Cohesin enables post-replicative DNA repair and chromosome segregation by holding sister chromatids together from the time of DNA replication in S-phase until mitosis1. There is growing evidence that cohesin also forms long range chromosomal cis-interactions2–4 and may regulate gene expression2–10 in association with CTCF (ref. 8, 9), mediator4 or tissue-specific transcription factors10. Human cohesinopathies like Cornelia de Lange Syndrome are thought to result from impaired non-canonical cohesin functions7, but a clear distinction between cohesin's cell division-related and cell division-independent functions as exemplified in Drosophila11–13 has not been demonstrated in vertebrate systems. To address this, we deleted the cohesin locus Rad21 in mouse thymocytes at a time in development when these cells stop cycling and rearrange their T cell receptor alpha locus (Tcra). Rad21 deficient thymocytes had a normal lifespan and retained the ability to differentiate, albeit with reduced efficiency. Loss of Rad21 led to a defective chromatin architecture at the Tcra locus, where cohesin binding sites flank the TEA promoter and the Eα enhancer, and demarcate Tcra from interspersed Tcrd elements and neighbouring housekeeping genes. Cohesin was required for long-range promoter-enhancer interactions, Tcra transcription, H3K4me3 histone modifications that recruit the recombination machinery14,15 and Tcra rearrangement. Provision of pre-rearranged T cell receptor transgenes largely rescued thymocyte differentiation, demonstrating that among thousands of potential target genes across the genome4,8–10 defective Tcra rearrangement was limiting for the differentiation of cohesin-deficient thymocytes. These findings firmly establish a cell division-independent role for cohesin in Tcra locus rearrangement and provide a comprehensive account of the mechanisms by which cohesin enables cellular differentiation in a well-characterised mammalian system.
The somatic rearrangement of lymphocyte receptor loci is central to adaptive immunity16. Gene segments distributed over millions of base pairs of genomic DNA are transcribed, brought into proximity with each other, and recombined in a cell lineage- and developmental stage-specific fashion17–19. In developing thymocytes, proliferation and differentiation are tightly linked and the activity of Rag (recombinase activating gene) proteins is restricted to the G1 phase of the cell cycle20. Early thymocytes at the CD4− CD8− double negative (DN) stages 1 and 2 proliferate in response to cytokines and briefly arrest at the DN3 stage, where they rearrange the T cell receptor (TCR) beta locus (Fig. 1a). Pre-T cell receptor signals drive a phase of proliferation that extends to the early CD4+ CD8+ double positive (DP) stage. Shortly after the acquisition of CD4 and CD8, DP thymocytes lose the expression of the transferrin receptor CD71 (ref. 21) and become small, non-proliferating CD71− DP cells (Fig. 1a), which represent the great majority of thymocytes. During their life span of 3 to 4 days, DP thymocytes undergo multiple rounds of TCR alpha (Tcra) rearrangement16,18,19. Successful TCR expression and engagement selects a minority (3–5%) of DP thymocytes for differentiation via a CD4+ CD8lo intermediate stage towards long-lived CD4 or CD8 SP cells, again with minimal proliferation16,22 (Fig. 1a).
