150–12155. doi: 10.1073/pnas.201407398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Burke P, Yang Y, Seiss K, Beamon J, et al. Soluble HLA-G inhibits myeloid dendritic cell function in HIV-1 infection by interacting with leukocyte immunoglobulin-like receptor B2. J Virol. 2010;84:10784–10791. doi: 10.1128/JVI.01292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935–944. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 21.Le Rond S, Azema C, Krawice-Radanne I, Durrbach A, Guettier C, et al. Evidence to support the role of HLA-G5 in allograft acceptance through induction of immunosuppressive/regulatory T cells. J Immunol. 2006;176:3266–3276. doi: 10.4049/jimmunol.176.5.3266. [DOI] [PubMed] [Google Scholar]
- 22.LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040–2048. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 23.Matte C, Lajoie J, Lacaille J, Zijenah LS, Ward BJ, et al. Functionally active HLA-G polymorphisms are associated with the risk of heterosexual HIV-1 infection in African women. AIDS. 2004;18:427–431. doi: 10.1097/00002030-200402200-00008. [DOI] [PubMed] [Google Scholar]
- 24.Segat L, Catamo E, Fabris A, Morgutti M, D'Agaro P, et al. HLA-G*0105N allele is associated with augmented risk for HIV infection in white female patients. AIDS. 2010;24:1961–1964. doi: 10.1097/QAD.0b013e32833c3324. [DOI] [PubMed] [Google Scholar]
- 25.Lajoie J, Hargrove J, Zijenah LS, Humphrey JH, Ward BJ, et al. Genetic variants in nonclassical major histocompatibility complex class I human leukocyte antigen (HLA)-E and HLA-G molecules are associated with susceptibility to heterosexual acquisition of HIV-1. J Infect Dis. 2006;193:298–301. doi: 10.1086/498877. [DOI] [PubMed] [Google Scholar]
- 26.Aikhionbare FO, Kumaresan K, Shamsa F, Bond VC. HLA-G DNA sequence variants and risk of perinatal HIV-1 transmission. AIDS Res Ther. 2006;3:28. doi: 10.1186/1742-6405-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabris A, Catamo E, Segat L, Morgutti M, Arraes LC, et al. Association between HLA-G 3′UTR 14-bp polymorphism and HIV vertical transmission in Brazilian children. AIDS. 2009;23:177–182. doi: 10.1097/QAD.0b013e32832027bf. [DOI] [PubMed] [Google Scholar]
- 28.Lajoie J, Massinga LM, Poudrier J, Guedou F, Pepin J, et al. Blood soluble human leukocyte antigen G levels are associated with human immunodeficiency virus type 1 infection in Beninese commercial sex workers. Hum Immunol. 2010;71:182–185. doi: 10.1016/j.humimm.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Lajoie J, Fontaine J, Tremblay C, Routy JP, Poudrier J, et al. Persistence of high levels of blood soluble human leukocyte antigen-G is associated with rapid progression of HIV infection. AIDS. 2009;23:1437–1440. doi: 10.1097/QAD.0b013e32832d0825. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson R, Ramanakumar AV, Richardson H, Tellier PP, Coutlee F, et al. Human leukocyte antigen (HLA)-E and HLA-G polymorphisms in human papillomavirus infection susceptibility and persistence. Hum Immunol. 2011;72:337–341. doi: 10.1016/j.humimm.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Yie SM, Li LH, Xiao R, Librach CL. A single base-pair mutation in the 3′-untranslated region of HLA-G mRNA is associated with pre-eclampsia. Mol Hum Reprod. 2008;14:649–653. doi: 10.1093/molehr/gan059. [DOI] [PubMed] [Google Scholar]
- 32.LeMaoult J, Zafaranloo K, Le Danff C, Carosella ED. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J. 2005;19:662–664. doi: 10.1096/fj.04-1617fje. [DOI] [PubMed] [Google Scholar]
- 33.Morel E, Bellon T. HLA class I molecules regulate IFN-gamma production induced in NK cells by target cells, viral products, or immature dendritic cells through the inhibitory receptor ILT2/CD85j. J Immunol. 2008;181:2368–2381. doi: 10.4049/jimmunol.181.4.2368. [DOI] [PubMed] [Google Scholar]
- 34.McIntire RH, Morales PJ, Petroff MG, Colonna M, Hunt JS. Recombinant HLA-G5 and -G6 drive U937 myelomonocytic cell production of TGF-beta1. J Leukoc Biol. 2004;76:1220–1228. doi: 10.1189/jlb.0604337. [DOI] [PubMed] [Google Scholar]