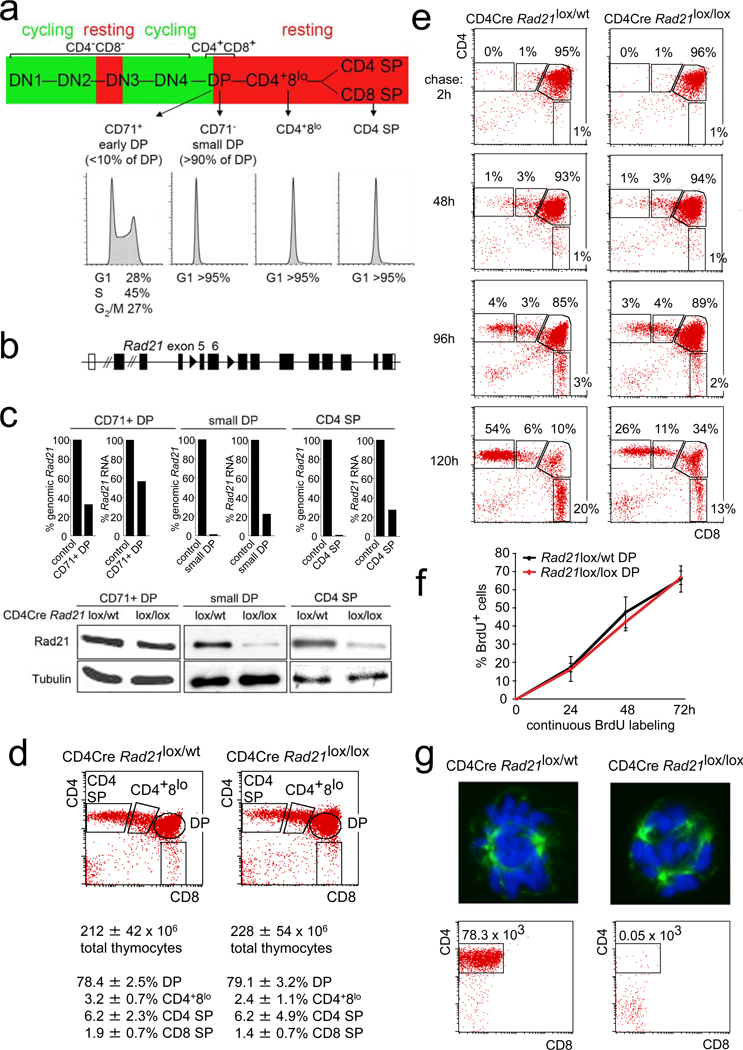
Figure 1. Genetic cohesin depleted in non-dividing thymocytes.
a) Thymocyte differentiation from left to right: CD4− CD8− double negative (DN) stages 1 to 4; CD4+ CD8+ double positive (DP), CD4+8lo; CD4 or CD8 single positive (SP) cells. Proliferation is in green, cell cycle arrest in red. Histograms show DNA content.
b) Conditional Rad21 allele (see supplementary fig. 1a).
c) Real time genomic PCR of Rad21 locus deletion, RT-PCR of Rad21 RNA and western blotting of Rad21 protein.
d) Cell numbers and flow cytometric analysis of thymocyte subsets in 6 week-old CD4Cre Rad21lox/lox and CD4Cre Rad21lox/lwt mice (mean ± SD, n = 12).
e) Pulse chase analysis of CD4Cre Rad21lox/wt and CD4Cre Rad21lox/lox thymocytes. Dot blots are gated on BrdU+ cells (see supplementary fig. 2b).
f) Continuous BrdU labelling for DP thymocyte turnover. See supplementary Fig. 2c for CD4+8lo and CD4 SP subsets (mean ± SD, n = 3–5 per data point).
g) Top: metaphase spreads of 2 day activated thymocytes stained for alpha-tubulin (green) and DNA (DAPI, blue, see supplementary fig. 3). Bottom: Cells recovered after 5 days.
To address the role of cohesin in Tcra rearrangement and thymocyte differentiation we combined a conditional allele encoding the cohesin subunit Rad21 (Rad21lox, fig. 1b, Supplementary Fig. 1a) with a Cre transgene under the control of Cd4 regulatory elements (CD4Cre), which becomes active at the transition from the CD4− CD8− DN to the DP stage23. Pilot experiments with YFP reporters showed CD4Cre-dependent accumulation of YFP after the CD71+ proliferative stage in non-dividing DP thymocytes (Supplementary fig. 1b). Proliferating CD4Cre Rad21lox/lox CD71+ DP cells showed partial locus deletion but retained >50% Rad21 mRNA and protein (Fig. 1c). Rad21 genomic deletion was essentially complete (>97%) and Rad21 RNA and protein levels were substantially reduced in non-dividing DP thymocytes (Fig. 1c). Hence, cohesin was selectively depleted from non-dividing thymocytes. Importantly, CD4Cre Rad21lox/lox DP thymocyte numbers were normal (Fig. 1d). Intermediate CD4+8lo and mature CD4 SP and CD8 SP thymocytes accumulated slowly in CD4Cre Rad21lox/lox mice (Supplementary fig. 2a) but were present in normal numbers by 6 weeks of age (Fig. 1d).
Bromodeoxyuridine (BrdU) incorporation into replicating DNA can identify proliferating thymocyte populations and track their differentiation22. Pulse-chase experiments labeled proliferating (CD71+) DP but not non-proliferating CD4+8lo, CD4 SP or CD8 SP thymocytes22 (Fig. 1e, 2 hour time point). During the subsequent chase period, BrdU-labeled DP cells differentiated to become CD4+8lo and eventually CD4 or CD8 SP (Fig. 1e, left panel). This sequence of differentiation was preserved in CD4Cre Rad21lox/lox thymocytes, but the proportion of DP thymocytes that became CD4 SP or CD8 SP was reduced (Fig. 1e, right panel, and supplementary fig. 2b).
In continuous BrdU labeling experiments, the percentage of BrdU+ cells indicates population turnover22. Importantly, cohesin-deficient and control DP thymocytes labeled with similar kinetics (Fig. 1f). Consistent with the pulse-labeling data (Fig. 1e), the accumulation of CD4+8lo, and CD4 SP subsets was reduced in CD4Cre Rad21lox/lox mice (Supplementary fig. 2c). Hence, cohesin depletion impaired the differentiation of DP thymocytes, but not their survival.
Unlike many other differentiated cell types, mature thymocytes can be induced to re-enter the cell cycle. In vitro activated CD4Cre Rad21lox/lox CD4 SP thymocytes showed abnormal mitotic figures with multiple spindles, chromosome segregation defects (Fig. 1g, supplementary fig. 3), and poor survival (Fig. 1g). CD4Cre-mediated deletion of Rad21 therefore generates thymocytes that die when forced to divide, yet have a normal lifespan as non-dividing cells in vivo. This affords the interrogation of cohesin functions in interphase, independent of essential cohesin functions during cell division.
Rad21 chromatin immunoprecipitation and sequencing (ChIP-seq) mapped cohesin to key positions within the Tcra locus in DP thymocytes (Fig. 2a). Cohesin was abundant at the locus control region24, which separates the Tcra enhancer, Eα, from the neighbouring Dad1 housekeeping gene25,26. Other prominent cohesin sites separated the Tcra TEA promoter from the Tcrd enhancer, Eδ, which controls Tcrd gene segments that are interspersed within the Tcra locus but follow a distinct developmental stage-specific programme18. Cohesin colocalisation with the insulator protein CTCF (ref. 8, 9) is found at the Dad1 site 10kb downstream of Eα, while at the Jα49 promoter cohesion associates with its loading protein Nipbl and mediator subunits4 more than with CTCF (Supplementary fig. 4). Interestingly, the major Tcra regulatory elements Eα and TEA bound copious amounts of cohesin, Nipbl, and mediator as well as CTCF (Supplementary fig. 4).
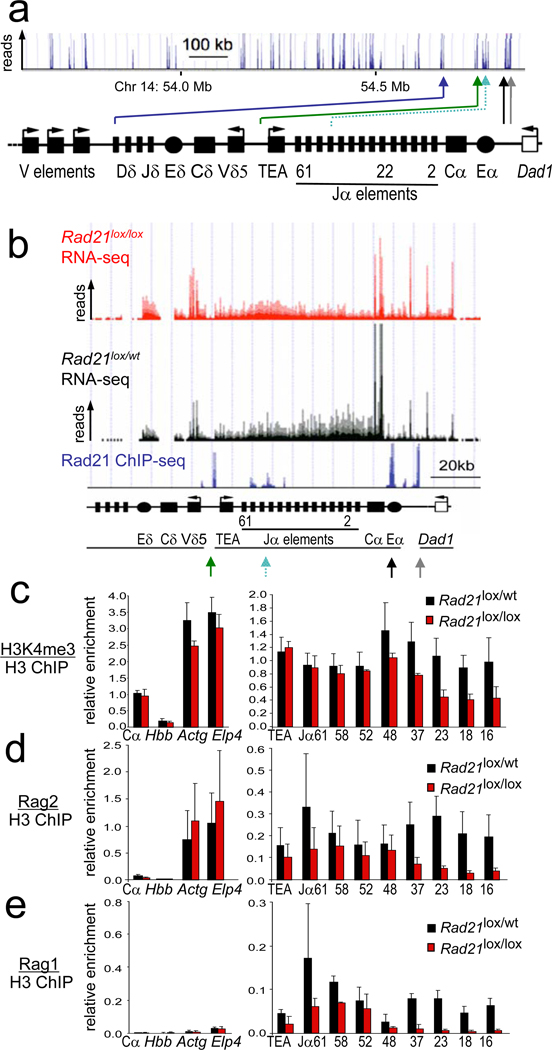
Figure 2. Cohesin affects Tcra transcription Rag recombinase recruitment.