- 35.Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, et al. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol. 1999;11:803–811. doi: 10.1093/intimm/11.5.803. [DOI] [PubMed] [Google Scholar]
- 36.van der Meer A, Lukassen HG, van Cranenbroek B, Weiss EH, Braat DD, et al. Soluble HLA-G promotes Th1-type cytokine production by cytokine-activated uterine and peripheral natural killer cells. Mol Hum Reprod. 2007;13:123–133. doi: 10.1093/molehr/gal100. [DOI] [PubMed] [Google Scholar]
- 37.Liang S, Ristich V, Arase H, Dausset J, Carosella ED, et al. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6–STAT3 signaling pathway. Proc Natl Acad Sci U S A. 2008;105:8357–8362. doi: 10.1073/pnas.0803341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanai T, Fujii T, Kozuma S, Yamashita T, Miki A, et al. Soluble HLA-G influences the release of cytokines from allogeneic peripheral blood mononuclear cells in culture. Mol Hum Reprod. 2001;7:195–200. doi: 10.1093/molehr/7.2.195. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Houser BL, Nicotra ML, Strominger JL. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci U S A. 2009;106:5767–5772. doi: 10.1073/pnas.0901173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:e9. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lajoie J, Poudrier J, Massinga-Loembe M, Guedou F, Agossa-Gbenafa C, et al. Differences in immunoregulatory cytokine expression patterns in the systemic and genital tract compartments of HIV-1-infected commercial sex workers in Benin. Mucosal Immunol. 2008;1:309–316. doi: 10.1038/mi.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lajoie J, Poudrier J, Massinga LM, Guedou F, Leblond F, et al. Chemokine expression patterns in the systemic and genital tract compartments are associated with HIV-1 infection in women from Benin. J Clin Immunol. 2010;30:90–98. doi: 10.1007/s10875-009-9343-3. [DOI] [PubMed] [Google Scholar]
- 43.Gumbi PP, Nkwanyana NN, Bere A, Burgers WA, Gray CM, et al. Impact of mucosal inflammation on cervical human immunodeficiency virus (HIV-1)-specific CD8 T-cell responses in the female genital tract during chronic HIV infection. J Virol. 2008;82:8529–8536. doi: 10.1128/JVI.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Low N, Chersich MF, Schmidlin K, Egger M, Francis SC, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med. 2011;8:e1000416. doi: 10.1371/journal.pmed.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thurman AR, Doncel GF. Innate immunity and inflammatory response to Trichomonas vaginalis and bacterial vaginosis: relationship to HIV acquisition. Am J Reprod Immunol. 2011;65:89–98. doi: 10.1111/j.1600-0897.2010.00902.x. [DOI] [PubMed] [Google Scholar]
- 47.Mirmonsef P, Gilbert D, Zariffard MR, Hamaker BR, Kaur A, et al. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2011;65:190–195. doi: 10.1111/j.1600-0897.2010.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen CR, Plummer FA, Mugo N, Maclean I, Shen C, et al. Increased interleukin-10 in the the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? AIDS. 1999;13:327–332. doi: 10.1097/00002030-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 49.Yasodhara P, Raghunath M, Sreeramulu D, Venu L, Hemalatha R, et al. Local immunity in Indian women with bacterial vaginosis. J Reprod Immunol. 2006;70:133–141. doi: 10.1016/j.jri.2005.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
sHLA-G genital levels according to the presence or absence of vaginosis in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1- uninfected non-CSW control subjects.
(DOC)
Spearman's correlations between soluble HLA-G and cytokine genital levels in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1-uninfected non-CSW women.
(DOC)
Spearman's correlations between soluble HLA-G and chemokine genital levels in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1- uninfected non-CSW women.
(DOC)
Cytokine genital levels according to the presence or absence of bacterial vaginosis in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1- uninfected non-CSW women.
(DOC)
Chemokine genital levels according to the presence or absence of bacterial vaginosis in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-1- uninfected non-CSW women.
(DOC)