a) Rad21 ChIP-seq of the 3' part of the Tcra locus in DP thymocytes. Arrowheads highlight cohesin sites at the Eα enhancer (black), the Tcra locus control region (grey), Jα promoters (turquoise), the TEA promoter (green) and between Tcrd elements and V gene segments (blue).
b) RNA-seq of Tcra in CD4Cre Rad21lox/lox (red) and control Rad21lox/wt (black) DP thymocytes. Rad21 ChIP-seq is in blue.
c) ChIP of H3K4me3 relative to total H3 in CD4Cre Rad21lox/lox and control DP thymocytes. Hbb is a negative and Actg and Elp4 are positive control loci (mean ± SE of 2 independent experiments). p=0.016 for all Jα elements; p=0.38 (NS) for proximal (Jα61–48) and p=0.004 for distal (Jα37–16) Jα elements.
d) ChIP of Rag2 relative to total H3 as in c) (mean ± SE of 3 independent experiments). p=0.0001 for all Jα elements; p=0.054 (NS) for proximal (Jα61–48) and p=0.0001 for distal (Jα37–16) Jα elements.
e) ChIP of Rag1 relative to total H3 as in c) (mean ± SE of 2 independent experiments). p=0.005 for all Jα elements; p=0.17 (NS) for proximal (Jα61–48) and p=0.003 for distal (Jα37–16) Jα elements.
RNA-sequencing (RNA-seq) indicated that Tcra constant region (Cα) transcripts were considerably more abundant than transcripts from the neighbouring Dad1 gene and the Tcrd constant region (Cδ) in control DP thymocytes. In cohesin-depleted small DP thymocytes, Cδ and Dad1 transcripts were elevated at the expense of Cα transcripts (Fig. 2b) as confirmed by real time RT-PCR (Supplementary fig. 5a). Moreover, transcription across the Tcra joining elements, Jα, was skewed: control DP thymocytes preferentially transcribed distal (3') Jα elements, while cohesin-depleted DP thymocytes preferentially transcribed proximal (5') Jα elements (Fig. 2b).
Transcription of lymphocyte receptor loci facilitates rearrangement27 in part via the trimethylation of histone H3 at lysine 4 (H3K4me3). H3K4me3 recruits Rag2 protein14,15,28, which together with Rag1 forms the recombinase complex18. ChIP showed reduced H3K4me3 deposition (Fig. 2c) and Rag binding (Fig. 2d,e) at distal Jα elements in CD4Cre Rad21lox/lox DP thymocytes. Hence, cohesin deficiency affected Tcra transcription, H3K4me3 histone modifications and the recruitment of Rag recombinases.
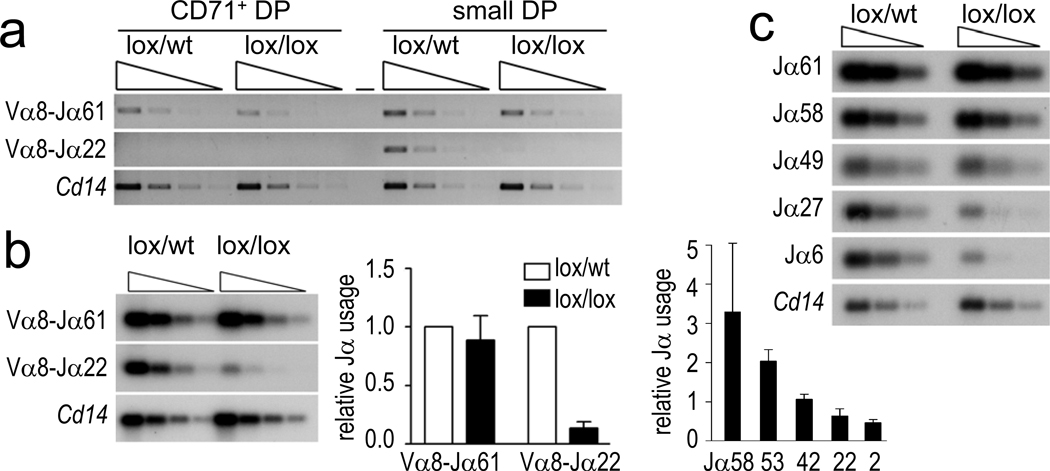
Primary Tcra rearrangements involve proximal (5’) Jα elements and occur in early CD71+ DP thymocytes, while secondary rearrangements involve progressively more distal (3’) Jα elements in non-dividing DP thymocytes18 (Supplementary fig. 1b). CD71+ DP CD4Cre Rad21lox/lox thymocytes had near-normal Rad21 protein levels (Fig. 1c) and Cδ, Cα and Dad1 transcription (Supplementary fig. 5b), and primary rearrangements of proximal Jα elements were present at normal levels (Fig. 3a, b). In contrast, secondary Tcra rearrangements were substantially impaired in non-dividing CD4Cre Rad21lox/lox DP thymocytes (Fig. 3a, b), which were depleted of cohesin (Fig. 1c). The usage of the distal Jα22 element, for example, was reduced on average by 86% (Fig. 3b, middle), reflecting a progressive under-representation of 3’ Jα segments (Fig. 3b, right). This was confirmed by analysis of additional Vα gene families (Supplementary fig. 6a) and by RT-PCR-based copy number analysis of mature Tcra transcripts (Supplementary fig. 6b). Therefore, primary rearrangements occurred before the depletion of cohesin, while reduced cohesin expression impaired secondary rearrangements in non-dividing DP thymocytes. Normal BrdU labeling kinetics (Fig. 1f) exclude decreased lifespan as an explanation for aberrant Tcra rearrangement29. Consistent with the defective recruitment of Rag proteins to the Tcra locus (Fig2d, e), double strand breaks were reduced in cohesin-deficient thymocytes (Fig. 3c). This identifies Rag cleavage, rather than double strand break repair as the limiting step for Tcra rearrangements in cohesin-deficient thymocytes.
Figure 3. Cohesin affects Tcra rearrangement.
a) Three-fold dilutions of genomic Vα8-Jα PCR products from CD71+ or CD71− DP thymocytes visualised with ethidium bromide. Cd14 is a genomic control.
b) Genomic PCR products from small DP thymocytes visualised by Southern blotting with Jα-specific probes (left). Usage of the distal Jα22 element was reduced by 86% (middle, mean ± SE, n=3). Southern blotting of Vα8-Jα RT-PCR products from CD4Cre Rad21lox/lox normalised to control DP thymocytes (right, mean ± SE, n=3).
c) Double strand breaks in three-fold serially diluted genomic DNA from CD4Cre Rad21lox/lox and control DP thymocytes detected by ligation-mediated PCR.
Since Tcra rearrangement changes the positioning of regulatory elements18, altered Tcra transcription (Fig. 2b) could either be a direct consequence of cohesin depletion, or result indirectly from defective rearrangement. To distinguish between these possibilities we compared intronic Tcra transcript copy numbers in the absence of rearrangement in Rag1-deficient CD4Cre Rad21lox/wt and CD4Cre Rad21lox/lox DP thymocytes. Cohesin depletion reduced the transcription (Fig. 4a) and H3K4me3 (Supplementary fig. 7). at Jα independently of Tcra rearrangement.
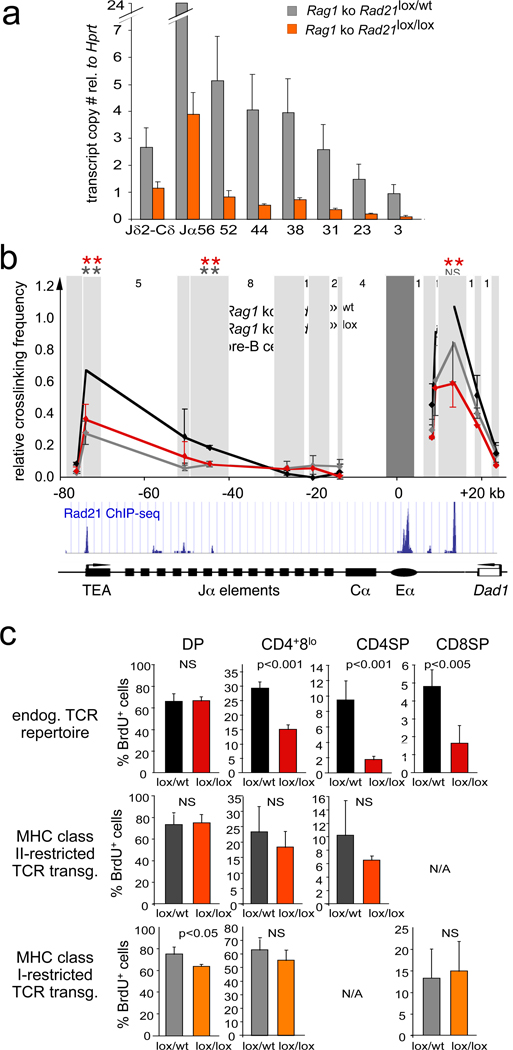
Figure 4. Cohesin mediates long-range interactions between regulatory elements that control Tcra transcription.
a) Transcript copy number of the unrearranged Tcra J region in Rag1-deficient CD4Cre Rad21lox/lox and Rad21lox/wt DP thymocytes (mean ± SE, n=3).
b) 3C analysis of long-range interactions between Eα and Tcra restriction fragments (shaded) in Rag1-deficient CD4Cre Rad21lox/wt (black, mean ± SD, n=3), CD4Cre Rad21lox/lox (red, mean ± SD, n=3) DP thymocytes and pre-B cells (grey, mean ± SD, n=3). Intervening HindIII fragment numbers and genomic distances are indicated. Stars: p < 0.05 (grey: control thymocytes versus pre-B cells; red: control versus cohesin-depleted thymocytes; NS = not significant).
c) TCR transgenes rescue the differentiation of cohesin-depleted thymocytes. Top: Percentages BrdU+ cells in CD4+8lo, CD4 SP and CD8 SP thymocytes. The differentiation of cohesin-deficient thymocytes is rescued by MHC class II-restricted (middle) and MHC class I-restricted TCR transgenes (bottom), n=3–5 per data point ± SD.
To explore how cohesin affects Tcra transcription we analysed long-range interactions between the TEA promoter and Eα, which are separated by approximately 80 kb of genomic DNA and together regulate the transcription of Tcra. In chromosome conformation capture (3C) assays30, Eα interacted strongly with TEA in DP thymocytes (Fig. 4b) but cohesin depletion reduced these interactions to the level found in pre-B cells, where Tcra is not detectably transcribed (Fig. 4b). Hence, the extent of Tcra enhancer-promoter interactions was cell type-specific, correlated with Tcra transcription, and was cohesin-dependent. A role for cohesin in additional enhancer-promoter interactions during sequential Tcra rearrangements is suggested by Eα contacts with promoters between Jα49 and Jα37 (Fig. 4b), which can drive Jα transcription in the absence of TEA18, and by the cohesin binding to numerous Vα promoters (Fig. 2a). Cohesin depletion also affected Eα interactions with the neighbouring Dad1 cohesin site, and therefore the topology of the Tcra locus control region24, which has CTCF-dependent transcriptional insulator function25,26 (Fig. 4b). As cohesin mediates CTCF-dependent transcriptional insulation8,9, increased Dad1 expression at the expense of Cα (Fig. 2b) may indicate impaired insulator function.
To test whether aberrant Tcra rearrangement caused inefficient differentiation we equipped cohesin-deficient thymocytes with transgenes encoding rearranged TCRs. Compared to endogenously rearranged TCRs (Fig. 4c, top), the expression of MHC class II (Fig. 4c, middle) or MHC class I restricted TCRs (Fig. 4c, bottom) markedly improved the generation of CD4Cre Rad21lox/lox CD4+8lo and SP thymocytes.
In summary, cohesin shapes the chromatin architecture of the Tcra locus by mediating cell type-specific long-range interactions between enhancer and promoter elements that control transcription, H3K4me3 deposition, Rag recombinase recruitment, and ultimately Tcra rearrangement. These defects compromise thymocyte differentiation by limiting the number and diversity of sequential Tcra rearrangements. Hence, cohesin contributes to cellular differentiation in a well-characterised mammalian system.
Methods summary
The conditional Rad21 allele was generated by inserting LoxP sites into introns 4 and 6 (Supplementary fig. 1a). Methods used for RT- and genomic PCR (ref. 2), flow cytometry2, 3C analysis2, ChIP for cohesin2, histone modifications30 and Rag proteins30 have been described. See supplementary methods for other mouse strains, BrdU labeling and detection, ChIP-seq and RNA-seq protocols, ligation-mediated PCR, confocal microscopy, Tcra rearrangement assays and copy number measurements.
Supplementary Material
Acknowledgements
We thank Drs. S. Hadjur, D. Tough, L. Williams, Z. Webster, J. Godwin and H-Y Shih for help and advice, L. Game and M. Jones for high throughput sequencing, A. Giess for sequence alignment, and J. Elliott and P. Hexley for cell sorting. Supported by the Medical Research Council, UK (VS, TL, HM-B, KEB, TC, AT, LA, AGF, KN, MM), the European Union FP6 integrated project HEROIC (HM), EU and the Marie Curie Research Training Network Chromatin Plasticity (HM-B), the Boehringer Ingelheim Fonds (TL), the Wellcome Trust (DJA, KN) and the National Institutes of Health (BH, MSK, GT, DGS). DGS is an investigator of the Howard Hughes Medical Institute.
Footnotes
Author contributions. VS and MM conceived the study with critical input from DGS, LA, AGF, MSK and KM. VS, BH, KTK, TL, HMB, KEB, GT, KH and MM conducted experiments, KTK, DJA, KM, GT and DGS designed and generated novel materials, TC, AT and HM analysed data, VS and MM wrote the paper and all authors discussed the results and commented on the manuscript.
Author information. The authors have no competing financial interests.
References
- 1.Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annu Rev Genet. 2009;43:525–558. doi: 10.1146/annurev-genet-102108-134233. [DOI] [PubMed] [Google Scholar]
- 2.Hadjur S, et al. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460:410–413. doi: 10.1038/nature08079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degner SC, et al. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1019391108. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kagey MH, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagstrom KA, Meyer BJ. Condensin and cohesin: more than chromosome compactor and glue. Nat Rev Genet. 2003;4:520–534. doi: 10.1038/nrg1110. [DOI] [PubMed] [Google Scholar]
- 6.Rollins RA, Morcillo P, Dorsett D. Nipped-B, a Drosophila homologue of chromosomal adherins participates in activation by remote enhancers in the cut and Ultrabithorax genes. Genetics. 1999;152:577–593. doi: 10.1093/genetics/152.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strachan T. Cornelia de Lange Syndrome and the link between chromosomal function, DNA repair and developmental gene regulation. Curr Opin Genet Dev. 2005;15:258–264. doi: 10.1016/j.gde.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Parelho V, et al. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell. 2008;132:422–433. doi: 10.1016/j.cell.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Wendt KS, et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008;451:796–801. doi: 10.1038/nature06634. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt D, et al. A CTCF-independent role for cohesin in tissue-specific transcription. Genome Res. 2010;20:578–588. doi: 10.1101/gr.100479.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuldiner O, et al. piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning. Dev. Cell. 2008;14:227–238. doi: 10.1016/j.devcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pauli A, et al. Cell-type-specific TEV protease cleavage reveals cohesin functions in Drosophila neurons. Dev. Cell. 2008;14:239–251. doi: 10.1016/j.devcel.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauli A, et al. A direct role for cohesin in gene regulation and ecdysone response in Drosophila salivary glands. Curr Biol. 2010;20:1787–1798. doi: 10.1016/j.cub.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews AG, et al. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature. 2007;450:1106–1110. doi: 10.1038/nature06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Subrahmanyam R, Chakraborty T, Sen R, Desiderio S. A plant homeodomain in RAG-2 that binds hypermethylated lysine 4 of histone H3 is necessary for efficient antigen-receptor-gene rearrangement. Immunity. 2007;27:561–571. doi: 10.1016/j.immuni.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kisielow P, von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- 17.Stanhope-Baker P, Hudson KM, Shaffer AL, Constantinescu A, Schlissel MS. Cell type-specific chromatin structure determines the targeting of V(D)J recombinase activity in vitro. Cell. 1996;85:887–897. doi: 10.1016/s0092-8674(00)81272-6. [DOI] [PubMed] [Google Scholar]
- 18.Krangel MS. Mechanics of T cell receptor gene rearrangement. Curr Opin. Immunol. 2009;21:133–139. doi: 10.1016/j.coi.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jhunjhunwala S, van Zelm MC, Peak MM, Murre C. Chromatin architecture and the generation of antigen receptor diversity. Cell. 2009;138:435–448. doi: 10.1016/j.cell.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desiderio S, Lin WC, Li Z. The cell cycle and V(D)J recombination. Curr Top Microbiol Immunol. 1996;217:45–59. doi: 10.1007/978-3-642-50140-1_4. [DOI] [PubMed] [Google Scholar]
- 21.Brekelmans P, et al. Transferrin receptor expression as a marker of immature cycling thymocytes in the mouse. Cell Immunol. 1994;159:331–339. doi: 10.1006/cimm.1994.1319. [DOI] [PubMed] [Google Scholar]
- 22.Huesmann M, Scott B, Kisielow P, von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991;66:533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 24.Diaz P, Cado D, Winoto A. A locus control region in the T cell receptor alpha/delta locus. Immunity. 1994;1:207–217. doi: 10.1016/1074-7613(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 25.Magdinier F, Yusufzai TM, Felsenfeld G. Both CTCF-dependent and -independent insulators are found between the mouse T cell receptor alpha and Dad1 genes. J Biol Chem. 2004;279:25381–25389. doi: 10.1074/jbc.M403121200. [DOI] [PubMed] [Google Scholar]
- 26.Zhong XP, Krangel MS. Enhancer-blocking activity within the DNAse I hypersensitivity site 2 to 6 region between the T cell receptor α and Dad1 genes. J. Immunol. 1999;163:295–300. [PubMed] [Google Scholar]
- 27.Abarrategui I, Krangel MS. Germline transcription: a key regulator of accessibility and recombination. Adv Exp Med Biol. 2009;650:93–102. doi: 10.1007/978-1-4419-0296-2_8. [DOI] [PubMed] [Google Scholar]
- 28.Ji Y, et al. The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell. 2010;141:419–431. doi: 10.1016/j.cell.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo J, et al. Regulation of the TCRalpha repertoire by the survival window of CD4(+)CD8(+) thymocytes. Nat Immunol. 2002;3:469–476. doi: 10.1038/ni791. [DOI] [PubMed] [Google Scholar]
- 30.Dekker J. The three 'C' s of chromosome conformation capture: controls, controls, controls. Nat Methods. 2006;3:17–21. doi: 10.1038/nmeth823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.